Abstract
Fibrosis is an important feature of inflammatory bowel diseases (IBD), particularly Crohn's disease (CD), but its pathogenesis is poorly understood. To determine the postulated involvement of epithelial−mesenchymal transition (EMT) in the development of fibrosis in IBD, we analysed the expression profiles of the miR‐200 family which has been shown to induce EMT in experimental models and various human diseases. We also analysed the expression of Snail and Slug, postulated targets of the investigated microRNAs. Ten patients with ulcerative colitis (UC) and 10 patients with CD who underwent colon resection were included. From each, two tissue samples were chosen (one with the most severely and one with the least affected or normal mucosa) for analysis of microRNAs expression using real‐time polymerase chain reaction, and Snail and Slug expression using immunohistochemistry. We found significant down‐regulation of all investigated microRNAs in CD, and of three investigated microRNAs in UC, in comparison to the normal or the least affected mucosa. Comparing UC and CD, four microRNAs were significantly more down‐regulated in CD than in UC. Snail and Slug were expressed in the injured epithelium and occasionally in mesothelial cells and submesothelial fibroblasts. Our finding of down‐regulation of the miR‐200 family and up‐regulation of transcription repressors Snail and Slug supports the postulated role of EMT in the pathogenesis of fibrosis in IBD. The described expression patterns are consistent with the notion that fibrosis does not occur only in CD but also in UC, being much more severe in CD.
Keywords: Crohn's disease, ulcerative colitis, fibrosis, epithelial−mesenchymal transition, microRNA, Snail, Slug
Introduction
Fibrosis is an important feature of inflammatory bowel diseases (IBD), with serious clinical consequences. There are significant differences between Crohn's disease (CD) and ulcerative colitis (UC). In CD, inflammation and fibrosis are usually transmural, often leading to stenosis and stricture formation 1. Stricture formation necessitating surgery will occur in up to 50% of patients with CD within 10 years of disease onset 2. Moreover, stricture commonly recurs at surgical anastomosis 3, requiring multiple surgeries. In UC, on the contrary, inflammation and fibrosis are restricted to mucosa and submucosa. Fibrosis may contribute to shortening and stiffening of the colon, but rarely results in clinically important stricture formation 4.
A significant progress has been made in the treatment of IBD, mainly targeting inflammation. In contrast, little progress has been made in the treatment of fibrosis 5. Cosnes et al. 6 reported an unchanged incidence of strictures and need for surgery during the last 25 years despite the more frequent use of immunosuppressive therapy. There is, therefore, an urgent need to develop new treatment modalities to prevent stricture.
One of the reasons for poor success in the treatment of fibrosis in IBD is that its pathogenesis is poorly understood. Similar to fibrosis in other diseases, it is believed to result from tissue damage due to chronic inflammation and impaired mechanisms of wound healing 7, 8. Fibrosis is characterized by proliferation of activated myofibroblasts and an excessive deposition of extracellular matrix proteins. The mechanisms by which inflammation leads to fibrosis are only beginning to be understood, but most likely involve epithelial−mesenchymal transition (EMT) 9. EMT is believed to be a key contributor to the pool of activated fibroblasts in fibrosis in various organs, such as the kidney, lung and liver 7, 10. There are limited data regarding EMT in IBD.
MicroRNAs are small, non‐coding RNAs that regulate gene expression by post‐transcriptional regulation of target genes. Due to incomplete complementarity, binding of miRNA to target 3′‐UTR causes the absence of protein synthesis by inhibition of translation of its mRNA 11. They are involved in a variety of physiological functions as well as in various human diseases including IBD 12, 13.
To analyse the postulated involvement of EMT in the development of fibrosis in IBD, we analysed the expression profiles of microRNAs of the miR‐200 family which have been shown in previous studies to induce EMT in experimental models and in human diseases 14, 15, 16, 17. We also analysed the expression of Snail and Slug, the postulated targets of the investigated miRNAs 14, 18, 19.
Materials and methods
Patients
Our study, which was approved by the Institute's Review Board, included 40 tissue samples from 20 patients who underwent colon resection (10 patients with UC, 10 patients with CD). The diagnosis of IBD was made on the basis of clinical, radiological, endoscopic and histological findings 20, 21, 22. For the purpose of this study, the most important demographic and clinical data were collected (IBD type, duration and maximal extent of the disease, therapy, indication for surgery).
Resection specimens had been handled according to standard procedures 22. Samples had been taken from inflamed mucosa and ulcers as well as from macroscopically normal mucosa. All samples had been embedded in paraffin and cut at 4 μm, stained with haematoxylin and eosin and analysed according to standard criteria 22.
For the purpose of this study, all slides were reviewed. Two slides with corresponding paraffin blocks were chosen from each case for immunohistochemistry and quantitative real‐time PCR (qPCR): one with most severe fibrosis upon histological examination and one from the normal mucosa, if present, or from the least affected mucosa, if normal mucosa was not present in the resection specimen. It may have contained mild inflammation, but not erosions, ulcers or fibrosis.
Immunohistochemistry
For immunohistochemistry, we used antibodies against Snail+Slug (dilution 1:200, ab85936; Abcam, Cambridge, UK) and E‐cadherin (dilution 1:20, SPM471; Labvision, Fremont, CA, USA). Sections were treated with biotinylated secondary antibody, followed by incubation with peroxidase conjugated streptavidin (iVIEW™ DAB Detection Kit; Ventana Medical System, Tucson, AZ, USA). Visualization of the immunoreaction was carried out with 3.3′ diaminobenzidine. Finally, sections were counterstained with haematoxylin. Adult fibrosarcoma served as a positive control for Snail and Slug, and oral mucosa for E‐cadherin. Positive controls and negative controls omitting the primary antibodies were also included.
RNA isolation
Tissue samples were cut at 10 μm from formalin‐fixed paraffin‐embedded tissue blocks using a microtome. Six to eight 10‐μm sections were used for the isolation procedure. Total RNA isolation was performed with a miRNeasy FFPE kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. All the reagents were from Qiagen, except where otherwise indicated. Briefly, 1 ml of Xylene (Merck, Kenilworth, NJ, USA) was added for de‐paraffinization, followed by brief vortexing and centrifugation. After the ethanol‐washing step, pellets were air‐dried and digestion with proteinase K was performed at 55°C for 15 min, followed by 15 min incubation at 80°C in order to partially reverse formaldehyde modification of nucleic acid. After the gDNA elimination step, 100% ethanol (Merck) was added to the samples and the mixture was transferred to an RNeasy MiniElute spin column. After two washing steps, the RNA was eluted in 30 μl of nuclease‐free water. The concentration was measured NanoDrop‐1000.
Quantitative real‐time PCR
Looped primers for specific reverse transcription (RT) of miRNAs were utilized following the manufacturer's protocol. RNU6B was used as RG. MicroRNAs, miR‐141, miR‐200a, miR‐200b, miR‐200c and miR‐429 were tested relatively to RNU6B. Briefly, 10 μl RT reaction master mix was performed with 10 ng of total RNA sample, 1.0 μl of MultiScribe Reverse Transcriptase (50 U/μl), 1 μl of RT Buffer (10×), 0.1 μl of dNTP (100 mM), 0.19 μl RNAase inhibitor (20 U/μl) and 2 μl of RT primer (5×). The reaction conditions were: at 16°C for 30 min, at 42°C for 30 min, at 85°C for 5 min.
Quantitative real‐time PCR was carried out in 20 μl PCR master mix containing 5 μl TaqMan 2× Universal PCR Master Mix, 0.5 μl TaqMan assay and 4.5 μl RT products diluted 16‐fold. The qPCR reactions were performed in duplicates as following: initial denaturation at 95°C for 10 min, and 40 cycles for 15 s at 95°C (denaturation), for 60 s at 60°C (primers annealing and elongation). The signal was collected at the end‐point of every cycle.
Prior to qPCR analysis, four pools of RNA samples were created, obtained from UC and corresponding normal mucosa, and from CD and corresponding normal mucosa. After RT, the cDNA was diluted in five steps, ranging from 4‐point dilution to 1024‐point dilution, and the probes were tested for qPCR efficiency. All the qPCR efficiency reactions were performed in triplicate.
Statistical analysis
To present relative gene expression, the method referred as the 2−ΔΔCt corrected for PCR efficiencies was used 23. Relative gene expression presents the data of the gene of interest (GOI, CtGOI) relative to reference gene (RG, CtRG), named ΔCt. Calculated ΔCt of CD or UC and adjunct normal mucosa were compared to and tested for statistical significance. For comparison of CD and UC to each other, samples were normalized to corresponding normal mucosa to obtain ΔΔCt and tested for difference in expression.
For the calculation of statistical differences, the SPSS analytical software ver. 20, (SPSS Inc., Chicago, IL, USA) was used, more precisely two‐tailed with a cut‐off point at P < 0.05. Mann–Whitney test was used for comparison of UC and CD. Wilcoxon Rank test was used for comparison of UC or CD to corresponding normal mucosa. The data were presented as fold change in graph with error bars presenting calculated fold change error using SD of the Ct triplicates.
Results
The most important demographic and clinical data at the time of surgery are presented in Table 1. Among patients with UC, there were four men and six women, aged 16–70 years (50.6 ± 18.3). Among patients with CD, there were four men and six women, aged 25–59 years (41.7 ± 13.1). The duration of the disease from the initial diagnosis to surgery ranged from 1 month to 15 years for UC and from 1 month to 35 years for CD. The most frequent indication for resection was stenosis in patients with CD and poor response to therapy in patients with UC. Normal mucosa was available in four patients with UC and in six patients with CD. In the remaining six patients with UC and four patients with CD, samples from the least affected mucosa were included as controls.
Table 1.
The most important demographic and clinical data of patients with inflammatory bowel diseases
| Ulcerative colitis (n = 10) | Crohn's disease (n = 10) | |
|---|---|---|
| Male:female | 2:3 | 2:3 |
| Age (years) (mean ± SD) | 50.6 ± 18.28 | 41.7 ± 13.1 |
| Duration of disease (years) (mean ± SD) | 7.3 ± 5.1 | 11.9 ± 10.7 |
| Indication for surgery | ||
| Treatment failure | 10 (100) | 2 (20) |
| Perforation | 0 | 1 (10) |
| Stenosis | 0 | 6 (60) |
| Fistulas | 0 | 1 (10) |
| Extent of disease | ||
| Ileum | 0 | 0 |
| Colon | 8 (80) | 5 (50) |
| Ileum and colon | 0 | 5 (50) |
| Rectosigmoid | 2 (40) | 0 |
| Medication | ||
| Methotrexate | 0 | 2 (20) |
| Infliximab | 6 (60) | 5 (50) |
| Azathioprine | 7 (70) | 6 (60) |
| Mycophenolate mofetil | 0 | 1 (10) |
| Adalimumab | 3 (30) | 4 (40) |
Values in parentheses are percentages.
Expression of the miR‐200 family in ulcerative colitis and Crohn's disease compared to corresponding normal mucosa
MicroRNAs miR‐141, miR‐200a, miR‐200b, miR‐200c and miR‐429 (ΔCt analysis) were down‐regulated in CD (~80.1‐fold, P = 0.010; ~131.9‐fold, P = 0.008; ~86.0‐fold, P = 0.008; ~96.9‐fold, P = 0.010; and ~65.0‐fold, P = 0.010, respectively; Wilcoxon signed ranks test) when compared to corresponding normal or the least affected mucosa. The results are summarized in Fig. 1A.
Figure 1.
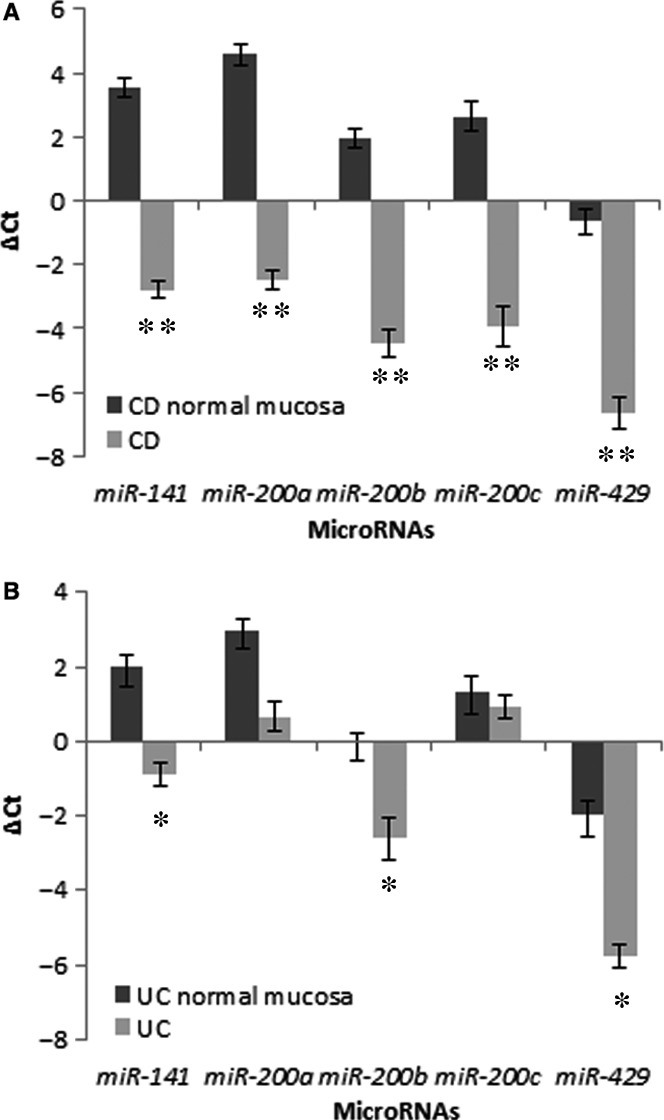
Expression of microRNAs of the miR‐200 family in Crohn's disease (A) and ulcerative colitis (B) in comparison to the corresponding normal mucosa. CD, Crohn's disease; UC, ulcerative colitis; *P ≤ 0.05; **P ≤ 0.01.
Similarly, microRNAs miR‐141, miR‐200b and miR‐429 were down‐regulated in UC (~7.5‐fold, P = 0.028; ~5.9‐fold, P = 0.047; and ~13.8‐fold, P = 0.022 respectively; Wilcoxon signed ranks test) when compared to corresponding normal mucosa. In contrast to CD, miR‐200a down‐regulation (~4.9‐fold) did not reach statistical significance, whereas expression of miR‐200c was similar as in corresponding normal or the least affected mucosa. The results are summarized in Fig. 1B.
Expression of the miR‐200 family in ulcerative colitis compared to Crohn's disease
Four of five investigated miRNAs, miR‐141, miR‐200a, miR‐200b and miR‐200c, were significantly down‐regulated in CD (ΔΔCt analysis) in comparison to UC (P = 0.041, P = 0.009, P = 0.041 and P = 0.007 respectively; Mann–Whitney test). Expression of miR‐429 was also down‐regulated but did not reach statistical significance. The results are summarized in Fig. 2.
Figure 2.
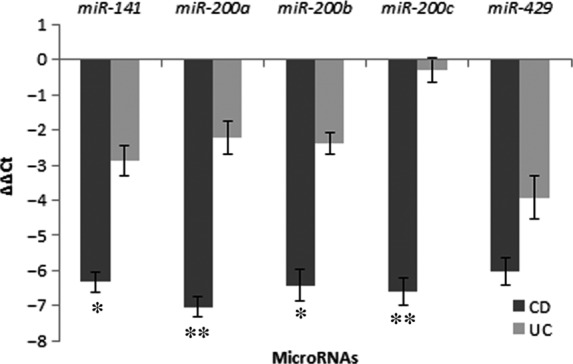
Expression of microRNAs of the miR‐200 family in Crohn's disease in comparison to ulcerative colitis. CD, Crohn's disease; UC, ulcerative colitis; *P < 0.05; **P ≤ 0.01.
Immunohistochemical expression of Snail, Slug and E‐cadherin
Snail and Slug expression was found in the crypt epithelium in 10 cases of CD (Fig. 3B) and in seven cases of UC (Fig. 4B), reaction was nuclear and cytoplasmic. They were also expressed in the mesothelial cells and in subserosal fibroblasts (Fig. 5B) in six and three cases of CD and UC respectively. The differences between CD and UC were not statistically significant.
Figure 3.
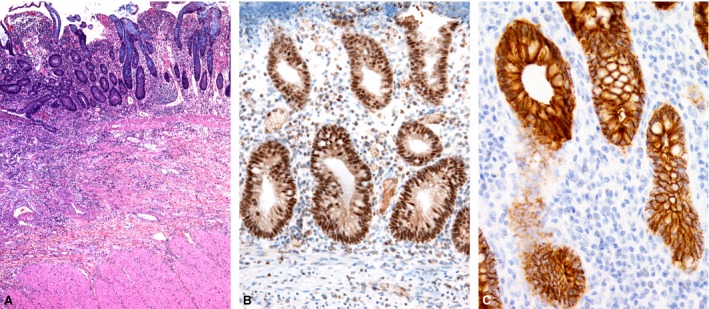
Crohn's disease. (A) Distorted crypt architecture, inflammation in the lamina propria and submucosa. (B) Immunohistochemistry for Snail and Slug: positive nuclear and cytoplasmic reaction for Snail and Slug in the crypt epithelium and some inflammatory cells. (C) Immunohistochemistry for E‐cadherin: positive membraneous reaction in the crypt epithelium, with a focal decreased intensity of staining.
Figure 4.
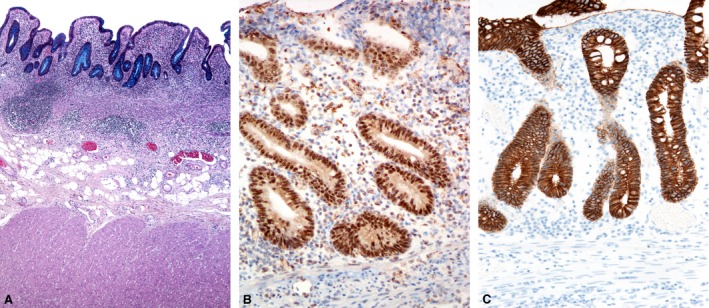
Ulcerative colitis. (A) Distorted crypt architecture, inflammation in the lamina propria and in the upper part of submucosa. (B) Immunohistochemistry for Snail and Slug: positive nuclear and cytoplasmic reaction for Snail and Slug in the crypt epithelium and some inflammatory cells. (C) Immunohistochemistry for E‐cadherin: positive membranous reaction in the crypt epithelium, with a focal decreased intensity of staining.
Figure 5.
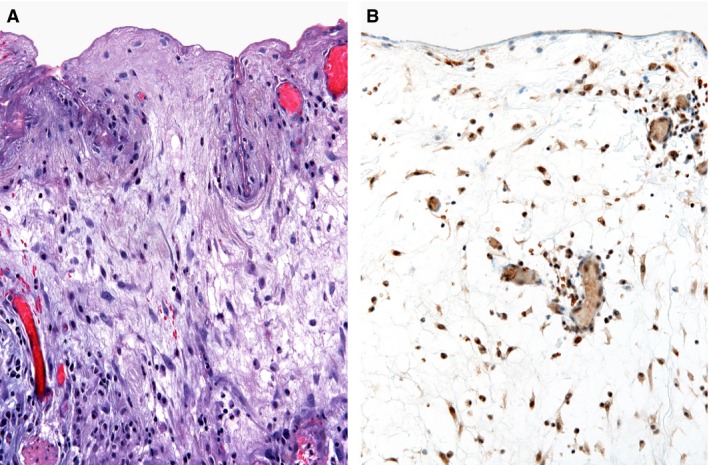
Crohn's disease. (A) Proliferation of myofibroblasts in the subserosal layer, with a positive immunohistochemistry for Snail and Slug (B).
In the normal mucosa, Snail and Slug were expressed in the nerve fibres, ganglion cells and in erythrocytes. No positivity was found in the epithelium, fibroblasts, mesothelial cells or smooth muscle cells.
Immunohistochemistry for E‐cadherin showed a decreased intensity of staining in areas of crypt destruction and cryptitis. In the preserved crypts, it exhibited almost a diffuse membranous reaction. There was a focal decrease in staining at the base and neck of the crypts (Figs 3C and 4C) in all cases.
Discussion
In this study, we analysed microRNAs of the miR‐200 family in UC and CD. The miR‐200 family comprises miR‐200a/b/c, miR‐141 and miR‐429 and has been shown to induce EMT in experimental models and in various human diseases 14, 15, 24. We found significant down‐regulation of the investigated microRNAs in both UC and CD in comparison to the normal or the least affected mucosa. These findings support the hypothesis that EMT does occur in IBD, probably contributing to the development of fibrosis, and that this is, at least partly, controlled by microRNAs of the miR‐200 family.
Comparison of microRNAs of the miR‐200 family showed that all miRNAs were down‐regulated in CD, whereas only three of five were down‐regulated in UC. It has been suggested that miRNAs from the same family may target the same process through co‐operativity to obtain more effective regulation 25. Speculatively, down‐regulation of all five miRNAs in CD might give a more efficient EMT induction leading to a more profound fibrosis compared to only three of five miRNAs down‐regulated in UC.
When we compared CD and UC, we found that all miRNAs were more down‐regulated in CD than in UC. Further comparison of miRNAs expression in CD and UC revealed that the expression of four of five investigated miRNAs (miR‐141, miR‐200a, miR‐200b and miR‐200c) was significantly lower in CD than in UC. This microRNA expression pattern is consistent with the notion that fibrosis does not occur only in CD but also in UC 4, 26, being much more common and severe in CD. The reported prevalence of clinically important fibrosis and strictures ranges from 30% to 50% for CD 27 and from 1% to 11% for UC, depending largely on its definition 4, 28. The difference between CD and UC is not surprising, as fibrosis in CD is mostly transmural and in UC, it mostly affects mucosa and submucosa, and only occasionally extends to deeper layers of the bowel wall. This pattern of fibrosis in IBD was also confirmed in our study. The possible explanation would be that miR‐200 family might regulate different target genes under different pathophysiological conditions 15.
The investigated microRNAs have been demonstrated in previous studies of various human diseases and experimental models to be strongly associated with EMT 14, 15, 16, 17. The significance of EMT in the pathogenesis of fibrosis in IBD is controversial. It likely contributes to the pool of myofibroblasts which are the key cells in the development of fibrosis. Once activated, they produce and secrete excessive amounts of extracellular matrix proteins, such as collagens, fibronectin and tenascin. They also exhibit increased proliferation and decreased apoptosis, and contribute to the self‐perpetuating process of fibrosis 27.
The key role of myofibroblasts in fibrogenesis in various diseases is well accepted 7. However, the source of these important cells remains controversial, particularly regarding the injured epithelium as their possible origin. There is mounting evidence that myofibroblasts may originate from the injured epithelium via EMT in various diseases of the kidney, lung, liver and other organs 7, 29, 30. The information about IBD is scarce, but in a mouse model of CD, Flier et al. 9 demonstrated that approximately one‐third of myofibroblasts were derived from intestinal epithelial cells through EMT. Some authors, on the contrary, believe that EMT does not contribute to the formation of myofibroblasts in vivo, and suggest that resident mesenchymal cells, e.g. fibrocytes and perivascular pericytes and not epithelial cells are the primary source of myofibroblasts in the kidney and other organs 31, 32, 33. However, even in these models, the injured epithelium seems to contribute to myofibroblast formation in a paracrine mode, through various ligands, cytokines and chemokines 31, 33. Both explanations seem acceptable regarding our results. According to one, the injured intestinal epithelial cells are a direct source of myofibroblasts via EMT, and according to the other, the EMT signalling deriving from the injured epithelium induces myofibroblast formation from resident mesenchymal cells (e.g. fibroblasts and pericytes) by paracrine cell signalling rather than cellular transition.
To further test the hypothesis that EMT is induced in IBD, we analysed the expression of markers of EMT — Snail and Slug which are believed to be regulated by the investigated microRNAs. We found overexpression of Snail and Slug in the injured crypt epithelium in both CD and UC and occasionally in mesothelial cell and subserosal fibroblasts, particularly in CD with severe transmural fibrosis. This finding indicates that in addition to the crypt epithelium, activation of the mesothelial cells and subserosal fibroblasts in cases with transmural inflammation might also be important in the development of fibrosis.
Snail and Slug mediate transcription repression which has emerged as a fundamental mechanism for induction of EMT. In experimental models, Snail, Slug and other transcription repressors induce a complete EMT at both morphological and behavioural levels. The exact mechanisms of transcription repressors have not been completely elucidated, but most likely include down‐regulation of E‐cadherin transcription, resulting in a functional loss of E‐cadherin 34, 35. Immunohistochemistry for E‐cadherin in our study showed only a focal decrease in staining of the crypt epithelium in all cases and is therefore not useful to confirm the proposed effect of transcription repression on intestinal mucosa.
MicroRNAs have emerged as important regulators of various human diseases including IBD 36, 37, 38. Previous studies confirmed that miRNA expression is dysregulated in intestinal mucosa and serum of patients with IBD 12, 39, especially microRNAs that play a role in the regulation of innate and adaptive immune response 12. Few studies have also focused on the role of microRNAs in the development of fibrosis and stenosis, particularly in CD, and presented some promising results. MicroRNAs of the miR‐29 family have been found to be down‐regulated in the mucosa overlying strictured areas in comparison to adjacent non‐strictured areas in CD 40. Lower serum levels of miR‐19 were found in CD patients with strictures in comparison to CD patients without strictures, suggesting that miR‐19 is a promising circulating biomarker of stricturing CD 39. Chen et al. 17, 41 reported on a decreased expression of miR‐200b in inflamed mucosa and overexpression of miR‐200b in the serum from patients with IBD, associated with a decreased expression of E‐cadherin and increased expression of vimentin. They also found that ZEB1, a transcription repressor of E‐cadherin and a direct target gene of miR‐200b, was decreased by miR‐200b. They concluded that miR‐200b plays a role in maintaining intact intestinal epithelium through inhibiting EMT.
There are several limitations in our study. The most important one is related to the selection criteria. Only patients who underwent surgery were included, resulting in a selection bias, with only the most severe cases of IBD being analysed. It is also not possible to exclude the effect of duration of the disease and treatment, and the effect of the treatment modalities on the histological, genetic and immunohistochemical features. Surgical resection specimens are more suitable for studying fibrosis in IBD, as the whole thickness of the bowel wall is needed, but in routine diagnostic work, we are dealing with endoscopic biopsies. To overcome these problems, future studies should focus on the expression of the investigated microRNAs in the serum and in endoscopic biopsies, to see whether they could be used as a marker of fibrosis.
Conclusion
Our finding of down‐regulation of microRNAs of the miR‐200 family supports the postulated role of EMT in the pathogenesis of fibrosis in UC and CD. These microRNAs act on transcription repressors which have been demonstrated to be up‐regulated in the analysed cases. These results add to our understanding of the pathogenetic mechanisms of fibrosis in IBD, which will hopefully enable to develop new treatment modalities. Furthermore, it is hoped that some of them will prove useful as markers to detect early in the course of the disease in patients who are at risk to develop severe fibrosis 42.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Funding source
None declared.
References
- 1. Latella G, Rogler G, Bamias G, et al Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis. 2014; 8: 1147–15. [DOI] [PubMed] [Google Scholar]
- 2. Thia KT, Sandborn WJ, Harmsen WS, et al Risk factors associated with progression to intestinal complications of Crohn's disease in a population‐based cohort. Gastroenterology. 2010; 139: 1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fichera A, Lovadina S, Rubin M, et al Patterns and operative treatment of recurrent Crohn's disease: a prospective longitudinal study. Surgery. 2006; 140: 649–54. [DOI] [PubMed] [Google Scholar]
- 4. Gordon IO, Agrawal N, Goldblum JR, et al Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis. 2014; 20: 2198–206. [DOI] [PubMed] [Google Scholar]
- 5. Latella G, Di Gregorio J, Flati V, et al Mechanisms of initiation and progression of intestinal fibrosis in IBD. Scand J Gastroenterol. 2015; 50: 53–65. [DOI] [PubMed] [Google Scholar]
- 6. Cosnes J, Nion‐Larmurier I, Beaugerie L, et al Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005; 54: 237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ‐specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013; 304: C216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rieder F, Kessler S, Sans M, et al Animal models of intestinal fibrosis: new tools for the understanding of pathogenesis and therapy of human disease. Am J Physiol Gastrointest Liver Physiol. 2012; 303: G786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flier SN, Tanjore H, Kokkotou EG, et al Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010; 285: 20202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thiery JP, Acloque H, Huang RY, et al Epithelial‐mesenchymal transitions in development and disease. Cell. 2009; 139: 871–90. [DOI] [PubMed] [Google Scholar]
- 11. Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015; 87: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalla R, Ventham NT, Kennedy NA, et al MicroRNAs: new players in IBD. Gut. 2015; 64: 504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis A, Nijhuis A, Mehta S, et al Intestinal fibrosis in Crohn's disease: role of microRNAs as fibrogenic modulators, serum biomarkers, and therapeutic targets. Inflamm Bowel Dis. 2015; 21: 1141–50. [DOI] [PubMed] [Google Scholar]
- 14. Korpal M, Kang Y. The emerging role of miR‐200 family of microRNAs in epithelial‐mesenchymal transition and cancer metastasis. RNA Biol. 2008; 5: 115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mongroo PS, Rustgi AK. The role of the miR‐200 family in epithelial‐mesenchymal transition. Cancer Biol Ther. 2010; 10: 219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zidar N, Boštjančič E, Gale N, et al Down‐regulation of microRNAs of the miR‐200 family and miR‐205, and an altered expression of classic and desmosomal cadherins in spindle cell carcinoma of the head and neck ‐ hallmark of epithelial‐mesenchymal transition. Hum Pathol. 2011; 42: 482–8. [DOI] [PubMed] [Google Scholar]
- 17. Chen Y, Xiao Y, Ge W, et al miR‐200b inhibits TGF‐β1‐induced epithelial‐mesenchymal transition and promotes growth of intestinal epithelial cells. Cell Death Dis. 2013; 4: e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burk U, Schubert J, Wellner U, et al A reciprocal repression between ZEB1 and members of the miR‐200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008; 9: 582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cano A, Pérez‐Moreno MA, Rodrigo I, et al The transcription factor snail controls epithelial‐mesenchymal transitions by repressing E‐cadherin expression. Nat Cell Biol. 2000; 2: 76–83. [DOI] [PubMed] [Google Scholar]
- 20. Van AsscheG, Dignass A, Panes J, et al ; European Crohn's and Colitis Organisation (ECCO) . The second European evidence‐based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis. 2010; 4: 7–27. [DOI] [PubMed] [Google Scholar]
- 21. Dignass A, Eliakim R, Magro F, et al Second European evidence‐based consensus on the diagnosis and management of ulcerative colitis part 1: Definitions and diagnosis. J Crohns Colitis. 2012; 6: 965–90. [DOI] [PubMed] [Google Scholar]
- 22. Magro F, Langner C, Driessen A, et al ; European Society of Pathology (ESP); European Crohn's and Colitis Organisation (ECCO) . European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013; 7: 827–51. [DOI] [PubMed] [Google Scholar]
- 23. Latham GJ. Normalization of microRNA quantitative RT‐PCR data in reduced scale experimental designs. Methods Mol Biol. 2010; 667: 19–31. [DOI] [PubMed] [Google Scholar]
- 24. Gregory PA, Bert AG, Paterson EL, et al The miR‐200 family and miR‐205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008; 10: 593–601. [DOI] [PubMed] [Google Scholar]
- 25. Barnes MR, Deharo S, Grocock RJ, et al The micro RNA target paradigm: a fundamental and polymorphic control layer of cellular expression. Expert Opin Biol Ther. 2007; 7: 1387–99. [DOI] [PubMed] [Google Scholar]
- 26. de Bruyn JR, Meijer SL, Wildenberg ME, et al Development of fibrosis in acute and longstanding ulcerative colitis. J Crohns Colitis. 2015; 9: 966–72. [DOI] [PubMed] [Google Scholar]
- 27. Li C, Kuemmerle JF. Mechanisms that mediate the development of fibrosis in patients with Crohn's disease. Inflamm Bowel Dis. 2014; 20: 1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gumaste V, Sachar DB, Greenstein AJ. Benign and malignant colorectal strictures in ulcerative colitis. Gut. 1992; 33: 938–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeisberg M, Yang C, Martino M, et al Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007; 282: 23337–47. [DOI] [PubMed] [Google Scholar]
- 30. Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010; 21: 1247–53. [DOI] [PubMed] [Google Scholar]
- 31. Duffield JS. Epithelial to mesenchymal transition in injury of solid organs: fact or artifact? Gastroenterology. 2010; 139: 1081–3. [DOI] [PubMed] [Google Scholar]
- 32. Campanholle G, Ligresti G, Gharib SA, et al Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol. 2013; 304: C591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hutchison N, Fligny C, Duffield JS. Resident mesenchymal cells and fibrosis. Biochim Biophys Acta. 2013; 1832: 962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peinado H, Olmeda D, Cano A. Snail, ZEB and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev. 2007; 7: 415–28. [DOI] [PubMed] [Google Scholar]
- 35. Moreno‐Bueno G, Cubillo E, Sarrió D, et al Genetic profiling of epithelial cells expressing E‐cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial‐mesenchymal transition. Cancer Res. 2006; 66: 9543–56. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Kowdley KV. MicroRNAs in common human diseases. Genomics Proteomics Bioinformatics. 2012; 10: 246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Guire V, Robitaille R, Tétreault N, et al Circulating miRNAs as sensitive and specific biomarkers for the diagnosis and monitoring of human diseases: promises and challenges. Clin Biochem. 2013; 46: 846–60. [DOI] [PubMed] [Google Scholar]
- 38. Chapman CG, Pekow J. The emerging role of miRNAs in inflammatory bowel disease: a review. Therap Adv Gastroenterol. 2015; 8: 4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lewis A, Mehta S, Hanna LN, et al Low serum levels of microRNA‐19 are associated with a stricturing Crohn's disease phenotype. Inflamm Bowel Dis. 2015; 21: 1926–34. [DOI] [PubMed] [Google Scholar]
- 40. Nijhuis A, Biancheri P, Lewis A, et al In Crohn's disease fibrosis‐reduced expression of the miR‐29 family enhances collagen expression in intestinal fibroblasts. Clin Sci (Lond). 2014; 127: 341–50. [DOI] [PubMed] [Google Scholar]
- 41. Chen Y, Ge W, Xu L, et al miR‐200b is involved in intestinal fibrosis of Crohn's disease. Int J Mol Med. 2012; 29: 601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rieder F, de Bruyn JR, Pham BT, et al Results of the 4th scientific workshop of the ECCO (Group II): markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis. 2014; 8: 1166–78. [DOI] [PubMed] [Google Scholar]


