Abstract
Introduction
The commensal gut microbiota have been shown to have an impact on human health as aberrant gut microbiota have been linked to disease. Dietary constituents are influential in shaping the gut microbiota. Diet-specific therapeutic strategies may therefore play a role in optimising human health via beneficial manipulation of the gut microbiota. Research has suggested that an individual's baseline gut microbiota composition may influence how the gut microbiota respond to a dietary intervention and individuals with differing habitual dietary intakes appear to have distinct baseline gut microbiota compositions. The responsiveness of the gut microbiota may therefore be influenced by habitual dietary intakes. This study aims to investigate what influence differing habitual dietary fibre intakes have on the responsiveness of the gut microbiota to a prebiotic intervention.
Methods and analysis
In this randomised, double-blind, placebo-controlled, cross-over, single-centre study, 20 low dietary fibre (dietary fibre intake <18 g/day for females and <22 g/day for males) and 20 high dietary fibre (dietary fibre intake ≥25 g/day for females and ≥30 g/day for males) consumers will be recruited. Participants will be randomised to a placebo (Glucidex 29 Premium) or a prebiotic (Synergy 1) intervention for 3 weeks with a 3-week washout followed by 3 weeks of the alternative intervention. Outcome measures of gut microbiota composition (using 16S rRNA gene sequencing) and functional capacity (faecal short chain fatty acid concentrations and Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt)) as well as appetite (visual analogue scale appetite questionnaire) will be assessed at the beginning and end of each intervention phase.
Ethics and dissemination
The Massey University Human Ethics Committee approved this study (Massey University HEC: Southern A application—15/34). Results will be disseminated through peer-review journal publications, conference presentations and a summary of findings will be distributed to participants.
Trial registration number
ACTRN12615000922572; Pre-results.
Keywords: Prebiotic, Gut microbiota, Appetite, 16S rRNA gene sequencing, NUTRITION & DIETETICS
Strengths and limitations of this study.
This study will be the first to provide robust data on the influence habitual dietary fibre intake has on how an individual and their gut microbiota respond to a prebiotic intervention.
Very few studies have used 16S rRNA gene sequencing to conduct detailed taxonomy characterisation of specific bacterial changes secondary to a prebiotic intervention.
A challenge of this study is the reliance on the dietary fibre intake food frequency questionnaire to accurately classify participants as low, moderate and high dietary fibre consumers.
Introduction
Humans and their gut microbiota have, over time, established a symbiotic relationship. The human host provides a steady supply of nutrients within an environment which favours microbial growth while the gut microbiota protect their human host against enteropathogenic organisms,1 2 extract nutrients from undigested dietary components,2 3 modulate the immune system2 and synthesise essential vitamins.4 It has been hypothesised that an aberrant host–microbe relationship is associated with a number of disease states including obesity, type 2 diabetes and inflammatory bowel disease.5–8 There are a number of factors which can influence the composition and therefore balance of the gut microbiota including diet,9 genetics,10 life stage,11 gender,12 antibiotic use9 13 and disease,5 with diet being particularly important.
Researchers are investigating ways of targeting beneficial gut microbiota to enhance human health. Since dietary components are particularly influential in shaping the gut microbiota, diet-specific therapeutic strategies could help optimise human health and well-being via their influence on the community structure and function of the gut microbiota. Prebiotics are ‘selectively fermented ingredients that result in specific changes in composition and/or activity in the gastrointestinal microbiota, thus conferring benefit(s) upon human health’.14 Established prebiotics, such as galacto-oligosaccharides, lactulose and inulin-type fructans (eg, inulin, oligofructose and fructo-oligosaccharides), have been shown to be effective in eliciting beneficial alterations in gut microbiota composition and function (ie, short chain fatty acid (SCFA) production)15–21 and regulating appetite.22–24 Generally, the target of prebiotic interventions is the enhancement of Bifidobacteria and Lactobacillus species; however, other beneficial bacteria such as Roseburia intestinalis, Ruminococcus bromii and Faecalibacterium prausnitzii are emerging as bacteria associated with good health.25
Principal coordinate analysis data have revealed that gut microbiota samples cluster by participant rather than by dietary intervention, suggesting that an individual's gut microbiota do not respond in a consistent manner to a particular dietary intervention.26 27 Baseline Bifidobacteria concentrations appear to have an impact on how effective a dietary intervention is in modifying the gut microbiota. Individuals with low baseline Bifidobacteria levels have been shown to have greater increases in Bifidobacteria concentrations after prebiotic intervention than individuals with high baseline Bifidobacteria levels.16 28 One study collated the results of three separate dietary intervention and gut microbiota studies to determine whether the composition of the gut microbiota may prove informative in predicting how the gut microbiota will respond to a dietary intervention.29 They established from these studies that individuals with lower baseline Bifidobacteria or particularly low or high abundance of Eubacterium ruminantium and Clostridium felsineum had a gut microbiota community which was more responsive to a dietary intervention than individuals with higher baseline abundance of Bifidobacteria and moderate abundance of E. ruminantium and C. felsineum. They also demonstrated that the dietary intervention needed to elicit changes in the gut microbiota to have an influence on lowering serum cholesterol, which suggests that there is a connection between the gut microbiota and human host responsiveness. They did not, however, determine whether there were any habitual dietary intake differences between responders and non-responders to the intervention. Baseline microbial gene count (MGC) has also been shown to affect host responsiveness in overweight and obese individuals. Individuals with low MGC had higher levels of insulin resistance and fasting triglycerides than individuals with high MGC. Dietary differences were demonstrated between the low and high MGC groups, with the low MGC group consuming lower quantities of fruit, vegetables and fish with a trend towards lower dietary fibre intakes. After a weight loss promoting, energy restricted diet, individuals with high MGC at baseline had a more significant improvement in inflammatory markers, insulin resistance and triglycerides than individuals with low MGC.30 MGC may therefore help predict how effective a dietary intervention may be on host outcomes.
An individual's habitual dietary intake has been shown to influence baseline gut microbiota composition.30–35 It is therefore plausible that an individual's habitual dietary intake, particularly dietary fibre intake, may influence how their gut microbiota respond to a particular dietary intervention. The impact of palm date intake was studied in a group of healthy participants to test its prebiotic potential. Fluorescence in situ hybridisation analysis showed that there were no significant differences in any of the bacterial groups after palm date consumption when compared with the control. The researchers then grouped participants as high dietary fibre consumers and low dietary fibre consumers and found that at baseline their Bacteroides concentrations were significantly different. Palm date consumption lead to a significant increase in total bacteria, Lactobacillus/Enterococcus group, Bacteroides, C. coccoides–E. rectale group, R. bromii+R. flavefaciens group and Roseburia+E. rectale group in the low dietary fibre group however there was no change in any of the bacterial groups analysed in the high dietary fibre group.36
Although these studies suggest that baseline gut microbiota composition and habitual diet may influence the responsiveness of the gut microbiota to a dietary intervention, no studies have specifically investigated whether differing habitual dietary intakes lead to gut microbiota which respond to a dietary intervention in a distinct manner. Given the limited research undertaken in this area until now, the primary objective of this study is to investigate whether differing habitual dietary fibre intakes influence how the gut microbiota respond to a prebiotic intervention.
Methods and analysis
Study design
This is a randomised, double-blind, placebo-controlled, cross-over, single-centre study in healthy individuals with differing habitual dietary fibre intakes. The study will investigate whether low versus high habitual dietary fibre intake influences bacterial relative abundance, diversity and faecal SCFA concentrations and appetite in a distinct manner in response to a prebiotic intervention.
Primary objective
To determine the effect of low versus high habitual intakes of dietary fibre on the way in which the composition (bacterial relative abundance) and diversity (α and β diversity) of the gut microbiota change in response to a prebiotic as measured by 16S rRNA gene sequencing.
Secondary objectives
To determine the effect of low versus high habitual intakes of dietary fibre on the way in which the functional capacity of the gut microbiota change in response to a prebiotic as predicted by Phylogenetic Investigation of Communities by Recon (PICRUSt) and faecal SCFA concentrations.
To determine whether baseline gut microbiota relative abundance, bacterial diversity, predicted relative abundance of bacterial gene functions and SCFA concentrations differ between individuals with low versus high dietary fibre intakes.
To determine whether low versus high habitual dietary fibre intakes alter the efficacy of a prebiotic to change appetite scores.
Primary hypothesis
The bacterial relative abundance and diversity of individuals with a low habitual dietary fibre intake will change more significantly in response to a prebiotic than individuals with a high habitual dietary fibre intake.
Secondary hypotheses
The predicted relative abundance of bacterial gene function and SCFA production of individuals with a low habitual dietary fibre intake will change more significantly in response to a prebiotic than those of individuals with a high habitual dietary fibre intake.
Individuals with low habitual dietary fibre intake will have baseline bacterial relative abundance, diversity and predicted relative abundance of bacterial gene function and SCFA concentrations which are distinctive from individuals with high dietary fibre intakes.
The efficacy of a prebiotic to influence appetite will be more pronounced in individuals with a low habitual dietary fibre intake than in individuals with a high habitual dietary fibre intake.
Study setting
The study will be undertaken at the Massey University Human Nutrition Research Unit (HNRU) which is located in Palmerston North, New Zealand.
Exclusion criteria
Less than 19 or >65 years of age;
Taken antibiotics within the past 6 months;
Taken laxatives, gastric motility medications, prebiotic or probiotic containing foods or supplements within the past month;
Medical history of clinically significant disease, that is, cancer, gastrointestinal disorders (irritable bowel syndrome, inflammatory bowel disease, coeliac disease, constipation, diarrhoea, excessive bloating), autoimmune disorders, diabetes, heart disease, renal failure or previous gastrointestinal surgery;
Body mass index of <18.5 or >30 kg/m2;
Significant weight loss or weight gain (>5% of total body weight) within the past year;
Significant dietary change within the past year (ie, has become vegetarian, removed gluten from their diet, actively trying to lose weight, etc);
Pregnant, breast feeding or planning a pregnancy in the next 3 months;
Food intolerance causing gastrointestinal symptoms (ie, lactose intolerance, gluten sensitivity);
Smokers;
High alcohol consumers (>15 standard drinks per week for males and >10 standard drinks per week for females AND fewer than two alcohol-free days per week—New Zealand Ministry of Health guidelines).
If a potential participant is found to be ineligible to participate in the study because they are taking prebiotic or probiotic containing foods or supplements, but are otherwise eligible, they can be included in the study if they are willing to discontinue the probiotic and prebiotic containing foods and supplements for a month prior to starting the study and during the study period.
Study duration
The study length is 10 weeks and will be split into four separate study phases:
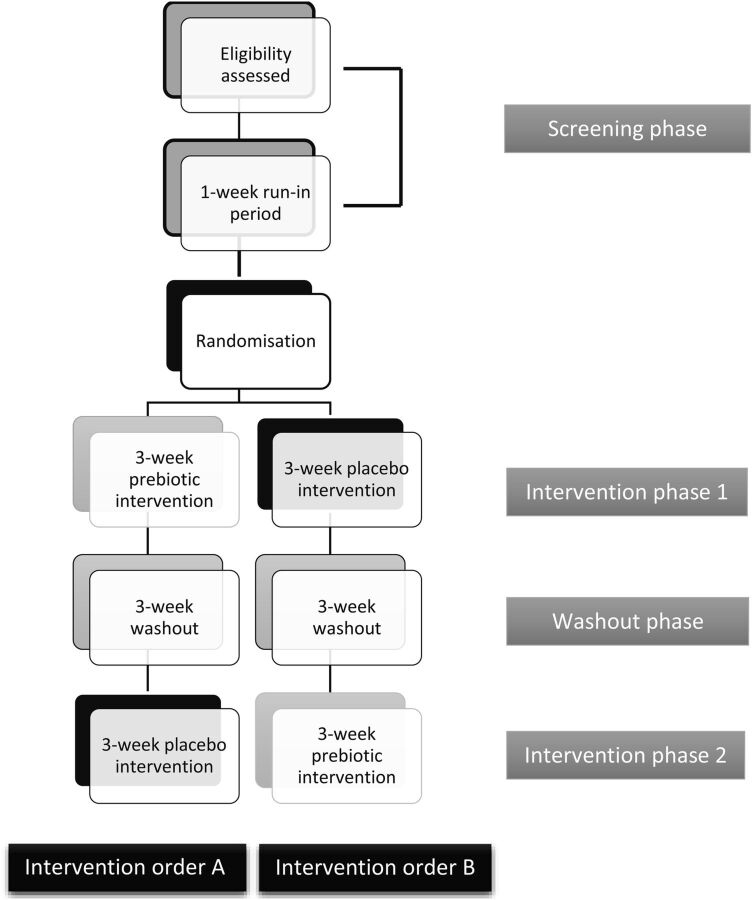
Screening phase (weeks −1 to 0);
Intervention phase 1 (weeks 0 to 3);
Washout phase (weeks 4 to 6);
Intervention phase 2 (weeks 7 to 9).
A summary of the four separate study phases is provided in figure 1.
Figure 1.
Flow diagram summarising the four separate study phases including the two possible intervention orders. The intervention orders may not be as described within the figure as they are blinded to the lead researcher, analysts and participants.
Sample size calculations
In order to detect a significant difference in responsiveness of the key phylum and genera (ie, Actinobacteria, Ruminococcus, Faecalibacterium, Bifidobacteria, etc) to the prebiotic intervention (difference of 3% in bacterial composition with a variance of 9% between and within individuals) between the low and high dietary fibre intake groups (with a power of 80% and significance of 5%), 17 participants per dietary fibre intake group need to be recruited. To allow for participant withdrawal, a total of 40 participants will be recruited: 20 with low dietary fibre intakes and 20 with high dietary fibre intakes.
Participant recruitment
Study participants will be recruited via a number of avenues including email, radio and newspaper advertising and flyer displays around the Palmerston North area.
Participant screening
Screening questionnaire
Eligibility will initially be assessed using a screening questionnaire. Once participants have provided written consent to the lead researcher to participate in the study, a link to an online screening questionnaire will be provided. Each participant will be asked to complete the screening questionnaire which will collect information relating to the participant's gender, health status, age, weight, height, medication use, prebiotic and probiotic intake, recent weight and dietary changes, habitual dietary fibre intake, drinking patterns, smoking status and food intolerances. If potential participants are considered eligible to participate in the study, then on the basis of the screening questionnaire, they will be invited to attend the screening phase visit (first research unit visit).
Habitual dietary fibre intake screening
An online dietary fibre intake food frequency questionnaire (FFQ) will be used to assess the habitual dietary fibre intake of participants. This is a component of the screening questionnaire. Only participants assessed as having low (dietary fibre intake <18 g/day for females and <22 g/day for males) or high (dietary fibre intake ≥25 g/day for females and ≥30 g/day for males) dietary fibre intakes will be eligible for inclusion in the study. The high dietary fibre intake cut-offs were chosen to reflect the New Zealand recommended dietary fibre intake which is >25 g/day for females and >30 g/day for males.37 The low dietary fibre intake cut-offs were chosen as the median dietary fibre intake in New Zealand is 17.5 g/day for females and 22.1 g/day for males, which is below recommended amounts.38
Screening phase visit
Once a participant is assessed as being eligible to take part in the study, then on the basis of the online screening questionnaire, they will be invited to attend a screening phase visit which will take place at the HNRU. At the screening phase visit, participants will provide a blood sample for baseline health screening (liver and kidney function tests, blood glucose levels, electrolytes, complete blood count, calcium and C reactive protein) and will have their weight, height and body composition, using air displacement plethysmography (BodPod), measured. Participants will also be provided with written and oral instructions on how to complete a 3-day diet record and appetite questionnaire, and a fructan intake FFQ (FI-FFQ)39 by a registered dietitian, as well as materials and instructions on how to collect a faecal sample. The 3-day diet record and appetite questionnaire will be completed during the 3 days leading up to the start of the intervention phase 1 (IP1) visit (second visit). The FI-FFQ will be completed and a faecal sample collected on the day before the start of the IP1 visit. The results of the blood test will be received and interpreted (any abnormal blood results will be reviewed by the research clinician) prior to the start of the IP1 visit, as individuals with blood results which may suggest chronic disease (ie, liver disease, kidney disease, diabetes) will be excluded from the study. The screening visit will take place around 1 week prior to the initiation of IP1 and will provide a short run-in period.
Interventions
Each participant will consume 16 g (as two 8 g doses; 8 g 30 min before breakfast and 8 g 30 min before dinner) of powdered fructan prebiotic (Beneo Orafti Synergy 1–50:50 inulin to fructo-oligosaccharide mix) each day for 3 weeks. Participants will also consume 16 g (as two 8 g doses; 8 g 30 min before breakfast and 8 g 30 min before dinner) of powdered placebo (Roquette Glucidex 29 Premium) each day for 3 weeks. The prebiotic and placebo are low in calories and provide 17 and 31 kcal, respectively, per dose. The prebiotic and placebo will be mixed into hot or cold beverages that the participants regularly consume. There will be a 3-week washout phase between the two intervention phases. Previous research has shown that a 3-week intervention provides sufficient time for the gut microbiota to respond to a fructan prebiotic15 and that a 3-week washout provides sufficient time for the gut microbiota to revert back to a baseline composition.40 Participants will be asked to continue their usual food intake and physical activities throughout the duration of the study.
Randomisation and intervention concealment
Participants will be randomly allocated one of two intervention orders: intervention order A or B (figure 1). The intervention order will be randomised using a computer-based pregenerated intervention order as participants will be recruited one at a time over a number of months. Randomisation will be the responsibility of the research unit manager who will not be involved in administering the intervention to participants, assessing the outcomes or analysing the data. Randomisation will be blinded from the lead researcher, analysts and participants. Unblinding may be permitted if medically relevant. The placebo and prebiotic will be in opaque sachets within sealed paper bags and are similar in appearance and taste.
Start of the intervention phase 1 visit
Eligible participants will visit the HNRU ∼1 week after their screening visit for their start of the IP1 visit. Participants will provide a completed 3-day diet record and appetite questionnaire, FI-FFQ and a faecal sample at this visit. Body weight will be measured and 1 week of either the placebo or prebiotic will be provided to each participant. The remaining placebo or prebiotic allocation will be mailed to the participants on a weekly basis. The participants will also be asked to complete a daily diary over the following three intervention weeks to help assess compliance to the intervention and to report any adverse gastrointestinal symptoms that may develop.
End of the intervention phase 1 visit
Three weeks after the start of the IP1 visit, participants will be invited back to the HNRU for the end of the IP1 visit. Another completed 3-day diet record and appetite questionnaire and FI-FFQ will be collected along with an end of the IP1 faecal sample. Body weight will again be measured and the completed daily diaries will be collected. Participants will then enter the 3-week washout phase where they will continue their usual food intake and physical activities but will not be taking either of the interventions or completing a daily diary.
Start of the intervention phase 2 visit
At the end of the 3-week washout phase, participants will be invited back to the HNRU to attend the start of the intervention phase 2 (IP2) visit. At this visit, participants will provide a completed 3-day diet record and appetite questionnaire, FI-FFQ as well as a start of the IP2 faecal sample. Body weight will be measured and each participant will be provided with 1 week of either the placebo or prebiotic at this visit. The remaining placebo or prebiotic allocation will be mailed to the participants on a weekly basis. The participants will be asked to complete a daily diary over the following three intervention weeks.
End of the intervention phase 2 visit
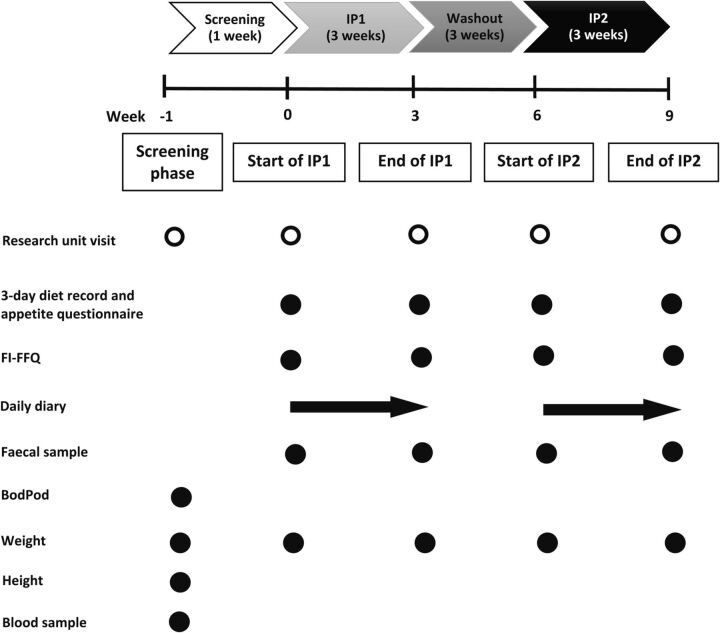
Three weeks after the start of the IP2 visit, participants will attend their final HNRU visit. Participants will provide the last 3-day diet record and appetite questionnaire, FI-FFQ, faecal sample, daily diaries and body weight measurement. Figure 2 provides an illustration of the participant flow through the study including the measurements, questionnaires and samples that will be obtained.
Figure 2.
Participant flow through the study including measurements, questionnaires and samples taken at each Human Nutrition Research Unit visit. BodPod, air displacement plethysmography; FI-FFQ, fructan intake food frequency questionnaire; IP, intervention phase.
Intervention compliance
Intervention adherence will be assessed at the end of each IP visit (end of IP1 visit and end of IP2 visit). Participants will be asked to bring back any unused sachets to each end of IP visit. A daily diary will be completed by the participants which allows them to report whether they had taken all of the allocated morning and afternoon interventions. If participants experience significant gastrointestinal symptoms that prevent them from complying with the treatment as instructed, they will be withdrawn from the study.
Adverse symptom monitoring
High intakes of inulin and fructo-oligosaccharides have been associated with mild gastrointestinal symptoms including flatulence, diarrhoea, borborygmi and bloating.23 41 Adverse symptoms relating to the consumption of the prebiotic and placebo will be monitored using the daily diary. Participants will be asked to report daily on whether they have experienced nausea, diarrhoea, flatulence, stomach rumbling, bloating or abdominal cramps or pain over the past 24 hours. For each symptom, the participants will be asked to indicate whether the symptom was absent, mild (nagging or annoying), moderate (strong negative influence on their daily living) or severe (disabling).
Outcome measures
Primary outcome measure
The primary outcome measure is gut microbiota composition after prebiotic intervention in individuals with low and habitual high dietary fibre intakes.
Compositional changes in gut microbiota will be analysed in the faecal samples collected before and after the prebiotic intervention. 16S rRNA gene sequencing methodology (Illumina MiSeq) and Quantitative Insights Into Microbial Ecology (QIIME) software will be used to analyse changes in bacterial relative abundance and α and β diversity.42
Secondary outcome measures
A secondary outcome is the functional changes in gut microbiota, as assessed by bacterial SCFA concentrations and relative abundance of bacterial gene function, after the prebiotic intervention in individuals with low and high dietary fibre intakes. 16S rRNA gene sequencing data will be further analysed using PICRUSt software to predict the relative abundance of bacterial gene function. Bacterial metabolic functionality will be determined by measuring faecal SCFA concentrations using gas chromatography.
The differences in baseline gut microbiota composition (bacterial relative abundance and diversity) and function (faecal SCFA concentrations and relative abundance of bacterial gene function) between individuals with low versus high dietary fibre intakes will be assessed as secondary outcomes. Compositional and functional differences in gut microbiota at baseline will be analysed in the faecal sample collected at the start of the IP1 (week 0) visit. 16S rRNA gene sequencing data and faecal SCFA concentrations will be analysed (methods outlined above).
Appetite will be assessed as a secondary outcome to determine whether the participant's habitual dietary fibre intake (low vs high) alters the efficacy of a prebiotic to influence appetite. A validated 100 mm anchored visual analogue scale appetite questionnaire43 will be used in conjunction with weight and dietary intake (assessed using the 3-day diet records) information.
Statistical analysis
Bacterial relative abundance and relative abundance of bacterial gene function differences (at baseline and between groups postprebiotic intervention) will be analysed using non-parametric Mann-Whitney tests. A Wilcoxon matched-pairs test will be used to analyse the bacterial relative abundance and relative abundance of bacterial gene function differences between the placebo and prebiotic intervention phases for the low and high dietary fibre groups. Bacterial diversity differences will be analysed using a non-parametric two-sample t-test. Analysis of variance (ANOVA) and discriminant analysis tests will be used to analyse the differences in SCFA concentrations. ANOVA tests will also be used to analyse differences in appetite ratings, dietary intake and weight measurements and a p value <0.05 will be considered significant.
Ethics and dissemination
Research ethics approval
A human ethics application was submitted.
Participants will provide signed informed consent before participating in the study. Participants are able to withdraw from the study at any point with no reason for withdrawal required.
Dissemination
The results of the study will be disseminated through a number of avenues including peer-reviewed journal publications, conference presentations and a summary of findings provided to participants.
Discussion
Habitual dietary intake plays a significant role in shaping the community of microbes which reside in the gastrointestinal tract; however, it is still unknown what impact distinctive habitual dietary intakes have on how responsive the gut microbiota are to a dietary intervention. This intervention study has been designed to investigate how differing habitual dietary fibre intakes influence how the gut microbiota respond to a prebiotic. This information may be invaluable as currently it is difficult to predict how an individual or their gut microbiota will respond to a prebiotic intervention. The high diversity of habitual dietary fibre intakes between individuals may assist in explaining why there is no consistent response by the gut microbiota to a particular prebiotic intervention. If our hypothesis is shown to be correct, researchers may need to take into consideration the habitual dietary fibre intake of their participants at baseline to ensure that potentially confounding factors are controlled for or eliminated in dietary intervention studies which aim to target the gut microbiota.
A limitation of this study is the reliance on the dietary fibre intake FFQ to accurately classify individuals as having low, moderate or high dietary fibre intakes. To verify that the participants have been classified correctly based on the dietary fibre intake FFQ, their first 3-day diet record (collected at the STP1 visit) will be analysed to ensure that each participant meets the low or high dietary fibre intake criteria.
The results generated from this study will provide information for future interventional studies which aim to beneficially modulate the gut microbiota, to help ensure that they are robustly designed so that the true efficacy of a dietary intervention can be determined.
Acknowledgments
The authors greatly appreciate the comments provided by Sarah Eady and Alison Wallace on the draft study protocol. They would also like to acknowledge Duncan Hedderley for conducting the power calculations and providing statistical expertise relating to the study protocol.
Footnotes
Contributors: GH was involved in the conception, design, writing and editing of the protocol. JC, CB, RM and LB were involved in the conception, design and editing of the protocol. KW was involved in the editing of the protocol. All authors read and approved the manuscript.
Funding: This work is supported by the Foods for Health programme (C11X1312). The funds for the Foods for Health programme were provided to a number of collaborating New Zealand organisations, including Plant and Food Research Limited, by the Ministry of Business, Innovation and Employment, New Zealand Government.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Massey University Human Ethics Committee in July 2015 (Massey University HEC: Southern A, Application 15/34).
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This article has been corrected since it was first published as the correspondence author name is changed from Genelle Healey to Genelle Lunken.
References
- 1.Candela M, Perna F, Carnevali P, et al. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol 2008;125:286–92. 10.1016/j.ijfoodmicro.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 2.Chow J, Lee SM, Shen Y, et al. Host–bacterial symbiosis in health and disease. Adv Immunol 2010;107:243–74. 10.1016/B978-0-12-381300-8.00008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonnenburg JL, Xu J, Leip DD, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 2005;307:1955–60. 10.1126/science.1109051 [DOI] [PubMed] [Google Scholar]
- 4.Tappenden KA, Deutsch AS. The physiological relevance of the intestinal microbiota- contributions to human health. J Am Coll Nutr 2007;26:679S–83S. 10.1080/07315724.2007.10719647 [DOI] [PubMed] [Google Scholar]
- 5.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–3. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009;457:480–4. 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett 2014;588:4223–33. 10.1016/j.febslet.2014.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Ma C, Han L, et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol 2010;61:69–78. 10.1007/s00284-010-9582-9 [DOI] [PubMed] [Google Scholar]
- 9.Walsh CJ, Guinane CM, O'Toole PW, et al. Beneficial modulation of the gut microbiota. FEBS Lett 2014;588:4120–30. 10.1016/j.febslet.2014.03.035 [DOI] [PubMed] [Google Scholar]
- 10.Campbell JH, Foster CM, Vishnivetskaya T, et al. Host genetic and environmental effects on mouse intestinal microbiota. ISME J 2012;6:2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012;486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller S, Saunier K, Hanisch C, et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol 2005;72:1027–33. 10.1128/AEM.72.2.1027-1033.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jernberg C, Löfmark S, Edlund C, et al. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007;1:56–66. 10.1038/ismej.2007.3 [DOI] [PubMed] [Google Scholar]
- 14.Gibson GR, Scott KP, Rastall RA, et al. Dietary prebiotics: current status and new definition. Food Sci Technol 2010;7:1–19. [Google Scholar]
- 15.Ramirez-Farias C, Slezak K, Fuller Z, et al. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr 2009;101:541–50. 10.1017/S0007114508019880 [DOI] [PubMed] [Google Scholar]
- 16.de Preter V, Vanhoutte T, Huys G, et al. Baseline microbiota activity and initial bifidobacteria counts influence responses to prebiotic dosing in healthy subjects. Aliment Pharmacol Ther 2008;27:504–13. 10.1111/j.1365-2036.2007.03588.x [DOI] [PubMed] [Google Scholar]
- 17.Costabile A, Kolida S, Klinder A, et al. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br J Nutr 2010;104:1007–17. 10.1017/S0007114510001571 [DOI] [PubMed] [Google Scholar]
- 18.Kolida S, Meyer D, Gibson GR. A double-blind placebo-controlled study to establish the bifidogenic dose of inulin in healthy humans. Eur J Clin Nutr 2007;61:1189–95. 10.1038/sj.ejcn.1602636 [DOI] [PubMed] [Google Scholar]
- 19.Kleessen B, Schwarz S, Boehm A, et al. Jerusalem artichoke and chicory inulin in bakery products affect faecal microbiota of healthy volunteers. Br J Nutr 2007;98:540–9. 10.1017/S0007114507730751 [DOI] [PubMed] [Google Scholar]
- 20.Bouhnik Y, Raskine L, Simoneau G, et al. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr 2004;80:1658–64. [DOI] [PubMed] [Google Scholar]
- 21.Depeint F, Tzortzis G, Vulevic J, et al. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study. Am J Clin Nutr 2008;87:785–91. [DOI] [PubMed] [Google Scholar]
- 22.Cani PD, Joly E, Horsmans Y, et al. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr 2006;60:567–72. 10.1038/sj.ejcn.1602350 [DOI] [PubMed] [Google Scholar]
- 23.Cani PD, Lecourt E, Dewulf EM, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 2009;90:1236–43. 10.3945/ajcn.2009.28095 [DOI] [PubMed] [Google Scholar]
- 24.Morel FB, Dai Q, Ni J, et al. Alpha-galacto-oligosaccharides dose-dependently reduce appetite and decrease inflammation in overweight adults. J Nutr Dis 2015;145:2052–9. 10.3945/jn.114.204909 [DOI] [PubMed] [Google Scholar]
- 25.Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015;7:17–44. 10.3390/nu7010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 2011;5:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salonen A, Lahti L, Salojärvi J, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J 2014;8:2218–30. 10.1038/ismej.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuohy KM, Kolida S, Lustenberger AM, et al. The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oligosaccharides—a human volunteer study. Br J Nutr 2001;86: 341–8. 10.1079/BJN2001394 [DOI] [PubMed] [Google Scholar]
- 29.Korpela K, Flint HJ, Johnstone AM, et al. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS ONE 2014;9:e90702. 10.1371/journal.pone.0090702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013;500:585–8. 10.1038/nature12480 [DOI] [PubMed] [Google Scholar]
- 31.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci 2010;107:14691–6. 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin A, Bik EM, Costello EK, et al. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS ONE 2013;8:e53838. 10.1371/journal.pone.0053838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr 2013;98:111–20. 10.3945/ajcn.112.056689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnorr SL, Candela M, Rampelli S, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun 2014;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matijašić BB, Obermajer T, Lipoglavšek L, et al. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr 2014;53:1051–64. 10.1007/s00394-013-0607-6 [DOI] [PubMed] [Google Scholar]
- 36.Eid N, Osmanova H, Natchez C, et al. Impact of palm date consumption on microbiota growth and large intestinal health: a randomised, controlled, cross-over, human intervention study. Br J Nutr 2015;114:1226–36. 10.1017/S0007114515002780 [DOI] [PubMed] [Google Scholar]
- 37. NHMRC. Nutrient Reference Values for Australia and New Zealand including Recommended Dietary intakes. Canberra: National Health and Medical Research Council; Wellington: Ministry of Health, 2006.
- 38.University of Otago and Ministry of Health. A Focus on Nutrition: Key findings of the 2008/09 New Zealand Adult Nutrition Survey. Wellington: Ministry of Health, 2011.
- 39.Dunn S, Datta A, Kallis S, et al. Validation of a food frequency questionnaire to measure intakes of inulin and oligofructose. Eur J Clin Nutr 2011;65:402–8. 10.1038/ejcn.2010.272 [DOI] [PubMed] [Google Scholar]
- 40.Ramnani P, Gaudier E, Bingham M, et al. Prebiotic effect of fruit and vegetable shots containing Jerusalem artichoke inulin: a human intervention study. Br J Nutr 2010;104:233–40. 10.1017/S000711451000036X [DOI] [PubMed] [Google Scholar]
- 41.Dewulf EM, Cani PD, Claus SP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013;62: 1112–21. 10.1136/gutjnl-2012-303304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flint A, Raben A, Blundell JE, et al. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes 2000;24:38–48. 10.1038/sj.ijo.0801083 [DOI] [PubMed] [Google Scholar]