Abstract
Recently, submandibular abscesses associated with Actinomyces denticolens have been reported in horses. The actinomycotic clumps have been observed in the tonsillar crypts. The aim of this study was to demonstrate colonisation of A denticolens in equine tonsils. Twelve equine tonsils obtained from a slaughterhouse were divided into two parts for histopathological examination and for isolation of A denticolens. When actinomycotic clumps were found in these tonsillar crypts, immunohistochemistry using hyperimmune serum against A denticolens (DMS 20671) was performed on the serial sections. To determine whether Actinomyces-like bacteria isolated using immunoantigenic separation technique were A denticolens, the isolates were analysed for the 16S rRNA gene sequence. Actinomycotic clumps were found in the tonsillar crypts of 11 (91.7 per cent) horses. The clumps were of the saprophytic type accompanied with the feedstuffs, but a few clumps were surrounded by inflammatory cells. A denticolens antigens were immunodetected not only in the clumps of 11 (100 per cent) tonsils, but also in the tonsillar parenchyma. Six isolates obtained from four tonsils showed 99.7–99.9 per cent similarity to A denticolens in the 16S rRNA gene sequence. In horses, the colonisation sites of A denticolens are the tonsils, thus the authors suggest that the tonsils provide the intrinsic infection site for A denticolens.
Keywords: Actinomyces denticolens, Diagnostics, Horses, intrinsic infection, tonsil
Introduction
In horses, as compared with other animals, actinomycosis has been thought to be a relatively uncommon disease. There had been a small number of reports on equine actinomycosis (Guard 1938, Kimball and Frank 1945, Burns and Simmons 1952, Specht and others 1991) before genetic analysis became a common tool for differential diagnosis. In recent years, the rapid identification of Actinomyces species has become possible using the 16S rRNA gene sequence. Consequently, several researchers have identified the Actinomyces species isolated from the abscesses of the cervicofacial region in horses with similar clinical signs of strangles as Actinomyces denticolens (Albini and others 2008, Fielding and others 2008, Beck and others 2011, Feary and others 2013). The cause of these abscesses is speculated to be an intrinsic infection caused by A denticolens invading from the equine oral cavity (Albini and others 2008, Fielding and others 2008, Beck and others 2011). Due to the presence of Actinomyces species in the upper (Bohra and others 2008) and lower respiratory tracts (Bailey and Love 1991), it has not been sufficiently clarified whether or not A denticolens is a member of the usual microbiota in the oral cavity of horses.
Actinomycotic clumps are often found in the tonsillar crypts in human beings (Ashraf and others 2011) or in tiny abscesses in fattening pigs (Murakami and others 1998). Although Actinomyces colonisation in the tonsils does not indicate an active infection in human beings, it can cause obstructive tonsillitis (Ozgursoy and others 2008). In addition, experimental inoculation in swine demonstrated that Actinomyces species isolated from porcine tonsils can cause actinomycotic mastitis (Murakami and others 1999). These reports prompted this current study on the possibility that the tonsillar crypts are the colonisation and/or the infection sites of A denticolens. In this study, pathological, immunohistochemical and genetic investigations were performed in order to reveal the presence of A denticolens in the tonsils of slaughtered fattening horses.
Materials and methods
Animals
A total of 27 horses were slaughtered (9 each day) in June, July and August 2012 at an equine slaughterhouse in Kumamoto Prefecture. The tonsils from the first 18 horses (Equine No. 887–901 and 911–919) were sent by the slaughterhouse although only tonsils from Equine No. 887, 888 and 915 were used to isolate Actinomyces due to the presence of noticeable clumps. In August, an additional 9 horses (Equine No. 941–949) were sampled to increase the sample size to 12 healthy, fattened horses for this study. Tissue samples included the pharynx, larynx and the posterior portion of the tongue (with the tonsil of the soft palate). The samples were immediately transferred in cooler boxes to the laboratory where this study was conducted. All samples were examined macroscopically upon arrival. Both the palatine and lingual tonsils were immediately resected from the samples. The soft palate was removed because tonsillar crypts were not observed in the soft palate (Kumar and Timoney 2006).
The palatine tonsils were divided into two parts (anterior and posterior) at the centre, and the lingual tonsils were divided into two parts (left and right) at the centre. The anterior part of the palatine tonsils and the left side of the lingual tonsils were immediately fixed with a 20 per cent formalin and methanol mixture (20 per cent FM) (Sakura Finetek Japan, Tokyo, Japan). The remaining tonsillar tissues were stored frozen at −80°C for the isolation of A denticolens.
Histopathology
In order to examine the tonsillar crypts for the presence of actinomycotic clumps, the formalin-fixed tonsils were cut from the anterior to the posterior margin at four locations and then embedded in paraffin. The deparaffined sections were 3–4 μm thick and were stained with haematoxylin and eosin (HE). Sections of actinomycotic clumps were stained by Gram's and Grocott's methods, when necessary, in order to observe the structure of the microorganisms.
Immunohistochemistry
When actinomycotic clumps were found in the tonsillar crypts in the HE specimens, the serial sections were immunostained using rabbit hyperimmune serum against A denticolens (DMS 20671). The immunohistochemistry was carried out using the same streptavidin-biotin-peroxidase conjugate (SAB) method as previously described (Murakami and others 1998). Sections of porcine lung without any lesions, which received a direct injection of A denticolens (DMS 20671), were used as positive controls. A section without the primary antiserum was used as a negative control.
As A bovis (Kimball and Frank 1945) and A viscosus (Specht and others 1991) have been reported as causative agents of actinomycosis in horses, they were used to examine the immunohistochemical cross-reactions between A denticolens and two species of the genus Actinomyces. The strains used for this analysis were A denticolens (DMS 20671), A bovis (ATCC 13683) and A viscosus (ATCC15987). The preparation of these tissue samples has already been described (Murakami and others 1998).
Sampling for the isolation of Actinomyces-like bacteria
In order to isolate the Actinomyces-like bacteria from the stored frozen tonsils corresponding to the immunopositive tonsils, symmetrical 1 mm thick sections were cut out from the frozen tonsils with a razor. After placing a piece of the tonsillar tissue on a glass slide, the cut tissue was frozen and stored at −80°C again. The remaining tissues (which mirrored the frozen tissues) were cut to a thickness of 2–3 mm, fixed with 20 per cent FM and embedded in paraffin. The deparaffined sections were stained with HE. When the actinomycotic clumps were found in HE specimens, the serial sections were immunostained using the same immunohistochemistry method in order to confirm the presence of A denticolens antigens. Small pieces of the tissues that had the immunodetected clumps were gouged out with a sterile needle from the same area of the symmetrical frozen tonsils.
Isolation of Actinomyces
An immunoantigenic separation technique (Olsvik and others 1994) was performed to isolate Actinomyces from small pieces of the raw tissues. The sample was homogenised by adding 3 ml brain heart infusion broth (Becton, Dickison and Company, Rutherford, USA). The solid components of the homogenate were then precipitated by centrifugation for three minutes at 1000 rpm, and the supernatant was dispensed into three microtubes (approximately 1 ml each). Then 20 μl of A denticolens antiserum (diluted at 1:100, 1:1000 and 1:10,000) was added to one of those three microtubes. Afterwards, the tubes were mounted on a magnetic rack (Dynal MPCTM-S Life Technologies, USA) and were shaken slowly for one hour in order to allow the immunoreaction to occur at room temperature. Then 20 μl of magnetic-beads-bound sheep anti-rabbit IgG was added into the tubes, and the tubes were again mounted on the magnetic rack and shaken for one hour. Following this, the magnet was set on the rack as per the manufacturer’s instructions. The beads were then concentrated by the magnet and the supernatants in the tubes were removed. Once the magnet was removed from the rack, 1 ml of 0.01 mol/l PBS with 0.05 per cent Tween 20 (PBS-Tween) was added to each tube. The tubes were shaken again for one hour in order to wash the beads. This process was repeated three times. After the supernatants were removed from the tubes, 150 μl PBS-Tween was added into each tube and mixed. A loopful of the suspension of each tube was streaked onto a 10 per cent horse blood agar (BA) plate for the detection of A denticolens, and then aerobically cultured at 37°C for four days. Isolates from the BA agar plates were examined for the predominantly Gram-positive diphtheroid rods, and were then stored in 10 per cent skim milk at −80°C.
16S rRNA gene sequencing
DNA detection of A denticolens was carried out using GenTEL high recovery (TaKaRa Bio, Tokyo, Japan) as per the manufacturer's protocol. The DNA pellet was washed in 70 per cent ethanol and resuspended in 20 μl of TE buffer; the DNA extracts were stored at −20°C. Partial 16S rRNA sequences (approximately 1390 bp in length) of six isolated strains (accession numbers LC086282, LC086283, LC086284, LC086285, LC086286 and LC086287) were determined by comparison with the homology of A denticolens (DMS 20671) (accession number LC086288).
PCR reactions were performed in a total volume of 25 µl. The reaction mix contained 1 µl DNA template, 0.5 µM of each primer, 2.5 mM of MgCl2, 1.2 mM of dNTP and 0.625U of KAPATaq Extra DNA polymerase (Kapa Biosystems, Wilmington, USA). PCR conditions for the amplification reactions were: initial denaturation at 94°C for two minutes; 30 cycles at 94°C for 20 seconds, 55°C for 30 seconds and 72°C for 30 seconds; and final extension at 72°C for five minutes. PCR products were separated on an agarose gel. Fragments 1400 bp in length were extracted by QIAquick gel extraction kit (Qiagen, Venlo, Netherlands). The sequence data were analysed via Sequencer Software (Gene Codes).
Results
The slaughtered horses were between two years and six years old with a median age of three years. Four of them were raised solely in Japan, while the other eight were imported from Canada and had stayed in the Kumamoto Prefecture in Japan for half a year for fattening. All horses were Norman breed.
Pathological findings
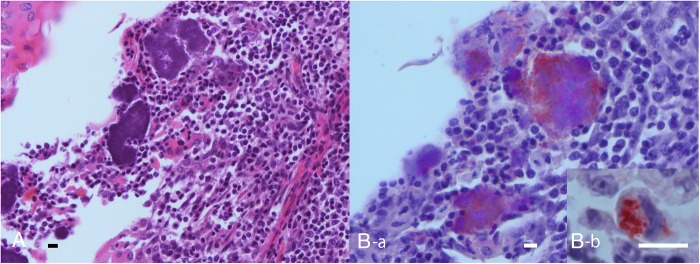
There were no macroscopic abnormalities in any of the tonsil samples other than fragments of feed and/or hairs stuck in the tonsillar crypts. Actinomycotic clumps were found in the tonsillar crypts of 11 out of the 12 tonsils examined (91.7 per cent): 10 (83.3 per cent) palatine tonsils and 6 (50 per cent) lingual tonsils (Table 1). Most colonised bacteria in the clumps were saprophytes and were often accompanied by fragments of feed or hair. Inflammatory cell reactions were not observed around the saprophytes. However, there were a few actinomycotic clumps surrounded by some infiltrated neutrophils and macrophages in the tonsillar crypts, although these clumps were not accompanied by ‘club-shaped’ structures (Fig 1A, 3A). When using Gram's staining, the areas around the saprophytes were not stained whilst the central area of the clumps and the filamentous organisms around the foreign substances were positively stained. The clumps surrounded by inflammatory cells were clearly positively stained using this method. Furthermore, the bacteria stained using Gram's method were also positively stained using Grocott's method; these consisted of various shapes such as bead-like cocci, bacillary cells and short-branching filaments (Fig 2). Another histopathological change observed was the mild swelling of the lymphoid follicles around the tonsillar crypts in almost all of the tonsils.
TABLE 1:
The confirmation of Actinomyces denticolens in equine tonsils of 12 horses using histopathology, immunohistochemistry and 16S rRNA gene sequence
| Formalin-fixed and paraffin-embedded tonsil |
Frozen tonsil |
|||||||
|---|---|---|---|---|---|---|---|---|
| Equine No. | Place of birth | Anatomical location of tonsil | Actinomycotic clump | Immunopositive antigen | Actinomycotic clump | Immunopositive antigen | Number of isolate | Sequence comparisons of partial 16S rRNA of DMS 20671 strain and isolates (per cent)* |
| 887 | Canada | P | + | + | + | + | 2 | LC086282†: (99.7), LC086287 (99.7) |
| L | + | + | + | + | ND | |||
| 888 | Japan | P | + | + | + | + | 1 | LC086283 (99.9) |
| L | + | + | + | + | ND | |||
| 915 | Canada | P | + | + | + | + | ND | |
| L | + | + | + | + | 1 | LC086284 (99.7) | ||
| 941 | Canada | P | + | + | − | |||
| L | − | |||||||
| 942 | Canada | P | + | + | − | |||
| L | − | |||||||
| 943 | Japan | P | + | + | − | |||
| L | + | + | − | |||||
| 944 | Japan | P | + | + | − | |||
| L | − | |||||||
| 945 | Japan | P | − | |||||
| L | − | |||||||
| 946 | Canada | P | − | |||||
| L | + | + | + | + | 2 | LC086285 (99.9), LC086286 (99.7) | ||
| 947 | Canada | P | + | + | − | |||
| L | − | |||||||
| 948 | Canada | P | + | + | − | |||
| L | − | |||||||
| 949 | Canada | P | + | + | + | + | ND | |
| L | + | + | − | |||||
*Accession number of A denticolens (DMS 20671) is LC086288
†Accession number of the isolate
–, negative; +, positive; L, lingual tonsil; ND, non-detected; P, palatine tonsil
FIG 1:
Formalin-fixed and paraffin-embedded tonsil. (A) Actinomycotic clumps surrounded by a few inflammatory cells in a tonsillar crypt in close contact with the area of lymphoepithelial symbiosis. HE. Bar=10 μm. (B-a) Microbial elements immunolabelled by Actinomyces denticolens antiserum in a serial section of A. Bar=10 μm. SAB method. (B-b) Immunolabelling showing A denticolens antigens in a macrophage in the lymphoepithelial symbiosis. Bar=10 μm. SAB method
FIG 3:
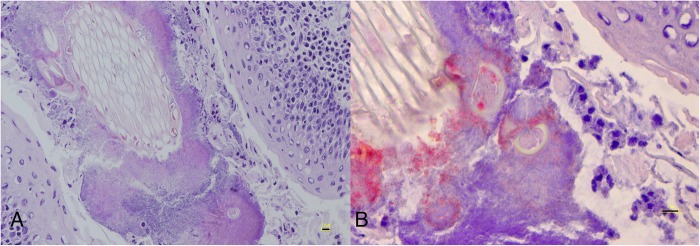
Frozen tonsil. (A) Microbial elements with plant fibers and surrounded by a small number of inflammatory cells in a tonsillar crypt. HE. Bar=10 μm. (B) Positive immunoperoxidase labelling for Actinomyces denticolens in one of the serial sections from A. SAB method. Bar=10 μm
FIG 2:
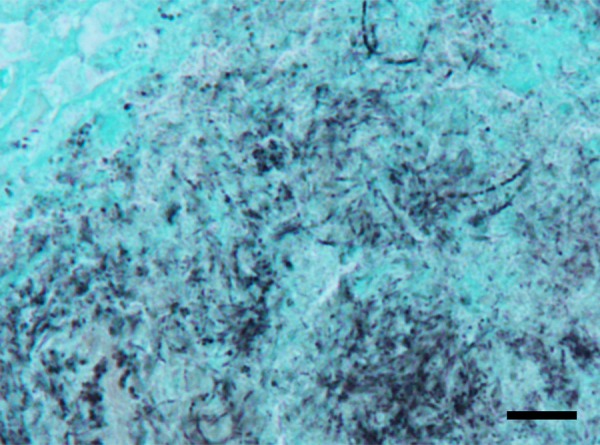
Cluster of the microbial elements consisting of bead-like cocci, bacillary cells or short-branching filaments. Grocott's method. Bar=10 μm
Immunohistochemical findings
Although there were cross-reactions between A denticolens (DMS 20671) anti-serum and the two Actinomyces species examined, the antigens of A denticolens (DMS 20671) were clearly immunolabelled with 1:128,000 diluted antibodies on the clumps in the tonsillar crypts (Fig 1B-a). The other bacteria were not immunostained even with a dilution of 1:64,000 or less. Consequently, immunopositive antigens were detected in all 11 tonsils (100 per cent) with actinomycotic clumps identified in the tonsillar crypts: 10 (90.9 per cent) palatine tonsils and 6 (54.5 per cent) lingual tonsils. The immunolabelled areas on the bacterial cells were almost consistent with the area positively stained using both Gram's and Grocott's methods. Interestingly, positive antigens were detected in the parenchymal area in contact with the tonsillar lymphoepithelial symbiosis and antigens were phagocytosed by the macrophages in the tonsils (Fig 1B-b).
The immunodetection of A denticolens in the frozen tonsils
Foreign substances were found in 5 of 11 tonsils with actinomycotic clumps (45.5 per cent) (Fig 3A): 4 (80 per cent) palatine and 4 (80 per cent) lingual. A denticolens antigens were immunodetected in all of the tonsils with clumps (Fig 3B).
Bacteriological findings and 16S rRNA gene analysis
Actinomyces-like bacteria were isolated in four tonsils. The isolates consisted of three strains in two palatine tonsils and three strains in two lingual tonsils. All of the isolates were sequenced using PCR analysis, and were found to be 99.7–99.9 per cent homologous with A denticolens.
Discussion
Strangles is a condition characterised by swelling and suppuration in the submandibular and/or retropharyngeal lymph nodes in horses. Since this disease is contagious it has been monitored specifically among equine bacterial infections (Sweeney and others 2005). When such clinical signs were observed in the cervicofacial regions in the past, it was assumed that the strangles was caused by Streptococcus equi and not by actinomycosis. In addition, when Actinomyces-like bacteria were isolated from the purulent lesions it might have been difficult to accurately identify the species in a general diagnostic laboratory due to the complexity of the bacteriological procedures or misdiagnosis due to using the ID-32-A-API gallery (bioMerieux) for anaerobes (Albini and others 2008). However, today's genetic analysis technology has solved these problems. As a result, abscesses have been reported as being caused by A denticolens infections in the cervicofacial region (Albini and others 2008, Fielding and others 2008, Beck and others 2011, Feary and others 2013). It is therefore unlikely that equine actinomycosis is actually a rare disease in horses.
In this study, actinomycotic clumps were found to be naturally present in the tonsils of fattened horses obtained from Japan and Canada and most were detected using A denticolens anti-serum. Furthermore, the isolates from the frozen materials consisting of the immunolabelled area of the tonsillar tissues were genetically identified as A denticolens. The immunohistochemistry and genetic analysis results demonstrated that A denticolens were commonly colonised in equine tonsillar crypts as part of the horses’ oral microbiota.
Factors associated with colonisation include not only the anatomical structure of equine tonsils, but also the fragments of feed and/or hair in the crypts. Casteleyn and others (2011) explained the anatomical structures of both the palatine and lingual tonsils in horses as follows:
The palatine tonsil is larger than the other four tonsils, and an elongated flat structure lies bilaterally on the floor of the oropharynx lateral to the tongue. Many tonsillar fossules leading to crypts are macroscopically visible on the surface of the palatine tonsil and the lingual tonsils.
Thus, equine tonsils provide the anatomical location and structure into which these foreign substances can be easily inserted. It is assumed that bacteria related to wound infections, such as Actinomyces, are able to colonise or infect the wound site (Schaal and others 2006).
With regard to experimental S equi infections in horses, it was reported that the organisms entered via the lingual and nasopharyngeal tonsils and, shortly thereafter, the organisms were carried to the regional lymph nodes (Timoney and Kumar 2008). A denticolens colonising in the tonsils may translocate from the tissues, particularly from the palatine and lingual tonsils to the lymph nodes distributed around the cervicofacial region, similar to S equi. If the intrinsic infection is established successfully in the immunosuppressed horses, it is reasonable to consider that A denticolens may cause abscess formations in the regional lymph nodes and/or the subcutaneous tissues, because of the invasion of the antigens in the tonsillar parenchyma.
Although A denticolens was first isolated from the dental plaque of cattle (Dent and Williams 1984) and from the gingival margins of normal felines (Love and others 1990), it is unclear whether A denticolens was pathogenic for these animals, as there have been no reports about pathogenesis. Therefore, A denticolens has been considered to be non-pathogenic to animal species (Schaal and others 2006). However, based on the results of this study and other recent case reports of equine actinomycosis (Albini and others 2008, Fielding and others 2008, Beck and others 2011, Feary and others 2013), it may be possible that A denticolens is pathogenic for horses. Contrastingly, actinomycosis associated with A bovis (Burns and Simmons 1952) and A viscosus (Specht and others 1991) has been reported in horses. However, neither species is considered a specific Actinomyces to horses because there have been no case reports published. When clinical signs with a differential diagnosis of strangles appear to be presenting in horses, it is necessary to include equine actinomycosis caused by A denticolens as another differential diagnosis.
This study demonstrated for the first time that A denticolens was found to be commonly colonising equine tonsillar crypts as a part of the oral microbiota. It is therefore suggested that equine tonsils provide the intrinsic infection site for A denticolens.
Acknowledgments
The authors thank K Inatu and K Tokusige of the Kumamoto Meat Center for their collaboration in this study.
Footnotes
Funding: Funded by the university.
Ethics approval: Approved by the ethics committee of the university (approval number 240052).
Data sharing statement: No additional data are available.
References
- Albini S., Korczak B. M., Abri C., Hüssy D., Limat S., Gerber V., Hermann M., Howald B., Miserez R. (2008) Mandibular lymphadenopathy caused by Actinomyces denticolens mimicking strangles in three horses. Veterinary Record 162, 158–159 doi:10.1136/vr.162.5.158 [DOI] [PubMed] [Google Scholar]
- Ashraf M. J., Azarpira N., Khademi B., Hashemi B., Shishegar M. (2011) Relation between actinomycosis and histopathological and clinical features of the palatine tonsils: An Iranian Experience. Iran Red Crescent Medical Journal 13, 499–502 http://ircmj.com/?page=article&article_id=1816 [PMC free article] [PubMed] [Google Scholar]
- Bailey G. D., Love D. N. (1991) Oral associated bacterial infection in horses: studies on the normal anaerobic flora from the pharyngeal tonsillar surface and its association with lower respiratory tract and paraoral infections. Veterinary Microbiology 26, 367–379 doi:10.1016/0378-1135(91)90030-J [DOI] [PubMed] [Google Scholar]
- Beck A., Baird J. D., Slavić D. (2011) Submandibular lymph node abscess caused by Actinomyces denticolens in a horse in Ontario. Canadian Veterinary Journal 52, 513–514 [PMC free article] [PubMed] [Google Scholar]
- Bohra D. L., Maherchandani S., Bahura C. L., Kashyap S. K. (2008) A comparative study of aerobic bacteria in upper respiratory tract of horses and donkeys. Veterinary Practitioner 9, 50–51 [Google Scholar]
- Burns R. H. G., Simmons G. C. (1952) A case of actinomycotic infection in a horse. Australian Veterinary Journal 28, 34–35 doi:10.1111/j.1751-0813.1952.tb05098.x [Google Scholar]
- Casteleyn C., Breugelmans S., Simoens P., Van den broeck W. (2011) The tonsils revisited: review of the anatomical localization and histological characteristics of the tonsils of domestic and laboratory animals. Clinical, Developmental Immunology 2011, Article ID 472460, 14 pages https://www.hindawi.com/journals/jir/2011/472460/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent V. E., Williams R. A. (1984) Actinomyces denticolens Dent, Williams sp. nov: a new species from the dental plaque of cattle. Journal Applied Bacteriology 56, 183–192 doi:10.1111/j.1365-2672.1984.tb01338.x [DOI] [PubMed] [Google Scholar]
- Feary D. J., Abraham S., Woolford D. J. (2013) Identification of Actinomyces denticolens as a cause of a soft tissue abscess in a horse. Australian Veterinary Journal 91, 416–417 doi:10.1111/avj.12102 [DOI] [PubMed] [Google Scholar]
- Fielding C. L., Magdesian K. G., Morgan R. A., Ruby R. E., Sprayberry K. A. (2008) Actinomyces species as a cause of abscesses in nine horses. Veterinary Record 162, 18–20 doi:10.1136/vr.162.1.18 [DOI] [PubMed] [Google Scholar]
- Guard W. F. (1938) Actinomycosis in a horse. Journal of American Veterinary Medical Association 93, 198–199 [Google Scholar]
- Kimball B. S., Frank E. R. (1945) The isolation of Actinomyces bovis from fistulous withers and poll evil. Journal of American Veterinary Research 6, 39–44 [Google Scholar]
- Kumar P., Timoney J. F. (2006) Histology, immunohistochemistry and ultrastructure of the tonsil of the soft palate of the horse. Anatomia Histologia Embryoliogia 35, 1–6 doi:10.1111/j.1439-0264.2005.00622.x [DOI] [PubMed] [Google Scholar]
- Love D. N., Vekselstein R., Collings S. (1990) The obligate and facultatively anaerobic bacterial flora of the normal feline gingival margin. Veterinary Microbiology 22, 267–275 doi:10.1016/0378-1135(90)90114-B [DOI] [PubMed] [Google Scholar]
- Murakami S., Azuma R., Koeda T., Oomi H., Watanabe T., Fujiwara H. (1998) Immunohistochemical detection for Actinomyces sp. in swine tonsillar abscess and granulomatous mastitis. Mycopathologia 141, 15–19 doi:10.1023/A:1006820611103 [DOI] [PubMed] [Google Scholar]
- Murakami S., Azuma R., Oomi H., Watanabe T., Suzuki S., Koeda T., Fujiwara H. (1999) Experimental actinomycosis caused by Actinomyces-like bacteria in mice and a sow. Journal of Veterinary Medicine A 46, 533–543 doi:10.1046/j.1439-0442.1999.00242.x [DOI] [PubMed] [Google Scholar]
- Olsvik O., Popovic T., Skjerve E., Cudjoe K. S., Hornes E., Ugelstad J., Uhlén M. (1994) Magnetic separation techniques in diagnostic microbiology. Clinical Microbiology Review 7, 43–54 doi:10.1128/CMR.7.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgursoy O. B., Kemal O., Saatci M. R., Tulunay O. (2008) Actinomycosis in the etiology of recurrent tonsillitis and obstructive tonsillar hypertrophy: answer from a histopathologic point of view. Journal of Otolaryngology Head Neck Surgery 37, 865–869 [PubMed] [Google Scholar]
- Schaal K. P., Yassin A. F., Stackebrandt E. (2006) The family Actinomycetaceae: Actinomyces, Actinobaculum, Arcanobacterium, Varibaculum, and Mobiluncus. In: Prokaryotes, 1st edn. Vol. 3. Eds Falkow S., Rosenberg E., Karl-heinz schleifer, Stackebrandt E., New York: Springer; pp 430–537 [Google Scholar]
- Specht T. E., Breuhaus B. A., Manning T. O., Miller R. T., Cochrane R. B. (1991) Skin pustules and nodules caused by Actinomyces viscosus in a horse. Journal of American Veterinary Medical Association 198, 457–459 [PubMed] [Google Scholar]
- Sweeney C. R., Timoney J. F., Newton J. R., Hines M. T. (2005) Streptococcus equi infections in horses: guidelines for treatment, control, and prevention of strangles. Journal of Veterinary Internal Medicine 19, 123–134 doi:10.1111/j.1939-1676.2005.tb02671.x [PubMed] [Google Scholar]
- Timoney J. F., Kumar P. (2008) Early pathogenesis of equine Streptococcus equi infection (strangles). Equine Veterinary Journal 40, 637–642 doi:10.2746/042516408X322120 [DOI] [PubMed] [Google Scholar]