Abstract
General obesity, typically measured using body mass index (BMI), has been associated with an increased risk of several cancers. However, few prospective studies have been conducted in Asian populations. Although central obesity, often measured using waist-hip ratio (WHR), is more predictive for type 2 diabetes and cardiovascular diseases (CVD) risk than BMI, knowledge of its association with cancer incidence is limited. In a cohort of 68, 253 eligible Chinese women, we prospectively investigated the association of BMI, WHR, and weight change during adulthood with risk of overall cancer and major site-specific cancers using multivariate Cox proportional hazard models. Compared to the BMI group of 18.5-22.9 kg/m2, obese (BMI≥30 kg/m2) women were at an increased risk of developing overall cancer (hazard ratio = 1.36, 95% confidence interval = 1.21-1.52), postmenopausal breast cancer (HR: 2.43, 95% CI: 1.73-3.40), endometrial cancer (HR: 5.34, 95% CI: 3.48-8.18), liver cancer (HR: 1.93, 95% CI: 1.14-3.27), and epithelial ovarian cancer (HR: 2.44, 95% CI: 1.37-4.35). Weight gain during adulthood (per 5 kg gain) was associated with increased risk of all cancers combined (HR: 1.05, 95% CI: 1.03-1.08), postmenopausal breast cancer (HR: 1.17, 95% CI: 1.10-1.24), and endometrial cancer (HR: 1.37, 95% CI: 1.27-1.48). On the other hand, WHR was not associated with cancer risk after adjustment for baseline BMI. These findings suggest that obesity may be associated with cancer risk through different mechanisms from those for type 2 diabetes and CVD and support measures of maintaining health body weight to reduce cancer risk in Chinese women.
Keywords: body mass index, cancer, obesity, waist-hip ratio, weight change trajectory
General obesity, often measured using body mass index (BMI), has been linked to increased risk of multiple cancers, including cancers of the colon, breast in postmenopausal women, corpus uteri, and kidney in studies conducted in United States and European countries, (1-5) including a recent large cohort study including 5.24 million UK adults.(3) However, very few cohort studies have been conducted in East Asian populations who have experienced a rapid increase in obesity in recent decades.(6;7) It has been shown that Asians are more susceptible to insulin resistance than European descendants for a given BMI, and Asians are at an elevated risk of developing type 2 diabetes and cardiovascular diseases at a relatively low BMI.(8-10) However, the association of BMI with cancer risk in Asians has been less well quantified.
Central obesity, commonly measured using waist circumference, waist-to-hip ratio (WHR), and waist-to-height ratio, has been shown to be more strongly related to the risk of cardiovascular diseases than general obesity in several previous studies,(11-14) including our study in Shanghai.(15) However, previous reports of central obesity and cancer risks have not been consistent,(16-18) and the literature lacks studies from Asian populations. Additionally, it has been shown that there are population differences in body fat distribution in comparisons between Asians and other ethnic and racial groups. Asians tend to have more abdominal and visceral adiposity than European descendants at a similar BMI level.(19) It is unclear, however, how this population difference in body fat distribution may affect cancer risk.
An additional issue in studies involving central adiposity is that accurate waist circumference measurement in very obese individuals (BMI≥35 kg/m2) is challenging. Substantial errors may be introduced in the measurement process because it is difficult to accurately palpate bony landmarks for an accurate waist circumference measurement in very obese individuals.(20) The higher prevalence of very obese individuals in the US as compared with China could increase the difficulty in assessing central obesity in the US population.
Weight gain trajectory has been a focus of multiple recent studies. Weight gain during adulthood is related to the risk of coronary heart disease,(21) type 2 diabetes,(22) and certain cancers.(23-25) However, very few studies have evaluated weight gain during adulthood in relation to the risk of overall cancer, particularly in Asians. Herein, we report a comprehensive evaluation of the association of BMI, WHR, and weight gain during adulthood with risk of overall cancer and site-specific common cancers in a large prospective cohort study conducted among Chinese women in Shanghai, the largest city on the east coast of China.
MATERIALS AND METHODS
Study population
Data used in this study are from the Shanghai Women’s Health Study (SWHS), a population-based cohort study that recruited 74,941 women, aged 40-70, interviewed between December 1996 and May 2000, living in seven urban communities in Shanghai, China. The design and methods for the SWHS have been previously described elsewhere.(26) In brief, a detailed baseline survey was conducted by trained interviewers using structured questionnaires to obtain information on demographics, personal and familial disease history, usual dietary intake and other lifestyle factors, menstrual and reproductive history, hormone use, and husband’s smoking status (only for married women). The study was approved by the relevant institutional review boards for human research in China and the United States.
Assessment of body mass index, waist-hip ratio and weight gain
At the baseline recruitment, all participants were asked to wear lightweight indoor clothing and were measured for weight, height, and circumferences of the waist and hips by trained interviewers, who were retired health professionals. The measurements were taken following a standard protocol. Waist circumference was measured at 2.5 cm above the umbilicus and hip circumference at the level of maximum width of the buttocks with the subject in a standing position. Circumferences and heights were measured to the nearest 0.1 cm. Weight was measured to the nearest 0.1 kg using a digital scale. All measurements were taken twice. A third measurement was taken if the difference of the first two measurements was greater than 1 kg for weight, 1 cm for height, or 0.5 cm for circumference measurements. BMI and WHR were calculated as the average of the two closest measurements. Participants were asked to recall their weight in kilograms at age 20. Weight change from age 20 until entry into the cohort was calculated as the difference in kilograms between body weight measured at baseline and the weight which participants reported for age 20.
Cohort follow-up and outcomes ascertainment
Data on site-specific cancer incidence and all-cause mortality were collected by record linkages to the Shanghai Cancer Registry and the Shanghai Vital Statistics Registry. For cohort members who were diagnosed with cancer, an in home visit was made to collect information on the date and hospital of diagnosis. Medical charts from the diagnosing hospital were reviewed to verify the diagnosis and to gather information on pathological characteristics of the tumor. In-person follow-up surveys are conducted every two to three years to assure timely and complete ascertainment of new cancer cases and participant vital status. Among all eligible participants contacted, the response rate for the baseline interview was 92.7%. Our cohort was followed up using a combination of record linkage and active follow-ups. The present analyses include outcome data obtained in the cohort follow-up through December 31, 2013 using record linkage to cancer and vital statistics registries; the follow-up was virtually complete given the very low out migration rate in Shanghai.
The primary endpoints in our analyses were all cancers combined (codes 140 to 208 of the International Classification of Diseases, Ninth Revision [ICD-9]) and site-specific common cancers, including cancers of the stomach (151), colon (153), rectum (154), liver and intrahepatic bile-duct (155), gall bladder and extrahepatic bile-duct (156), pancreas (157), lung (162), breast (174), endometrium (182), ovary (183), renal cell (189), and thyroid (193). We also grouped several cancers based on their anatomic sites or tissue origins, such as cancers of the digestive tract (150-159), colon/rectum (153-154), and urinary tract (188-189), as well as lymphoma (201-202), and lymphatic and hematopoietic malignancy (200-208). Analyses for ovarian cancer were also performed for epithelial cancer (183.0) that accounts for approximately 80 % of all ovarian cancer and has been shown to have an etiology different from other types of ovarian cancer.(27)
Statistical analysis
Participants were excluded from the analysis due to missing data for waist or hip circumference measurements (n=25), BMI measurement (n=26), or reproductive history at baseline (n=10), or loss to follow-up immediately following study recruitment (n=5). Subjects were also excluded if they had a prior history of cancer at time of baseline survey (n=1,598) or had an in situ cancer diagnosis (n=110) or incomplete information to confirm their cancer diagnosis (n=256). Few women reported to have ever smoked cigarettes (n=2,113), and thus they were excluded from the present analyses to eliminate confounding by cigarette smoking. To reduce the possible influence of reverse causation on our study results, we excluded patients who were diagnosed with cancer within one year after study enrollment (n=365). In addition, we excluded underweight women (BMI<18.5, n=2,571) from the analysis because the sample size was too small for an informative analysis of the association of cancer with underweight. After these exclusions (not mutually exclusive), data from 68,253 participants remained for our main analysis. Additionally, women who had had a hysterectomy (n=3,487) were excluded from analyses for endometrial cancer, and women who had had an oophorectomy (n=2,693) were excluded for analyses for ovarian cancer. Further, in the analyses of weight change, we excluded subjects with missing data on weight at age 20 (n=6,792).
Study participants were classified into five categories for analyses of BMI, WHR, and weight change trajectory. WHO’s criteria for obesity: normal weight (18.5- 24.9), overweight (25-29.9) and obesity (≥ 30.0) were applied on defining BMI-categories primarily. We additionally divided the broader categories defined by the WHO obesity criteria using a different obesity classification scheme has been proposed for Asians: overweight (23.0- 27.4) and obesity (≥27.5). (28) To facilitate the evaluation of potential dose-response association, we ended up with 5 BMI categories: 18.5-22.9 (referent), 23.0-24.9, 25.0-27.4, 27.5-29.9, and ≥ 30.0 kg/m2. The sample size for the two highest BMI categories (27.5-29.9, and ≥ 30.0 kg/m2) was relatively small for several cancers, and these two categories were combined if either category had fewer than 15 cancer cases. The categories for WHR were generated by quintiles: ≤ 0.77 (referent), 0.78-0.80, 0.81-0.82, 0.83-0.85, and > 0.85. The categories for weight change from age 20 to baseline were <0, 0-5.0 (referent), 5.0-9.9, 10.0-15.0, and > 15.0kg.
Potential confounding variables were identified a priori: education (elementary school or less, some middle school, high school graduate, some college or more), total energy intake (quintiles: <1,300 kcal/day, 1,300-1,499 kcal/day, 1,500-1,699 kcal/day, 1,700-1,899 kcal/day, >1,900 kcal/day), total vegetable and fruit intake (tertiles: <414 g/day, 414-640 g/day, >640 g/day), total meat intake (tertiles: <43 g/day, 43-74 g/day, >74 g/day), leisure-time exercise (no regular exercise, ≤ 15.0 metabolic equivalent (MET)-hour/week, >15.0 MET-hour/week), regular alcohol consumption (consumed alcohol at least 3 times per week for over 6 months: never, ever), menopausal status (premenopausal, postmenopausal), exposure to spousal cigarette smoke (yes, no, missing), parity (number of live births: 0, 1, 2, >2), and first degree relative diagnosed with cancer (yes, no). For all variables, the lowest category served as the reference category.
Cox proportional hazards regression models, with age as the time scale, were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of BMI, WHR and weight change with the risk of cancer after adjusting for potential confounders. Entry time was defined as age at the baseline interview, and exit time was defined as age at cancer diagnosis or censoring (December 31, 2013, or date of death), whichever came first. Statistical models were stratified by year of birth (categorized into seven five-year groups) and adjusted for the above-mentioned covariates. In addition, BMI and WHR were mutually adjusted to assess their independent association with cancer risk, and BMI at age 20 was adjusted to evaluate the association of weight change and incident cancer. We tested the proportional hazards assumption by the correlation of scaled Schoenfeld residuals with time and the assumptions held. Tests of linear trend were performed by entering an ordinal variable as a continuous term in the regression model and testing the significance of the term using the Wald chi-square test. We fitted models to estimate cancer risk associated with per 5 kg/m2 increase in BMI, a standard deviation (SD) increase in WHR, and a 5 kg increase in weight since age 20 (participants who lost weight were not included). We also evaluated associations of breast cancer with BMI, WHR, and weight change in stratified analyses based on their menopausal status at baseline because results from previous studies showed an increased risk of postmenopausal breast cancer but a reduced risk premenopausal breast cancer associated with obesity.(29)
We also investigated associations between risk of major cancers and two additional measures of central adiposity: waist circumference and waist-to-height ratio (modeled with per SD increase). We explored mutual adjustment between BMI (per SD increase) and measures of central obesity (waist circumference, WHR, and waist-to-height ratio) to assess their possible independent association with cancer risk using both standard and residual methods. To employ the standard method, we included terms for BMI and the measure of central obesity in the same statistical models. The residual method, which is commonly used in nutritional epidemiological studies for total energy intake adjustment,(30) was applied to address the correlations between the anthropometric measures. For instance, in this method, waist circumferences were regressed to the subjects’ BMI. The residuals from the regression represent the differences between each individual’s actual waist circumference and the waist circumference predicted by his BMI. The Cox model assessing the association of waist circumference and cancer risk included the waist circumference residual plus BMI variables, with other covariates. (30)
Further, we investigated the combined effect of baseline BMI (<25, and ≥25 kg/m2) and WHR (tertiles: <0.79, 0.79-0.83, >0.83), as well as weight change (5 categories as described above) and BMI at age 20 (<18.5, and ≥18.5 kg/m2). P-values for interactions were estimated using likelihood ratio tests comparing models with and without the interaction terms. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC). All statistical tests were based on 2-sided probability.
RESULTS
During a median follow-up time of 15.1 years (range: 1-17 years) 5,094 women were diagnosed with cancer among 68,253 cohort members eligible for this analysis. The mean BMI for these study participants at baseline was 24.2 kg/m2 (standard deviation = 3.2). Participants with lower adiposity measures were more likely to be younger, premenopausal, regular exercisers, and more highly educated, less likely to have spousal smoking exposure or a positive family history of cancer, and on average, had a lower total energy intake, and lower total fruit/vegetables intake. No significant differences were observed in leisure-time physical activity levels among BMI categories. There was no significant difference in the level of total meat intake or alcohol consumption among different BMI and WHR categories (Table 1).
Table 1.
Baseline Characteristics of Study Participants by Body Mass Index (BMI) and Waist-Hip Ratio (WHR) in the Shanghai Women's Health Study, 1996-2013
| Characteristic | BMI Categories |
P | ||||
|---|---|---|---|---|---|---|
| 18.5-22.9 | 23.0-24.9 | 25.0-27.4 | 27.5-29.9 | ≥30.0 | ||
| Number (%) | 26,689 (39.1) | 16,807 (24.6) | 14,481 (21.2) | 6,742 (9.9) | 3,534 (5.2) | |
| Age at baseline (years, median, (25th, 75th percentile)) |
47.3 (43.2, 55.6) |
49.9 (44.4, 59.5) |
52.7 (46.1, 62.5) |
55.3 (47.5, 64.0) |
57.8 (49.1, 64.9) |
<0.001 |
| Education (%) ab | <0.001 | |||||
| Elementary school or lower | 16.1 | 19.1 | 22.1 | 26.2 | 29.9 | |
| High school | 36.3 | 37.8 | 39.4 | 42.0 | 43.7 | |
| High school graduate | 30.9 | 28.7 | 26.7 | 22.5 | 19.7 | |
| Some college or higher | 16.8 | 14.4 | 11.7 | 9.4 | 6.7 | |
| Postmenopausal women (%) b | 47.6 | 48.3 | 48.4 | 49.1 | 49.8 | <0.001 |
| Total energy intake (kcal/day) b | 1,639.5 | 1,662.3 | 1,675.1 | 1,703.4 | 1,727.8 | <0.001 |
| Total meat intake (g/day) b | 63.4 | 64.4 | 64.1 | 64.2 | 63.6 | 0.218 |
| Total fruit/vegetables intake (g/day) b | 529.7 | 556.5 | 563.2 | 571.8 | 560.3 | <0.001 |
| Physical activity, regularly (yes, %) b | 35.1 | 36.2 | 34.4 | 33.0 | 33.7 | <0.001 |
| Leisure-time Physical activity (MET- h/week) b |
5.61 | 5.73 | 5.56 | 5.61 | 5.42 | 0.441 |
| Regular alcohol consumption (ever, %) b | 2.0 | 2.1 | 2.0 | 1.7 | 1.8 | 0.624 |
| Spouse smoking exposure (ever, %) b | 53.4 | 54.6 | 56.6 | 57.1 | 59.4 | <0.001 |
| Positive family history of cancer (%) b | 26.2 | 26.6 | 27.7 | 27.0 | 26.4 | 0.026 |
|
| ||||||
| Characteristic | WHR Quintile Categories |
P | ||||
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | ||
|
| ||||||
| Number (%) | 12,056 (17.7) | 14,748 (21.6) | 15,932 (23.3) | 12,263 (18.0) | 13,254 (19.4) | |
| Age (years, median, 25th, 75th percentile) | 46.3 (43.0, 52.9) |
47.9 (43.6, 55.6) |
49.7 (44.4, 59.0) |
52.5 (45.6, 62.2) |
58.0 (48.7, 65.0) |
<0.001 |
| Education (%) ab | <0.001 | |||||
| Elementary school or lower | 15.6 | 16.8 | 19.3 | 21.8 | 25.7 | |
| High school | 35.8 | 37.0 | 37.9 | 39.7 | 40.8 | |
| High school graduate | 31.0 | 30.3 | 28.9 | 26.2 | 24.1 | |
| Some college or higher | 17.7 | 15.9 | 14.0 | 12.3 | 9.5 | |
| Postmenopausal women (%) b | 46.8 | 47.5 | 48.1 | 48.8 | 50.2 | <0.001 |
| Total energy intake (kcal/day) b | 1,658.3 | 1,656.0 | 1,659.6 | 1,669.0 | 1,680.3 | <0.001 |
| Total meat intake (g/day) b | 64.4 | 64.4 | 63.6 | 63.9 | 63.5 | 0.294 |
| Total fruit/vegetables intake (g/day) b | 556.5 | 551.6 | 550.8 | 549.1 | 543.8 | 0.018 |
| Physical activity, regularly (yes, %) b | 37.6 | 35.8 | 35.0 | 33.5 | 33.0 | <0.001 |
| Leisure-time Physical activity (MET- h/week) b |
6.15 | 5.72 | 5.48 | 5.50 | 5.37 | <0.001 |
| Regular alcohol consumption (ever, %) b | 1.8 | 1.8 | 2.1 | 2.1 | 1.9 | 0.134 |
| Spouse smoking exposure (ever, %) b | 52.6 | 53.7 | 55.1 | 56.6 | 57.5 | <0.001 |
| Positive family history of cancer (%) b | 26.3 | 26.9 | 27.2 | 26.9 | 26.3 | 0.140 |
May not sum up to 1 due to rounding
Adjusted for age distribution
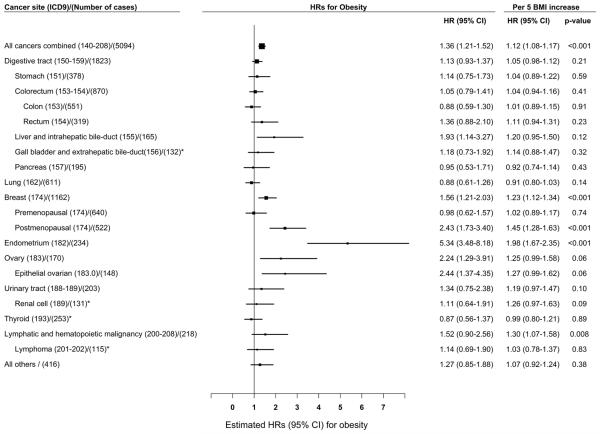
Significant linear associations were observed between higher BMI and increased risk of breast, endometrial cancer, and all cancers combined (p-trends < 0.001, Supplementary Table 1). BMI was positively associated with breast cancer risk in postmenopausal women (p-trend < 0.001), but not in premenopausal women (p-trend = 0.71, p for heterogeneity = 0.0004). Compared to the reference group (BMI: 18.5-22.9 kg/m2), statistically significant associations were observed for the association of obese BMI (≥ 30 kg/m2) with risk of total cancer (HR = 1.36, 95% CI = 1.21-1.52), liver cancer (HR = 1.93, 95% CI = 1.14-3.27), postmenopausal breast cancer (HR = 2.43, 95% CI = 1.73-3.40), endometrial cancer (HR = 5.34, 95% CI = 3.48-8.18), and epithelial ovarian cancer (HR = 2.44, 95% CI = 1.37-4.35). Results for the association per 5 kg/m2 increase in BMI showed a similar pattern. The strongest association per 5 kg/m2 increase was observed for endometrial cancer (HR = 1.98, 95% CI = 1.67-2.35), followed by postmenopausal breast cancer (HR = 1.45, 95% CI = 1.28-1.63), lymphatic and hematopoietic malignancy (HR = 1.30, 95% CI = 1.07-1.58), overall breast cancer (HR = 1.23, 95% CI = 1.12-1.34), and all cancer combined (HR = 1.12, 95% CI = 1.08-1.17) (Supplementary Table 1 and Figure 1). Additional adjustment for WHR did not materially change the associations between BMI and risk of cancer (Supplemental Table 2). The association per 5 kg/m2 increase for renal cell carcinoma was marginally significant before WHR adjustment, and became significant (HR = 1.33, 95% CI = 1.01-1.75) after additionally adjusting for WHR using standard method.
Figure 1.
Adjusted hazard ratios (HRs) for the associations of cancer risk with obesity (body mass index (BMI) ≥30 kg/m2) or per 5 kg/m2 increase in BMI* BMI group ≥ 27.5 kg/m2 was demonstrated due to a small sample size; HRs were adjusted for education, total energy intake, total vegetable and fruit intake, total meat intake, leisure-time physical activity, alcohol consumption, hormone replacement treatment, menopausal status, spouse smoking exposure, parity and family history of cancer; Women who had a hysterectomy were excluded (N = 3,487) for the endometrial cancer analysis; Women who had an oophorectomy were excluded (N = 2,693) for the ovarian cancer analysis.
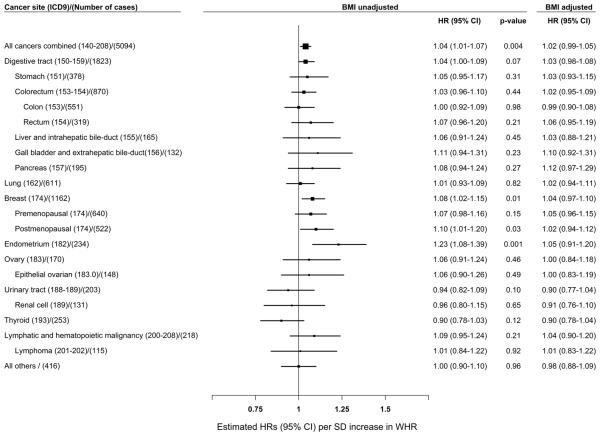
A significant linear association was observed between higher WHR and risk of all cancers combined, breast cancer (particularly postmenopausal breast cancer), and endometrial cancer (p-trends < 0.05, Supplementary Table 3). Further, the association per SD increase in WHR showed a similar pattern (p-values < 0.05, Supplementary Table 3 and Figure 2). These associations, however, were diminished after adjustment for baseline BMI (Supplemental Table 4 and Figure 2). No significant associations were found between WHR and risk of other cancers examined using either standard or residual method (Supplementary Table 5).
Figure 2.
Adjusted hazard ratios (HRs) for the associations of cancer risk per standard deviation (SD) increase in waist-hip ratio SD = 0.05; HRs were adjusted for education, total energy intake, total vegetable and fruit intake, total meat intake, leisure-time physical activity, alcohol consumption, hormone replacement treatment, menopausal status, spouse smoking exposure, parity, family history of cancer and BMI; Women who had a hysterectomy were excluded (N = 3,487) for the endometrial cancer analysis; Women who had an oophorectomy were excluded (N = 2,693) for the ovarian cancer analysis.
BMI was highly correlated with waist circumference and waist-to-height ratio. The correlation coefficients for BMI and waist circumference, BMI and WHR, and BMI and waist-to-height ratio were 0.83, 0.44, and 0.83, respectively (Table 2). The association per SD increase for all cancers combined, breast cancer (particularly postmenopausal breast cancer), and endometrial cancer (p < 0.001, Supplementary Table 5) were statistically significant with waist circumference and waist-to-height ratio before adjusting for BMI. These associations were attenuated after BMI adjustment. After adjustment for BMI, cancer risk associations were diminished for WHR and waist-to-height ratio; however, waist circumference was still found to be associated with all cancers combined and breast cancer (particularly postmenopausal breast cancer) (p < 0.05, Supplementary Table 5). Adjustment by measures of central obesity slightly attenuated the association of cancer risk with BMI. Interestingly, the strong association between BMI and all cancers combined diminished after waist circumference adjustment using the residual method, while the association between waist circumference and all cancers remained significant (Supplementary Table 5&6).
Table 2.
Correlations between four anthropometric measures in the Shanghai Women's Health Study, 1996-2013
| Body-mass index | Waist-to-hip ratio | Waist circumference | Waist-to-height ratio | |
|---|---|---|---|---|
| Body-mass index | 1.00 | |||
| Waist-to-hip ratio | 0.44 | 1.00 | ||
| Waist circumference | 0.83 | 0.70 | 1.00 | |
| Waist-to-height ratio | 0.83 | 0.71 | 0.95 | 1.00 |
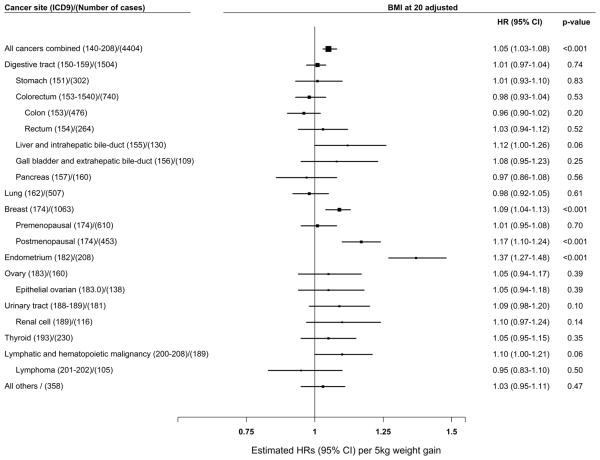
Weight change since age 20 was positively associated with increased risk of all cancers combined, postmenopausal breast cancer, and endometrial cancer in a dose-response manner (p-trend < 0.001, Supplementary Table 7). The highest risk associated with each 5 kg increase in weight was found for endometrial cancer (HR = 1.37, 95% CI = 1.27-1.48), followed by postmenopausal breast cancer (HR = 1.17, 95% CI = 1.10-1.24), and all cancer combined (HR = 1.05, 95% CI = 1.03-1.08) (Supplementary Table 7 and Figure 3). A positive association between weight change and breast cancer risk was found for postmenopausal women, but the association was null for premenopausal women (p-trends = 0.39) (p for heterogeneity = 0.0015).
Figure 3.
Adjusted hazard ratios (HRs) for the association of cancer risk with per 5 kg increase in weight change since age 20 years HRs were adjusted for education, total energy intake, total vegetable and fruit intake, total meat intake, leisure-time physical activity, alcohol consumption, hormone replacement treatment, menopausal status, spouse smoking exposure, parity, family history of cancer and BMI at age 20; Women who had a hysterectomy were excluded (N = 3,138) for the endometrial cancer analysis; Women who had an oophorectomy were excluded (N = 2,492) for the ovarian cancer analysis.
We did not find any evidence for possible interactions of BMI and WHR, or weight change during adulthood with BMI at age 20 in cancer risk, overall or by cancer types (data not shown). Additional adjustments for age at menarche, duration of breastfeeding, a prior history of benign breast disease (in the analyses of breast and endometrial cancers), and history of benign ovarian cyst (in the analysis of ovarian cancer) did not materially change the results for the association of these cancers with BMI, WHR and weight change (data not shown).
DISCUSSION
In this large prospective cohort study, we found that BMI was positively associated with risk of all cancers combined in a dose-response manner. Being overweight or obese was significantly associated with an elevated cancer risk. The strength of the association between BMI and cancer, however, differed by site and by menopausal status for breast cancer. Consistent with most previous studies, we showed that BMI was strongly associated with the risk of endometrial cancer and postmenopausal breast cancer. Notably, we found a more than 5-fold elevated risk of endometrial cancer associated with obesity, an association that is stronger than that found in many previous studies.(2;3;5;31) We found that being obese was associated with a nearly 80% increased risk of liver cancer and a more than 2-fold risk of ovarian cancer, particularly epithelial ovarian cancer, compared to having normal weight BMI. In addition, high BMI was associated with increased risk of kidney cancer, particularly renal cell cancer, and lymphatic and hematopoietic malignancy. The positive association of BMI with risk for a broad group of lymphatic and hematopoietic malignancies was likely due to an association of BMI with risk of leukemia and myeloma since no association for high BMI with lymphoma risk was found in our study. We found no association between BMI and risk of colon, rectum, gall bladder, pancreas, or thyroid cancers, which fails to support the positive association for these cancer reported in some but not all previous reports.(2;3;5) Circulating estrogen, synthesized primarily in adipose tissues after menopause, has been shown to be associated with a reduced risk of colorectal cancer. On the other hand, obesity may lead to insulin resistance and chronic inflammation, which in turn may be related to an increased colorectal cancer risk. Therefore, the association of colorectal cancer risk with BMI is complex and heterogeneous between genders, and null findings for associations of colorectal cancer risk with BMI for Asian females have been reported previously.(3;5;6;32;33) Further studies are warranted to determine whether the observed null associations are truly absent in our study population or veiled due to limited statistical power.
Central obesity, often measured using WHR, has been clearly demonstrated to be a strong risk factor for cardiovascular disease and type 2 diabetes. WHR has also been associated with an increased risk of death due to cancer in some previous studies.(13-15) Knowledge about the association of WHR in incident cancer risk is inadequate, especially scarce in Asian populations. Some previous studies reported that WHR might be an independent risk factor for cancer, but most of these studies used a case-control design, anthropometric measures were not taken directly but were self-reported or did not adjust for BMI in their analyses.(16-18) We found that all observed WHR-cancer associations disappeared after adjusting for BMI, which suggests that general obesity, probably through excessive endogenous sex hormone production in adipose tissue, might play a more important role in the etiology of cancer than central adiposity, which is more closely related to insulin resistance than general obesity.(4) We found similar cancer risk associations for waist circumference and BMI, which strengthen the hypothesis that waist circumference, similar to BMI, is more strongly correlated with total body fat than visceral fat, especially among elderly individuals.(34;35) Consequently, we consider WHR, which is less correlated with BMI and more correlated with visceral fat compared to waist circumference related measures, to be a preferable measurement of central obesity. Our study provides evidence that among middle aged and elderly Chinese females, total body fat, indicated by BMI and waist circumference, may be more closely related to cancer risk than visceral fat, indicated by WHR.
Previous studies have shown that weight gain during adulthood is a risk factor for endometrial cancer and postmenopausal breast cancer.(36) We found that an increased weight change trajectory during adulthood was associated with elevated risk of all cancers combined, breast cancer, particularly postmenopausal breast cancer, endometrial cancer, and possibly liver cancer and lymphatic and hematopoietic malignancy, independent of BMI at age 20 and other established risk factors. These results suggest that regardless of BMI at early adulthood, being obese in later life or gaining weight along the way increases the risk of several cancers, emphasizing the importance of maintaining a healthy weight during adulthood.
To our knowledge, our study is the largest prospective cohort study examining the association of central obesity with cancer risk across the whole cancer spectrum. Further, this is the first and largest prospective cohort study that comprehensively evaluated the risk of all common cancers with multiple obesity measurements, including general and central obesity and weight gain during adulthood. The follow-up rate for the study is high, reducing the likelihood of selection bias. Data on anthropometric measurements were carefully collected using standard protocols by experienced interviewers, which decreases the possibility of misclassification. In addition, availability of information on a large number of covariates with very little missing data enabled proper control for potential confounders.
As with any observational study, measurement errors, particularly errors related to the recall of weight at age 20 years, could be a concern. However, these errors are non-differential, which likely attenuate the association. Although this is one of the largest studies investigating the association of obesity with cancer risk, the sample size remains relatively small for certain cancer sites. Therefore, the null association observed for some cancers may be due to low statistical power. Having only two weight measurements may not capture the entire weight history. However, weight cycling is not common in our study population.
In conclusion, our study indicates that total body fat and weight gain during adulthood play important roles in the risk of development of overall cancer and several site-specific cancers among Chinese women. On the other hand, central obesity, as measured using WHR, may not be an important and independent risk factor for common cancers. Our study emphasizes the importance of maintaining normal body weight as a means of cancer prevention in China, particularly given the rapid increase in obesity in this populous country in recent decades.
Supplementary Material
Novelty and Impact.
Previous studies evaluating associations between obesity and cancer risk were conducted primarily in European-ancestry populations. Central obesity, a strong predictor for cardiovascular disease risk, was less well evaluated in relation to cancer risk among Asians. Using data from a large prospective study conducted among Chinese women, we provide strong evidence for a positive association of general obesity and weight gain with cancer risk and virtually no association of central obesity with risk of common cancers.
ACKNOWLEDGEMENTS
We would like to thank staff members and the study participants of the Shanghai Women’s Health Study for their contributions to this research. We also thank Ms. Kimberly A. Kreth for her assistance in preparing the manuscript. This work was supported by grants from the United States National Institutes of Health (R37 CA070867 and UM1 CA182910).
Abbreviations
- BMI
Body mass index
- CVD
cardiovascular disease
- WHR
waist-hip ratio
- HRs
hazard ratios
- CIs
confidence intervals
Reference List
- 1.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, Diem G, Oberaigner W, Weiland SK. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005 Oct 31;93(9):1062–7. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007 Dec 1;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014 Aug 30;384(9945):755–65. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004 Aug;4(8):579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008 Feb 16;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Jee SH, Yun JE, Park EJ, Cho ER, Park IS, Sull JW, Ohrr H, Samet JM. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008 Oct 15;123(8):1892–6. doi: 10.1002/ijc.23719. [DOI] [PubMed] [Google Scholar]
- 7.Kuriyama S, Tsubono Y, Hozawa A, Shimazu T, Suzuki Y, Koizumi Y, Suzuki Y, Ohmori K, Nishino Y, Tsuji I. Obesity and risk of cancer in Japan. Int J Cancer. 2005 Jan 1;113(1):148–57. doi: 10.1002/ijc.20529. [DOI] [PubMed] [Google Scholar]
- 8.Hsu WC, Araneta MR, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015 Jan;38(1):150–8. doi: 10.2337/dc14-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, He J, Gupta PC, Ramadas K, Tsugane S, Irie F, Tamakoshi A, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011 Feb 24;364(81):719–29. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013 Jun;36(6):1789–96. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjonneland A, Halkjaer J, Jensen MK, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008 Nov 13;359(20):2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 12.Song X, Jousilahti P, Stehouwer CD, Soderberg S, Onat A, Laatikainen T, Yudkin JS, Dankner R, Morris R, Tuomilehto J, Qiao Q. Comparison of various surrogate obesity indicators as predictors of cardiovascular mortality in four European populations. Eur J Clin Nutr. 2013 Dec;67(12):1298–302. doi: 10.1038/ejcn.2013.203. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008 Apr 1;117(13):1658–67. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 14.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, Sellers TA, Lazovich D, Prineas RJ. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Arch Intern Med. 2000 Jul 24;160(14):2117–28. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Shu XO, Yang G, Li H, Cai H, Gao YT, Zheng W. Abdominal adiposity and mortality in Chinese women. Arch Intern Med. 2007 May 14;167(9):886–92. doi: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JE, Rosner B, Speizer FE, Hankinson SE. Waist circumference, waist:hip ratio, and risk of breast cancer in the Nurses' Health Study. Am J Epidemiol. 1999 Dec 15;150(12):1316–24. doi: 10.1093/oxfordjournals.aje.a009963. [DOI] [PubMed] [Google Scholar]
- 17.Krebs EE, Taylor BC, Cauley JA, Stone KL, Bowman PJ, Ensrud KE. Measures of adiposity and risk of breast cancer in older postmenopausal women. J Am Geriatr Soc. 2006 Jan;54(1):63–9. doi: 10.1111/j.1532-5415.2005.00541.x. [DOI] [PubMed] [Google Scholar]
- 18.Park JY, Mitrou PN, Keogh RH, Luben RN, Wareham NJ, Khaw KT. Self-reported and measured anthropometric data and risk of colorectal cancer in the EPIC-Norfolk study. Int J Obes (Lond) 2012 Jan;36(1):107–18. doi: 10.1038/ijo.2011.61. [DOI] [PubMed] [Google Scholar]
- 19.Lim U, Ernst T, Buchthal SD, Latch M, Albright CL, Wilkens LR, Kolonel LN, Murphy SP, Chang L, Novotny R, Le ML. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr Diabetes. 2011;1:e6. doi: 10.1038/nutd.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank Hu. Obesity Epidemiology. Oxford University Press; New York City: 2008. Measurements of Adiposity and Body Composition; pp. 53–83. [Google Scholar]
- 21.Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, Hennekens CH. Weight, weight change, and coronary heart disease in women. Risk within the 'normal' weight range. JAMA. 1995 Feb 8;273(6):461–5. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997 Aug 1;146(3):214–22. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- 23.Ahn J, Schatzkin A, Lacey JV, Jr., Albanes D, Ballard-Barbash R, Adams KF, Kipnis V, Mouw T, Hollenbeck AR, Leitzmann MF. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007 Oct 22;167(19):2091–102. doi: 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- 24.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006 Jul 12;296(2):193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 25.Trentham-Dietz A, Nichols HB, Hampton JM, Newcomb PA. Weight change and risk of endometrial cancer. Int J Epidemiol. 2006 Feb;35(1):151–8. doi: 10.1093/ije/dyi226. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, Li HL, Wen W, Ji BT, Li Q, Shu XO, Gao YT. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005 Dec 1;162(11):1123–31. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 27.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004 Dec 9;351(24):2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 28.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004 Jan 10;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997 Nov 5;278(17):1407–11. [PubMed] [Google Scholar]
- 30.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997 Apr;65(4):1220S–8S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 31.Park SL, Goodman MT, Zhang ZF, Kolonel LN, Henderson BE, Setiawan VW. Body size, adult BMI gain and endometrial cancer risk: the multiethnic cohort. Int J Cancer. 2010 Jan 15;126(2):490–9. doi: 10.1002/ijc.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Yang G, Xiang YB, Zhang X, Zheng W, Gao YT, Shu XO. Body weight, fat distribution and colorectal cancer risk: a report from cohort studies of 134255 Chinese men and women. Int J Obes (Lond) 2013 Jun;37(6):783–9. doi: 10.1038/ijo.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otani T, Iwasaki M, Inoue M. Body mass index, body height, and subsequent risk of colorectal cancer in middle-aged and elderly Japanese men and women: Japan public health center-based prospective study. Cancer Causes Control. 2005 Sep;16(7):839–50. doi: 10.1007/s10552-005-4573-z. [DOI] [PubMed] [Google Scholar]
- 34.Wannamethee SG, Shaper AG, Morris RW, Whincup PH. Measures of adiposity in the identification of metabolic abnormalities in elderly men. Am J Clin Nutr. 2005 Jun;81(6):1313–21. doi: 10.1093/ajcn/81.6.1313. [DOI] [PubMed] [Google Scholar]
- 35.Harris TB, Visser M, Everhart J, Cauley J, Tylavsky F, Fuerst T, Zamboni M, Taaffe D, Resnick HE, Scherzinger A, Nevitt M. Waist circumference and sagittal diameter reflect total body fat better than visceral fat in older men and women. The Health, Aging and Body Composition Study. Ann N Y Acad Sci. 2000 May;904:462–73. doi: 10.1111/j.1749-6632.2000.tb06501.x. [DOI] [PubMed] [Google Scholar]
- 36.Han X, Stevens J, Truesdale KP, Bradshaw PT, Kucharska-Newton A, Prizment AE, Platz EA, Joshu CE. Body mass index at early adulthood, subsequent weight change and cancer incidence and mortality. Int J Cancer. 2014 Dec 15;135(12):2900–9. doi: 10.1002/ijc.28930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.