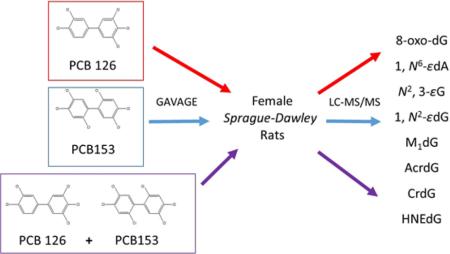
Abstract
Polychlorinated biphenyls (PCBs) are organic chemicals that were traditionally produced and widely used in industry as mixtures and are presently formed as byproducts of pigment and dye manufacturing. They are known to persist and bioaccumulate in the environment. Some have been shown to induce liver cancer in rodents. Although the mechanism of the toxicity of PCBs is unknown, it has been shown that they increase oxidative stress, including lipid peroxidation. We hypothesized that oxidative stress-induced DNA damage could be a contributor for PCB carcinogenesis and analyzed several DNA adducts in female Sprague–Dawley rats exposed to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126), 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153), and a binary mixture (PCB 126 + 153) for 14, 31, and 53 wks. Eight adducts were measured to profile oxidative DNA lesions, including 8-oxo-deoxyguanosine (8-oxo-dG), 1,N6-ethenodeoxyadenosine (1,N6-εdA), N2,3-ethenoguanine (N2,3-εG), 1,N2-ethenodeoxyguanosine (1,N2-εdG), as well as malondialdehyde (M1dG), acrolein (AcrdG), crotonaldehyde (CrdG), and 4-hydroxynonenal-derived dG adducts (HNEdG) by LC–MS/MS analysis. Statistically significant increases were observed for 8-oxo-dG and 1,N6-εdA concentrations in hepatic DNA of female rats exposed to the binary mixture (1000 ng/kg/day + 1000 μg/kg/day) but not in rats exposed to PCB 126 (1000 ng/kg/day) or PCB 153 (1000 μg/kg/day) for 14 and 31 wks. However, exposure to PCB 126 (1000 ng/kg/day) for 53 wks significantly increased 8-oxo-dG, 1,N6-εdA, AcrdG, and M1dG. Exposure to PCB 153 (1000 μg/kg/day) for 53 wks increased 8-oxo-dG, and 1,N6-εdA. Exposure to the binary mixture for 53 wks increased 8-oxo-dG, 1,N6-εdA, AcrdG, 1,N2-εdG, and N2,3-εG significantly above control groups. Increased hepatic oxidative DNA adducts following exposure to PCB 126, PCB 153, or the binary mixture shows that an increase in DNA damage may play an important role in hepatic toxicity and carcinogenesis in female Sprague–Dawley rats.
Graphical Abstract
INTRODUCTION
Polychlorinated biphenyls (PCBs) are industrial chemicals manufactured as mixtures that have been commercially available since 1929.1 These compounds are relatively stable under a broad range of chemical, thermal, and electrical conditions. This stability has fostered their wide use in commercial applications, including dielectrics in transformers and capacitors, cooling fluids in hydraulic systems, and plasticizers in paints, copying paper, adhesives, sealants, plastics, and so forth.1 Although production of PCBs was banned in 1979, some of these chemicals are still detectable in waters contaminated by paint manufacturing facilities and commercial paint pigments.2 Because of their high lipophilicity and stability, these chemicals persist in the environment. Chronic exposure to PCBs has been shown to adversely affect the immune, reproductive, nervous, and endocrine systems in animals and, specifically, to induce cancer.3,4 Additionally, the International Agency for Research on Cancer has recently classified PCBs, as a group, as a Group 1 Human Carcinogen due to their carcinogenic effects on animals and humans.5
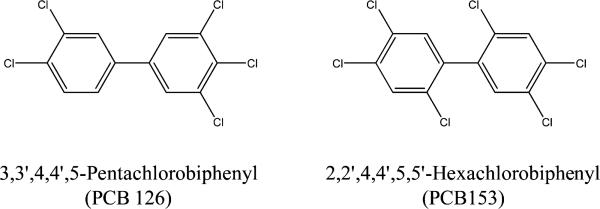
PCBs are generally classified as either dioxin- or nondioxin-like depending on the location of the chlorination. Dioxin-like PCBs act through a similar mechanism of action as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) by binding to the aryl hydrocarbon receptor (AhR), which upon activation has been reported to cause severe weight loss, thymic atrophy, hepatotoxicity, immunotoxicity, and enzyme induction in rodents.4,6 Available in vivo and in vitro data show that 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) has a toxic equivalency factor (TEF) value of 0.1 relative to that of the TCDD toxicity.6 As the most potent PCB in the environment, PCB 126 accounts for 40–90% of the toxic potency of dioxin-like PCBs.7 A nondioxin-like PCB, 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153), is shown to have the highest concentrations in human samples on a molar basis. Nondioxin-like PCBs are not included in the TEF methodology, and therefore, PCB 153 has no TEF value.6
The role of DNA adducts in the tumorigenesis of nongenotoxic chemicals such as PCBs has been studied, leading to one possible mechanism of PCB carcinogenesis occurring through the production of reactive oxygen species (ROS) or reactive oxygenated metabolites (ROM).8,9 Generation of ROS or ROM is followed by oxidative attack on DNA or proteins.4 ROS-induced damage during PCB exposure/metabolism has been reported in numerous in vitro10–23 and in vivo studies.3,24–45 Bioaccumulation of PCBs in target tissues may cause upregulation of CYP450 and subsequent DNA damage due to oxidative stress. Although other mechanisms of PCB carcinogenesis have been suggested, such as induction of metabolic enzymes, inhibition of gap junction intercellular communication, stimulation of mitosis or inhibition of apoptosis, and enhanced cell proliferation, the accumulation of promutagenic lesions in DNA may also be a key factor.46–49
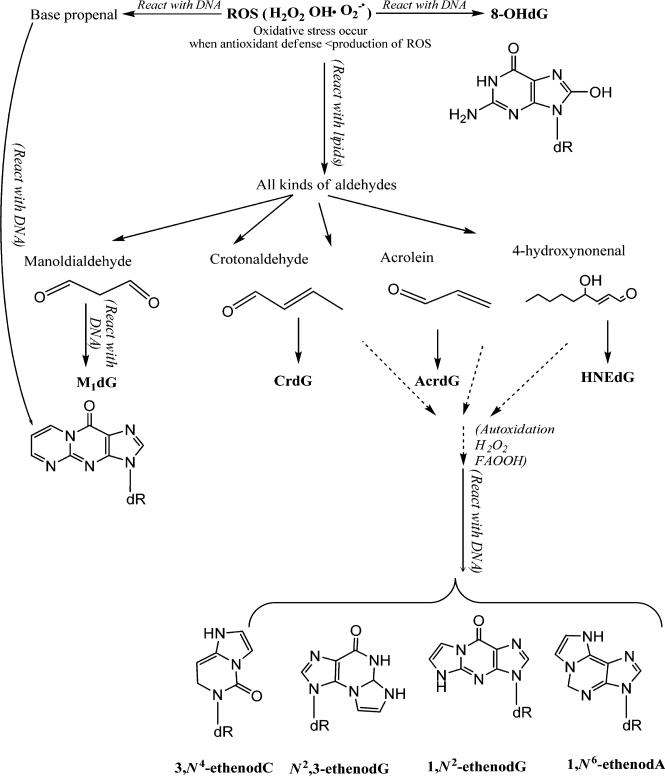
In this study, we collaborated with the National Toxicology Program (NTP) to understand the oxidative DNA adduct profile in hepatic DNA of female Sprague–Dawley rats that were exposed to PCB 126, PCB 153 (Figure 1), and a binary mixture of PCB 126 and PCB 153 for 14, 31, and 53 wks. To date, several DNA adducts have been specified as potential key endogenous oxidative DNA adducts in cancer studies and include 8-oxo-deoxyguanosine (8-oxo-dG), 1,N6-ethenodeoxyadenosine (1,N6-εdA), N2,3-ethenoguanine (N2,3-εG), 1,N2-ethenodeoxyguanosine (1,N2-εdG), malondialdehyde (MDA)-derived dG adducts (M1dG), acrolein-derived dG adducts (AcrdG), crotonaldehyde-derived dG adducts (CrdG), and 4-hydroxynonenal (HNE)-derived dG adducts (HNEdG).39,41–44,50–61 These adducts are summarized in Figure 2. Site-directed mutagenicity studies found that these DNA adducts could induce specific transition and/or transversion point mutations in bacteria and/or mammalian cells.50–57 Growing evidence supports that these DNA adducts are significantly induced in patients or animals with various chronic inflammatory diseases, including cancer.39,52,58–61 Because these adducts have different formation and repair pathways in vivo and can cause different mutations in cells, their profile study will provide more comprehensive information on oxidative DNA lesions to estimate the toxicity of PCBs. The knowledge gained from this study will improve the scientific basis of human risk assessment of PCBs in the environment.
Figure 1.
Chemical structures of PCB 126 and PCB 153.
Figure 2.
An illustration of the major DNA adducts induced by ROS.
EXPERIMENTAL PROCEDURES
Chemicals
Nucleic acid purification grade lysis buffer, protein precipitation solution, and proteinase K were purchased from Gentra Systems (Minneapolis, MN). HPLC grade water and methanol were from Thermo Fisher Scientific Company (Raleigh, NC). 15N5-8-oxo-dG, 15N5-dG and 13C10-dG were purchased from Cambridge Isotope Laboratories (Andover, MA, USA). Other chemical reagents were from Sigma-Aldrich Chemical Company (St. Louis, MO). 15N5-1,N6-εdA standard was synthesized as described by Ham et al.62 1,N2-εdG and 13C10-1,N2-εdG were synthesized as reported by Kusmierek et al.63 MDA-modified 15N5 and 14N5 DNA were made by the method in Jeong's study.64 AcrdG, CrdG, and HNEdG standards and their 15N5-labeled internal standards were synthesized according to previously published methods.65–67
Animal Exposure and DNA Isolation
Rat liver tissues were provided by Battelle Laboratories (Columbus, OH) and State University of New York at Buffalo from a series of studies conducted for the National Toxicology Program's Dioxin Toxic Equivalency Factor (TEF) initiative according to the NIEHS contract N01-ES-75411.7,68,69 Female Harlan Sprague–Dawley rats were treated by gavage (2.5 mL/kg) with PCB 126 alone, PCB 153 alone, or the binary mixture of PCB 126 and PCB 153 five days per week for 14, 31, and 53 weeks. The Sprague–Dawley female rat was used for these studies based upon the prior observation of high hepatocarcinogenic potency of TCDD within this strain and the extensive literature on the effects of TCDD and related compounds in this model.7,68–70 Dose formulations were prepared fresh on a monthly basis by formulating the test articles in a corn-oil vehicle containing 1% USP-grade acetone. Control groups, dosed with a corn-oil:acetone (99:1) vehicle were included in each of the studies. Doses used were based on values of the TEF selected by the World Health Organization.6,71 The design and dosages for these studies are as outlined in the original NTP reports and were selected to evaluate the dose response of a constant ratio of each of the PCB congeners and evaluate the effect of increasing concentrations of PCB 153 on the PCB 126-induced responses. The dosages of PCB 126 were designed to match the dose range used for the studies of carcinogenicity of TCDD with an adjustment for the TEF of 0.1. The doses of PCB 153 are similar to those previously used in tumor promotion studies.7,68,69 The doses were as follows: PCB 126 (0 and 1000 ng/kg/day for 14 and 31 wks; 0, 550, and 1000 ng/kg/day for 53 wks), PCB 153 (0 and 1000 μg/kg/day for 14 and 31 wks; 0, 300, and 1000 μg/kg/day for 53 wks), and the binary mixture of PCB 126 and PCB 153 (0 and 1000 ng/kg/day + 1000 μg/kg/day for 14 and 31 wks; 0, 300 ng/kg/day + 300 μg/kg/day, and 1000 ng/kg/day + 1000 μg/kg/day for 53 wks). Liver tissues were collected from 5–8 female rats per group/day following the final exposure and stored frozen at −80 °C. DNA was isolated as described previously.72
8-oxo-dG and 1,N6-εdA Assay
The assay was performed as previously described by Pang et al. with minor modifications.58 First, 100 μg of DNA in NaOAc buffer I (30 mM sodium acetate, 0.2 mM ZnCl2, pH 5.6) was incubated with nuclease P1 (5 μg) at 37 °C for 1 h. Immediately after incubation, DNA solutions were spiked with 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO, 5 μL, 1.5 M), 15N5-8-oxo-dG (500 fmol), and 15N5-1,N6-εdA (20 fmol) followed by the addition of NaOAc buffer II (30 mM sodium acetate, pH 8.1), alkaline phosphatase (20 units), and phosphodiesterase (0.012 units) and then incubated at 37 °C for an additional hour. Enzymes and undigested DNA were removed by Microcon-10 filtration (11500 rpm, 4 °C, 50 min), and the filtrate was concentrated by SpeedVac.
Samples were enriched for 8-oxo-dG and 1,N6-εdA using an Agilent 1200 HPLC system equipped with an Atlantis T3 column (5 μm, 4.6 mm × 150 mm). The nucleosides were monitored at 264 nm. The column was eluted at a flow rate of 1 mL/min at 30 °C with a 5–80% methanol gradient in 10 mM ammonium acetate buffer as follows: hold at 5% methanol for 5 min, 5–10% methanol over 5 min, 10–20% methanol over 10 min, and 20–80% over 10 min and re-equilibrate at 5% for 5 min. 8-oxo-dG and 1,N6-εdA fractions were collected at 24–26 min and 33–34 min, respectively.
AcrdG, 1,N2-εdG, M1dG, CrdG, and HNEdG Assay
A similar assay to that of 8-oxo-dG and 1,N6-εdA was applied to AcrdG, 1,N2-εdG, M1dG, CrdG, and HNEdG with minor modifications. First, 100 μg of DNA in NaOAc buffer I was incubated with nuclease P1 (5 μg) at 37 °C for 1 h. Immediately before incubation, DNA solutions were spiked with TEMPO (5 μL, 1.5 M), 15N5–AcrdG (50 fmol), 13C10-1,N2-εdG (100 fmol), MDA-modified internal standard corresponding to 400 fmol 15N5-M1dG, 15N5–CrdG (50 fmol), and 15N5–HNEdG (50 fmol) followed by the addition of NaOAc buffer II, alkaline phosphate (20 units), and phosphodiesterase (0.012 units) and then incubated at 37 °C for an additional hour. Enzymes and undigested DNA were removed by Microcon-10 filtration (11500 rpm, 4 °C, 50 min), and the filtrate was concentrated by SpeedVac.
Samples were enriched for AcrdG, 1,N2-εdG, and M1dG by the same HPLC method as described for 8-oxo-dG and 1,N6-εdA with a 100% methanol and 5 mM ammonium formate–0.1% formic acid mobile phase. CrdG and HNEdG were eluted at a flow rate of 0.5 mL/min with a 35–70% methanol mobile phase in 10 mM ammonium acetate buffer over 25 min. Fraction collection times for AcrdG, 1,N2-εdG, CrdG, and HNEdG were 28–30, 32–34, 15–18, and 34–36 min, respectively.
LC–MS/MS Analysis
8-oxo-dG was analyzed by a Waters Acquity UPLC coupled to a Thermofinnigan TSQ Quantum Ultra triple-quadrupole mass spectrometer in a positive selected reaction mode (SRM) monitoring the signals m/z 284.1 → 168.0 for 8-oxo-dG and m/z 289.1 → 173.0 for 15N5-8-oxo-dG. Separation was performed on a T3 HSS column (1.7 μm, 2.1 mm × 100 mm) with a flow rate of 200 μL/min using mobile phase (A) 0.1% acetic acid in water and (B) 0.1% acetic acid in methanol. MS settings were as follows: electrospray voltage (3000 V), ion transfer capillary temperature (285 °C), vaporizer temperature (250 °C), sheath and auxiliary gas pressures (35 and 30 arbitrary units), and collision energy (12 eV). 1,N6-εdA, AcrdG, 1,N2-εdG, M1dG, CrdG, and HNEdG were analyzed by nanoAcquity UPLC coupled to a Thermofinnigan TSQ Quantum Ultra triple-quadrupole mass spectrometer in positive SRM monitoring the signals m/z 276.0 → 160.0 for 1,N6-εdA; m/z 281.0 → 165.0 for 15N5-1,N6-εdA; m/z 304.0 → 188.0 for M1dG; m/z 308.0 → 193.0 for 15N5-M1dG; m/z 292.0 → 176.0 for 1,N2-εdG; m/z 302.0 → 181.0 for 13C10-1,N2-εdG; m/z 424.0 → 308.0 for HNEdG; m/z 429.0 → 313.0 for 15N5- HNEdG; m/z 338.0 → 222.0 for CrdG; m/z 343.0 → 227.0 for 15N5- CrdG; m/z 324.0 → 208.0 for AcrdG; and m/z 329.0 → 213.0 for 15N5- AcrdG. Separation was performed on a UPLC BEH C18 column (1.7 μm, 100 μm × 100 mm) with a flow rate of 1 μL/min using mobile phase (A) 5 mM ammonium formate in water and (B) 1% formic acid in acetonitrile for 1,N6-εdA and (A) 0.1% formic acid in water and (B) acetonitrile for AcrdG, 1,N2-εdG, M1dG, CrdG, and HNEdG. MS settings were as follows: emitter tip voltage (1500 V), ion transfer capillary temperature (285 °C), and collision energy (12 eV). N2,3-εG was analyzed as previously described.58
Statistical Analysis
Statistical analyses were performed using R (2.11). Considering the limited sample size in certain groups, the nonparametric test was used to assess the differences between control and PCB-treated rats or various control groups for the number of adducts by Wilcox Rank Sum test.72 Two-sided and one-sided p values were considered significant if they were less than 0.05.
RESULTS AND DISCUSSION
In this study, we examined the relationship between PCB exposure and oxidative DNA adduct formation. Female Sprague–Dawley rats were exposed to PCB 126, PCB 153, or the binary mixture of PCB 126 and PCB 153 by gavage five days per week for 14, 31, and 53 wks.
8-oxo-dG and 1,N6-εdA formation in the liver of female rats exposed to PCB 126, PCB 153, and binary mixture are shown in Table 1. After exposure to only PCB 126 or PCB 153 for 14 or 31 wks, neither 8-oxo-dG nor 1,N6-εdA was significantly increased. For the longer exposure period of 53 wks, PCB 126 or PCB 153 alone showed significant increases for both 8-oxo-dG and 1,N6-εdA (Table 1). However, exposure to the binary mixture showed a statistically significant increase in each exposure group for both adducts. Specifically, exposure to the binary mixture (1000 ng/kg + 1000 μg/kg) produced roughly a 1.5–2.5-fold increase in 8-oxo-dG in hepatic DNA after 14 wks (p = 0.005), 31 wks (p = 0.009), and 53 wks (p = 0.029) compared to their respective control groups. More impressively, a 6–20-fold increase of 1,N6-εdA was observed in the same exposure groups.
Table 1.
Number of 8-oxo-dG Adducts/106 dG and 1,N6-εdA Adducts/108 dA Measured in Sprague-Dawley Rat Hepatic DNA Following Exposure to PCB 126 (1000 ng/kg/day), PCB 153 (1000 μg/kg/day), or the Binary Mixture of PCB 126 + PCB 153 (1000 ng/kg/day + 1000 μg/kg/day) for 14, 31, or 53 weeks
| PCB 126 |
PCB 153 |
binary (PCB 126 + 153) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 14 wks | 31 wks | 53 wks | 14 wks | 31 wks | 53 wks | 14 wks | 31 wks | 53 wks | ||
| 8-oxo-dG add/106 dG | control | 2.65 ± 1.84 | 2.46 ± 0.82 | 3.61 ± 0.70 | 3.28 ± 0.82 | 2.67 ± 0.84 | 2.37 ± 0.28 | 3.45 ± 1.15 | 4.88 ± 1.06 | 4.96 ± 0.66 |
| exposeda | 3.82 ± 2.39 | 3.63 ± 1.71 | 6.80 ± 0.31b | 3.65 ± 0.64 | 3.21 ± 0.49 | 4.27 ± 1.71b | 6.01 ± 1.14b | 7.08 ± 1.38b | 12.50 ± 2.52b | |
| 1,N6-εdA add/108 dA | control | 2.24 ± 1.11 | 1.47 ± 0.17 | 1.90 ± 0.37 | 1.28 ± 0.23 | 1.56 ± 0.26 | 2.19 ± 0.71 | 0.56 ± 0.24 | 1.67 ± 0.74 | 2.89 ± 0.79 |
| exposeda | 1.47 ± 0.18 | 1.81 ± 0.50 | 4.36 ± 1.34b | 1.33 ± 0.41 | 1.43 ± 0.31 | 3.96 ± 0.98b | 11.30 ± 2.75b | 18.00 ± 7.10c | 18.30 ± 8.00c | |
Indicates exposure to PCB 126 (1000 ng/kg/day), PCB 153 (1000 μg/kg/day), or binary (PCB 126 + 153) (1000 ng/kg/day + 1000 μg/kg/day).
Indicates p ≤ 0.05.
Indicates p ≤ 0.01.
The induction of the DNA adduct N2,3-εG in hepatic DNA of female rats exposed to PCB 126, PCB 153, or the binary mixture for 53 wks are shown in Table 2. Although there was no increase observed for the lower doses of PCB 126 (550 ng/kg/day), PCB 153 (300 μg/kg/day), or the binary mixture (PCB 126 + PCB 153, 300 ng/kg/day + 300 μg/kg/day), approximately a 1.5–2-fold significant increase was observed for higher doses of PCB 126 (1000 ng/kg/day, p < 0.05), PCB 153 (1000 μg/kg/day, p < 0.01), and the binary mixture (PCB 126 + PCB 153, 1000 ng/kg/day + 1000 μg/kg/day, p < 0.01) compared to their respective control groups.
Table 2.
Number of N2-3εG Adducts/108 G Measured in Sprague-Dawley Rat Hepatic DNA Following Exposure to Multiple Concentrations of PBC 126, PCB 153, or the Binary Mixture of PCB 126 + PCB 153 for 53 Weeks
| N2,3εG add/108 G | ||
|---|---|---|
| PCB 126 53 wks | control | 1.67 ± 0.30 |
| 550 ng/kg | 1.44 ± 0.70 | |
| 1000 ng/kg | 2.55 ± 0.56a | |
| PCB 153 53 wks | control | 1.48 ± 0.37 |
| 300 μg/kg | 1.59 ± 0.63 | |
| 1000 μg/kg | 2.55 ± 0.44b | |
| binary (PCB 126 + 153) 53 wks | control | 1.18 ± 0.26 |
| 300 ng/kg + 300 μg/kg | 1.70 ± 0.41 | |
| 1000 ng/kg + 1000 μg/kg | 2.59 ± 0.78b |
Indicates p ≤ 0.05.
Indicates p ≤ 0.01.
In this study, we also analyzed several other lipid peroxidation-induced DNA adducts such as AcrdG, 1,N2-εdG, M1dG, CrdG, and HNEdG in the hepatic DNA of female rats exposed to PCB 126 (1000 ng/kg/day) and the binary mixture (PCB 126 + PCB 153, 1000 ng/kg/day + 1000 μg/kg/day) for 53 wks (Table 3). Concentrations of AcrdG (p = 0.029) and 1,N2-εdG (p = 0.029) were significantly higher in animals exposed to the binary mixture (PCB 126 + PCB 153, 1000 ng/kg/day + 1000 μg/kg/day) compared to their respective control groups. M1dG (p = 0.026) and AcrdG (p = 0.009) were observed to increase in animals that were exposed to PCB 126 (1000 ng/kg/day) alone for 53 wks.
Table 3.
Number of 1,N2-εdG Adducts/108 dG, M1dG Adducts/108 dG, CrdG Adducts/108 dG, HNEdG Adducts/108 dG, and AcrdG Adducts/108 dG Measured in Sprague-Dawley Rat Hepatic DNA Following Exposure to PCB 126 (1000 ng/kg/day) or the Binary Mixture of PCB 126 + PCB 153 (1000 ng/kg/day + 1000 μg/kg/day) for 53 weeks
| PCB 126 add/108 dG | binary add/108 dG | |
|---|---|---|
| 1,N2-εdG control | 2.26 ± 0.15 | 2.59 ± 0.36 |
| 1,N2-εdG exposed | 2.74 ± 0.43 | 4.19 ± 0.98b |
| M1dG control | 4.02 ± 0.73 | 3.03 ± 0.48 |
| M1dG exposeda | 5.65 ± 1.86b | 5.80 ± 1.65 |
| CrdG control | 0.60 ± 0.14 | 0.34 ± 0.06 |
| CrdG exposeda | 0.68 ± 0.18 | 0.66 ± 0.20 |
| HNEdG control | 1.01 ± 0.11 | 0.93 ± 0.36 |
| HNEdG exposeda | 1.17 ± 0.25 | 2.27 ± 1.66 |
| AcrdG control | 9.32 ± 0.74 | 12.70 ± 2.93 |
| AcrdG exposeda | 11.90 ± 1.91c | 24.30 ± 4.03b |
Indicates exposure to PCB 126 (1000 ng/kg/day) and binary (PCB 126 + 153) (1000 ng/kg/day + 1000 μg/kg/day) for 53 wks.
Indicates p ≤ 0.05.
Indicates p ≤ 0.01.
Similar findings that implicate the role of PCBs in creating oxidative stress are consistent with our data.29,30,38,73,74 Fadhel et al.38 and Lamartinier et al.73 observed a significant increase in glutathione-S-transferase (GST) in Sprague–Dawley rats that were exposed to 150 μmol/kg PCB 153 for six days and an increase in hepatic lipid peroxidation with a single dose of PCB 153. Twaroski et al.30 also reported similar findings from rats that received intraperitoneal injections of PCB 153 (100 mmol/kg for 3 wks). Previous reports also showed Sprague–Dawley rats to have significant increases in oxidized vitamin E (α-tocopheryl quionone) after intraperitoneal PCB 153 injections (100 mmol/kg/injection)75 as well as a significant induction of hepatic vitamin A following subchronic exposure to 50 ppm of PCB 153.74 These studies, and others that showed the enhancement of ROS by several pathways,7 indicate the induction of oxidative stress by an AhR-independent pathway due to PCB 153 exposure.
Similarly, the significant induction of 8-oxo-dG, M1dG, 1,N6-εdA, and AcrdG that we observed in samples exposed to PCB 126 for 53 wks (1000 ng/kg/day) could be indicative of PCB 126-related oxidative stress. Although Hassaoun et al.25,26 observed increased oxidative stress in hepatic tissues of female rats exposed to PCB 126 for 13 and 31 wks, we found no significant increase in 8-oxo-dG or 1,N6-εdA in samples that were exposed to PCB 126 or PCB 153 for 14 and 31 wks. More severe accumulation of PCB 126 and PCB 153 were observed in the liver tissues of 53 wk exposure animals than in the 13 or 31 wk exposure ones.5,6 Because the increase in formation of these DNA adducts was only observed in the 53 wk exposure groups, we assume that long-term chronic exposure is necessary for induction of these oxidative lesions. Aqil et al. also reported a significant increase of 8-oxo-dG in the liver and lung tissues of female Sprague–Dawley rats after exposure to PCB 126 via polymeric implants up to 45 days.76
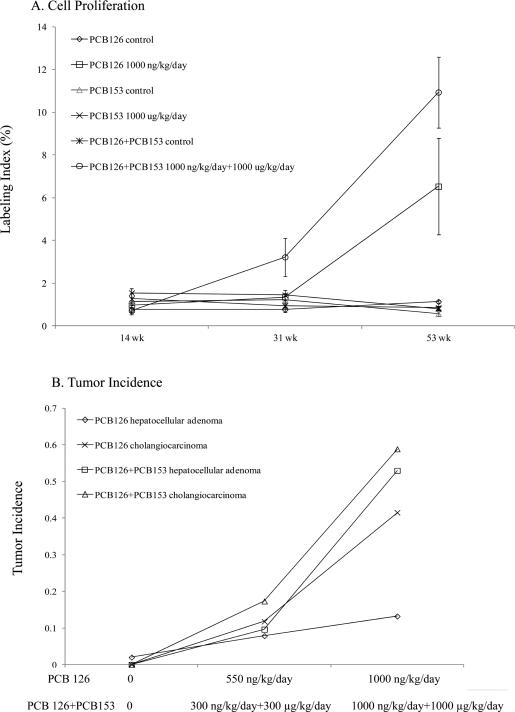
In this study, we also illustrated that the exposure to PCB 126 (1000 ng/kg/day) for 53 wks induced slightly more 8-oxo-dG and 1,N6-εdA adducts than those of PCB 153 (1000 μg/kg/day) for 53 wks. These findings are also in agreement with the induction of the milder hepatic lesions that are induced by exposure to PCB 153 compared to that of PCB 126 in these animals.7,68 The relative toxicity of PCB 126 and PCB 153 that was evaluated by tumor incidence and cell proliferation in those studies is also in accordance with our oxidative DNA adduct profile. The NTP reported that although a significant induction of cell proliferation and dose-dependent increases of tumor incidences were observed in PCB 126 exposed animals with the highest labeling index detected in the longest exposure group (Figure 3), there was only one report of hepatocellular adenoma (3000 μg/kg/day group), and no cholangiocarcinomas were observed in PCB 153-exposed animals.7,68
Figure 3.
Cell proliferation (A) in the liver of female Sprague–Dawley rats exposed to PCB 126 (1000 ng/kg/day), PCB 153 (1000 μg/kg/day), or the binary mixture of PCB 126 and PCB 153 (1000 ng/kg/day +1000 μg/kg/day) for 14, 31, and 53 weeks. Tumor incidence (B) in the liver of female Sprague–Dawley rats exposed to PCB 126 (0, 550, and 1000 ng/kg/day) or the binary mixture of PCB 126 and PCB 153 (0, 300 ng/kg/day + 300 μg/kg/day, and 1000 ng/kg/day + 1000 μg/kg/day);7 n = 8–10.7,68,69
Furthermore, we found that the binary mixture (PCB 126 + PCB 153, 1000 ng/kg/day + 1000 μg/kg/day) generally induced more DNA adducts in comparison to that of PCB 126 or PCB 153 alone, especially for the 53 wk exposure group. Similar results were also reported for M1dG formation by Jeong et al.39 Likewise, the histopathology results for the 2-year cancer bioassay from NTP (Figure 3) reported a higher hepatic tumor incidence in the animals that were exposed to the binary mixture in comparison to PCB 126 or PCB 153 alone. It is not known whether this reflects a pharmacokinetic or pharmacodynamic interaction. At 53 wks, the PCB 153 levels in the binary mixture (PCB 126 + PCB 153, 1000 ng/kg/day + 1000 μg/kg/day) exposed animals were over 3.5× higher than those in the comparable 1000 μg/kg/day PCB 153 group (6728 ng of PCB 153/g). In contrast, average liver PCB 126 levels showed less change in the rats exposed to the 1000 ng/kg/day + 1000 μg/kg/day binary mixture (780 ± 218 ng/g) than those exposed to 1000 ng/kg/day of PCB 126 alone (605 ± 19 ng/g). However, the EROD activities were higher with 3219 ± 271 and 2122 ± 132 pmol/min/mg in the livers of rats exposed to the binary mixture (PCB 126 + PCB 153, 1000 ng/kg/day + 1000 μg/kg/day) or PCB 126 alone (1000 ng/kg/day), respectively.7,68,69 Additionally, concentrations of PCB 126 and PCB 153 were determined in fat, liver, lung, and blood at the 14, 31, and 53 week interim evaluations and at the end of the 2-year study (105 weeks). PCB 126 was not detectable in vehicle control animals but increased with increasing dose of PCB 126 and duration of exposure in exposed animals with the highest concentrations being found in liver and fat and lower levels seen in lung and blood. Increasing the proportion of PCB 153 in the mixture relative to PCB 126 led to a general decrease in the amount of PCB 126 in liver and lung at the later time points, whereas in fat and blood, there was generally either no effect of PCB 153 on the disposition of PCB 126 or there was an increase in the amount of PCB 126 in the tissue. In vehicle control animals, PCB 153 was not detectable in the liver at any time points in the lung at the 14 wk interim evaluation or in the blood except for the 31 wk exposure group. PCB 153 was measurable in all examined tissues of treated animals with the highest concentrations found in fat at the end of the 2-year study in groups administered the highest doses. At 53 wks, the PCB 153 levels in the PCB 126 + PCB 153 (1000 ng/kg/day + 1000 μg/kg/day) group were 59450 ng/g, which is over 3.5× higher than those in PCB 153 alone (1000 μg/kg/day). Therefore, in the presence of PCB 126, there are much higher levels of PCB 153. In contrast, the presence of PCB 153 did not affect the amount of PCB 126.7,68,69
Oxidative DNA adducts may interfere with DNA sequence translation, ultimately resulting in modifications to gene expression that can lead to malignant cells if the DNA is not repaired prior to cell replication.50–55 Many studies reported the diverse mutation spectrum induced by the oxidative DNA adducts listed in this paper.50–55 The predominant mutation induced by 8-oxo-dG was G to T transversions in both bacterial and mammalian cells.52 1,N6-εdA mainly induced A to G transition in E. coli and kidney cells, and N2,3-εG produced specifically G to A transitions in vitro and in E. coli.51 Although various mutations were induced by 1,N2-εdG, G to T transversion was dominant in Chinese hamster ovary cells.54 This mutation was also found to be induced by AcrdG and CrdG49 as well as MDA in E. coli and 4-HNE in hamster cells.53,55 Increased oxidative DNA adducts were also detected in animal studies exposed to various chemical carcinogens.39,77–83 Significant increases in HNEdG, M1G, and 8-oxo-dG were detected in the liver of rats after an intraperitoneal injection of a single dose of CCl4,77–79 a widely applied model toxicant for hepatic carcinogenesis studies in animals. Trimethylarsine oxide, an organic metabolite of inorganic arsenics, was found to significantly induce the formation of hepatic 8-oxo-dG and hepatocellular adenomas in male F344 rats after two-year exposure with the dose of 200 ppm.80 A 2-fold significant increase of 8-oxo-dG was detected in mouse bone marrow DNA 1 h following oral administration of benzene (880 mg/kg).81 The induction of hepatic 8-oxo-dG, cell proliferation, and enzyme-altered hepatocellular foci by TCDD were observed after 30 wk exposure in female rats with diethylnitrosamine initiation.82,83 Significantly increased M1G was observed in the liver of female Sprague–Dawley rats following chronic exposure (53 weeks) to PCB 126 or the binary mixture of PCB 126 and PCB 153 at the highest dose with an almost 2-fold increase compared with controls. The increased M1G was accompanied by increased cell proliferation.39 A significant increase in 1,N6-εdA and 3,N4-εdC were found in premalignant target organs affected by various chronic diseases, such as genetic metal storage disorders (Wilson's disease and hemochromatosis), chronic pancreatitis, and chronic hepatitis.60 Elevated 8-oxo-dG has been detected in patients with diverse malignancies, acute leukemia, colorectal cancer, hepatic cancer, and breast cancer.57 Higher M1dG was detected in smoking patients with lung cancer compared with control smokers.61 All of these studies consistently support the importance of these oxidative DNA adducts as critical biomarkers in carcinogenesis. Moreover, Lehmann et al. found a significant increase in G to T transversions in the livers of male Fisher rats after 4-monochlorobiphenyl exposure,84 which agrees with the dominant mutation spectrum induced by several oxidative DNA adducts in this study and implies the possible importance of these adducts for the toxicity of PCBs as well.
The concentrations of the oxidative DNA adducts that were measured in this study reflect several complex factors such as exposure time, exposure dose, chemical metabolism, adduct repair pathway, tissue, age, sex, species, and so forth. Thus, the background concentration of each hepatic DNA adduct (8-oxo-dG > AcrdG > M1dG > 1,N2-εdG > 1,N6-εdA > N2,3-εG > HNEdG > CrdG) in female rats showed different distributions with a range from ~2 adducts/106 parent bases to ~1 adduct/109 parent bases. Similar findings were reported by Pang et al.58
Several age-dependent studies showed that the background levels of 8-oxo-dG and 1,N6-εdA increased with age.85,86 These results might be explained by the decreased capability of DNA repair in aged animals.87,88 Chung et al. also reported a variation of distribution of AcrdG and CrdG in several tissues (lung, kidney, colon, brain, etc.) and suggested that the significance of tissue specific and stereoselective patterns of the adducts plays a role in the background amounts of those DNA adducts.89
In summary, the present study consistently demonstrates an association between oxidative DNA damage and hepatic toxicity in female Sprague–Dawley rats exposed to PCB 126, PCB 153, and the binary mixture of these two compounds. The time-course study confirmed that accumulation of these lesions was time-dependent, which agrees with the tumor incidence and cell proliferation results reported by NTP.7,68,69 The DNA adduct profile study further confirmed the enhanced toxicity of PCB 126 by PCB 153 cannot be evaluated by the simplified TEF methodology. However, measurement of DNA adduct profiles in comparison with their relative repair potential, the specific susceptibilities of different tissue types or regions of tissue to specific types of DNA damage, or to associate the toxicity of chemicals with specific formation patterns of adducts is still underdeveloped. Further mechanistic studies on PCB-induced oxidative DNA adducts can provide more valuable information.
ACKNOWLEDGMENTS
We would like to thank Dr. Valeriy Afonin for his technical assistance in DNA isolation.
Funding
This work was supported by the NIEHS Superfund Basic Research Program P42-ES05948 and the NIEHS Center for Environmental Health and Susceptibility P30 ES 10126.
ABBREVIATIONS
- 1,N2-εdG
1,N2-ethenodeoxyguanosine
- 1,N6-εdA
1,N6-ethenodeoxyadenosine
- 8-oxo-dG
8-oxo-deoxyguanosine
- AcrdG
acrolein-derived dG adducts
- AHR
aryl hydrocarbon receptor
- CrdG
crotonaldehyde-derived dG adducts
- HNE
4-hydroxynonenal
- HNEdG
4-hydroxynonenal-derived dG adducts
- M1dG
malondialdehyde-derived dG adducts
- MDA
malondialdehyde
- N2,3-εG
N2,3-ethenoguanine
- NTP
National Toxicology Program
- PCB 126
3,3′,4,4′,5-pentachlorobiphenyl
- PCB 153
2,2′,4,4′,5,5′-hexachlorobiphenyl
- PCB
polychlorinated biphenyls
- ROM
reactive oxygenated metabolites
- ROS
reactive oxygen species
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TEF
toxic equivalency factor
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxy
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.WHO . Concise International Chemical Assessment Document. Vol. 55. Geneva, Switzerland: 2003. Polychlorinated biphenyls: Human health aspects; pp. 1–57. [Google Scholar]
- 2.Hu D, Hornbuckle KC. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2010;44:2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glauert HP, Tharappel JC, Lu Z, Stemm D, Banerjee S, Chan LS, Lee EY, Lehmler HJ, Robertson LW, Spear BT. Role of oxidative stress in the promoting activities of pcbs. Environ. Toxicol. Pharmacol. 2008;25:247–250. doi: 10.1016/j.etap.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson LW, Hansen LG. PCBs: Recent advances in environmental toxicology and health effects. University Press of Kentucky; Lexington, KY.: 2001. [Google Scholar]
- 5.IARC . IARC Monogr. Eval. Carcinog. Risks Hum. World Heath Organization; Lyon, France: 2015. Polychlorinated Biphenyls and Polybrominated Biphenyls; pp. 1–510. [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NTP . NTP technical report on the toxicology and carcinogenesis studies of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in female Harlan Sprague-Dawley rats (Gavage Studies) US Department of Health and Human Services; Washington, DC.: 2006. [PubMed] [Google Scholar]
- 8.Jarabek AM, Pottenger LH, Andrews LS, Casciano D, Embry MR, Kim JH, Preston RJ, Reddy MV, Schoeny R, Shuker D, Skare J, Swenberg J, Williams GM, Zeiger E. Creating context for the use of DNA adduct data in cancer risk assessment: I. Data organization. Crit. Rev. Toxicol. 2009;39:659–678. doi: 10.1080/10408440903164155. [DOI] [PubMed] [Google Scholar]
- 9.Melnick RL, Kohn MC, Portier CJ. Implications for risk assessment of suggested nongenotoxic mechanisms of chemical carcinogenesis. Environ. Health Perspect. 1996;104(Suppl 1):123–134. doi: 10.1289/ehp.96104s1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat. Toxicol. 2006;77:422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Spencer WA, Lehmler HJ, Robertson LW, Gupta RC. Oxidative DNA adducts after Cu(2+)-mediated activation of dihydroxy PCBs: role of reactive oxygen species. Free Radical Biol. Med. 2009;46:1346–1352. doi: 10.1016/j.freeradbiomed.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De S, Ghosh S, Chatterjee R, Chen YQ, Moses L, Kesari A, Hoffman EP, Dutta SK. PCB congener specific oxidative stress response by microarray analysis using human liver cell line. Environ. Int. 2010;36:907–917. doi: 10.1016/j.envint.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreiem A, Rykken S, Lehmler HJ, Robertson LW, Fonnum F. Hydroxylated polychlorinated biphenyls increase reactive oxygen species formation and induce cell death in cultured cerebellar granule cells. Toxicol. Appl. Pharmacol. 2009;240:306–313. doi: 10.1016/j.taap.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol. Appl. Pharmacol. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- 15.Lin CH, Lin PH. Induction of ROS formation, poly(ADP-ribose) polymerase-1 activation, and cell death by PCB126 and PCB153 in human T47D and MDA-MB-231 breast cancer cells. Chem.-Biol. Interact. 2006;162:181–194. doi: 10.1016/j.cbi.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Lee DW, Opanashuk LA. Polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. NeuroToxicology. 2004;25:925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Song E, Liu L, Ma X, Tian X, Dong H, Song Y. Polychlorinated biphenyl quinone metabolites lead to oxidative stress in HepG2 cells and the protective role of dihydrolipoic acid. Toxicol. In Vitro. 2012;26:841–848. doi: 10.1016/j.tiv.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Studies on the protective role of vitamin C and E against polychlorinated biphenyl (Aroclor 1254)–induced oxidative damage in Leydig cells. Free Radical Res. 2005;39:1259–1272. doi: 10.1080/10715760500308154. [DOI] [PubMed] [Google Scholar]
- 19.Mariussen E, Myhre O, Reistad T, Fonnum F. The polychlorinated biphenyl mixture aroclor 1254 induces death of rat cerebellar granule cells: the involvement of the N-methyl-D-aspartate receptor and reactive oxygen species. Toxicol. Appl. Pharmacol. 2002;179:137–144. doi: 10.1006/taap.2002.9353. [DOI] [PubMed] [Google Scholar]
- 20.Saed GM, Jiang ZL, Fletcher NM, Al Arab A, Diamond MP, Abu-Soud HM. Exposure to polychlorinated biphenyls enhances lipid peroxidation in human normal peritoneal and adhesion fibroblasts: a potential role for myeloperoxidase. Free Radical Biol. Med. 2010;48:845–850. doi: 10.1016/j.freeradbiomed.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katynski AL, Vijayan MM, Kennedy SW, Moon TW. 3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126) impacts hepatic lipid peroxidation, membrane fluidity and beta-adrenoceptor kinetics in chick embryos. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2004;137:81–93. doi: 10.1016/j.cca.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Kalen AL, Li L, Lehmler HJ, Robertson LW, Goswami PC, Spitz DR, Aykin-Burns N. Polychlorinated-biphenyl-induced oxidative stress and cytotoxicity can be mitigated by antioxidants after exposure. Free Radical Biol. Med. 2009;47:1762–1771. doi: 10.1016/j.freeradbiomed.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatesha VA, Venkataraman S, Sarsour EH, Kalen AL, Buettner GR, Robertson LW, Lehmler HJ, Goswami PC. Catalase ameliorates polychlorinated biphenyl-induced cytotoxicity in nonmalignant human breast epithelial cells. Free Radical Biol. Med. 2008;45:1094–1102. doi: 10.1016/j.freeradbiomed.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, Haschek WM, Ludewig G, Robertson LW. Acute toxicity of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environ. Int. 2010;36:918–923. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassoun EA, Li F, Abushaban A, Stohs SJ. Production of superoxide anion, lipid peroxidation and DNA damage in the hepatic and brain tissues of rats after subchronic exposure to mixtures of TCDD and its congeners. J. Appl. Toxicol. 2001;21:211–219. doi: 10.1002/jat.744. [DOI] [PubMed] [Google Scholar]
- 26.Hassoun EA, Li F, Abushaban A, Stohs SJ. The relative abilities of TCDD and its congeners to induce oxidative stress in the hepatic and brain tissues of rats after subchronic exposure. Toxicology. 2000;145:103–113. doi: 10.1016/s0300-483x(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 27.Hassoun EA, Periandri-Steinberg S. Assessment of the roles of antioxidant enzymes and glutathione in 3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126)-induced oxidative stress in the brain tissues of rats after subchronic exposure. Toxicol. Environ. Chem. 2010;92:301. doi: 10.1080/02772240902846660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hori M, Kondo H, Ariyoshi N, Yamada H, Hiratsuka A, Watabe T, Oguri K. Changes in the hepatic glutathione peroxidase redox system produced by coplanar polychlorinated biphenyls in Ah-responsive and -less-responsive strains of mice: mechanism and implications for toxicity. Environ. Toxicol. Pharmacol. 1997;3:267–275. doi: 10.1016/s1382-6689(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 29.Twaroski TP, O'Brien ML, Larmonier N, Glauert HP, Robertson LW. Polychlorinated biphenyl-induced effects on metabolic enzymes, AP-1 binding, vitamin E, and oxidative stress in the rat liver. Toxicol. Appl. Pharmacol. 2001;171:85–93. doi: 10.1006/taap.2000.9114. [DOI] [PubMed] [Google Scholar]
- 30.Twaroski TP, O'Brien ML, Robertson LW. Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes, and selenium status: implications for oxidative stress. Biochem. Pharmacol. 2001;62:273–281. doi: 10.1016/s0006-2952(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 31.Sipka S, Eum SY, Son KW, Xu S, Gavalas VG, Hennig B, Toborek M. Oral administration of PCBs induces proinflammatory and prometastatic responses. Environ. Toxicol. Pharmacol. 2008;25:251–259. doi: 10.1016/j.etap.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkataraman P, Krishnamoorthy G, Vengatesh G, Srinivasan N, Aruldhas MM, Arunakaran J. Protective role of melatonin on PCB (Aroclor 1,254) induced oxidative stress and changes in acetylcholine esterase and membrane bound ATPases in cerebellum, cerebral cortex and hippocampus of adult rat brain. Int. J. Dev. Neurosci. 2008;26:585–591. doi: 10.1016/j.ijdevneu.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Venkataraman P, Muthuvel R, Krishnamoorthy G, Arunkumar A, Sridhar M, Srinivasan N, Balasubramanian K, Aruldhas MM, Arunakaran J. PCB (Aroclor 1254) enhances oxidative damage in rat brain regions: protective role of ascorbic acid. NeuroToxicology. 2007;28:490–498. doi: 10.1016/j.neuro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Sridevi N, Venkataraman P, Senthilkumar K, Krishnamoorthy G, Arunakaran J. Oxidative stress modulates membrane bound ATPases in brain regions of PCB (Aroclor 1254) exposed rats: protective role of alpha-tocopherol. Biomed. Pharmacother. 2007;61:435–440. doi: 10.1016/j.biopha.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Selvakumar K, Bavithra S, Suganthi M, Benson CS, Elumalai P, Arunkumar R, Krishnamoorthy G, Venkataraman P, Arunakaran J. Protective role of quercetin on PCBs-induced oxidative stress and apoptosis in hippocampus of adult rats. Neurochem. Res. 2012;37:708–721. doi: 10.1007/s11064-011-0661-5. [DOI] [PubMed] [Google Scholar]
- 36.Banudevi S, Krishnamoorthy G, Venkataraman P, Vignesh C, Aruldhas MM, Arunakaran J. Role of alpha-tocopherol on antioxidant status in liver, lung and kidney of PCB exposed male albino rats. Food Chem. Toxicol. 2006;44:2040–2046. doi: 10.1016/j.fct.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Lee DW, Notter SA, Thiruchelvam M, Dever DP, Fitzpatrick R, Kostyniak PJ, Cory-Slechta DA, Opanashuk LA. Subchronic polychlorinated biphenyl (Aroclor 1254) exposure produces oxidative damage and neuronal death of ventral midbrain dopaminergic systems. Toxicol. Sci. 2012;125:496–508. doi: 10.1093/toxsci/kfr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fadhel Z, Lu Z, Robertson LW, Glauert HP. Effect of 3,3′,4,4′-tetrachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobi phenyl on the induction of hepatic lipid peroxidation and cytochrome P-450 associated enzyme activities in rats. Toxicology. 2002;175:15–25. doi: 10.1016/s0300-483x(02)00086-0. [DOI] [PubMed] [Google Scholar]
- 39.Jeong YC, Walker NJ, Burgin DE, Kissling G, Gupta M, Kupper L, Birnbaum LS, Swenberg JA. Accumulation of M1dG DNA adducts after chronic exposure to PCBs, but not from acute exposure to polychlorinated aromatic hydrocarbons. Free Radical Biol. Med. 2008;45:585–591. doi: 10.1016/j.freeradbiomed.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffman DJ, Melancon MJ, Klein PN, Rice CP, Eisemann JD, Hines RK, Spann JW, Pendleton GW. Developmental toxicity of PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl) in nestling American kestrels (Falco sparverius). Toxicol. Sci. 1996;34:188–200. doi: 10.1006/faat.1996.0189. [DOI] [PubMed] [Google Scholar]
- 41.Jin X, Kennedy SW, Di Muccio T, Moon TW. Role of oxidative stress and antioxidant defense in 3,3′,4,4′,5-pentachlorobiphenyl-induced toxicity and species-differential sensitivity in chicken and duck embryos. Toxicol. Appl. Pharmacol. 2001;172:241–248. doi: 10.1006/taap.2001.9150. [DOI] [PubMed] [Google Scholar]
- 42.Arzuaga X, Wassenberg D, Di Giulio R, Elskus A. The chlorinated AHR ligand 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) promotes reactive oxygen species (ROS) production during embryonic development in the killifish (Fundulus heteroclitus). Aquat. Toxicol. 2006;76:13–23. doi: 10.1016/j.aquatox.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Livingstone DR, Mitchelmore CL, O'Hara SC, Lemaire P, Sturve J, Forlin L. Increased potential for NAD(P)H-dependent reactive oxygen species production of hepatic subcellular fractions of fish species with in vivo exposure to contaminants. Mar. Environ. Res. 2000;50:57–60. doi: 10.1016/s0141-1136(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 44.Gunawickrama SH, Aarsaether N, Orbea A, Cajaraville MP, Goksoyr A. PCB77 (3,3′,4,4′-tetrachlorobiphenyl) co-exposure prolongs CYP1A induction, and sustains oxidative stress in B(a)P-exposed turbot, Scophthalmus maximus, in a long-term study. Aquat. Toxicol. 2008;89:65–74. doi: 10.1016/j.aquatox.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Schlezinger JJ, Stegeman JJ. Induction and suppression of cytochrome P450 1A by 3,3′,4,4′,5-pentachlorobiphenyl and its relationship to oxidative stress in the marine fish scup (Stenotomus chrysops). Aquat. Toxicol. 2001;52:101–115. doi: 10.1016/s0166-445x(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 46.Machala M, Blaha L, Vondracek J, Trosko JE, Scott J, Upham BL. Inhibition of gap junctional intercellular communication by noncoplanar polychlorinated biphenyls: inhibitory potencies and screening for potential mode(s) of action. Toxicol. Sci. 2003;76:102–111. doi: 10.1093/toxsci/kfg209. [DOI] [PubMed] [Google Scholar]
- 47.Jensen KG, Wiberg K, Klasson-Wehler E, Onfelt A. Induction of aberrant mitosis with PCBs: particular efficiency of 2, 3,3′,4,4′-pentachlorobiphenyl and synergism with triphenyltin. Mutagenesis. 2000;15:9–15. doi: 10.1093/mutage/15.1.9. [DOI] [PubMed] [Google Scholar]
- 48.Yang D, Lein PJ. Polychlorinated biphenyls increase apoptosis in the developing rat brain. Curr. Neurobiol. 2010;1:70–76. [PMC free article] [PubMed] [Google Scholar]
- 49.Vondracek J, Machala M, Bryja V, Chramostova K, Krcmar P, Dietrich C, Hampl A, Kozubik A. Aryl hydrocarbon receptor-activating polychlorinated biphenyls and their hydroxylated metabolites induce cell proliferation in contact-inhibited rat liver epithelial cells. Toxicol. Sci. 2005;83:53–63. doi: 10.1093/toxsci/kfi009. [DOI] [PubMed] [Google Scholar]
- 50.Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem. Res. Toxicol. 2009;22:759–778. doi: 10.1021/tx9000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moriya M, Zhang W, Johnson F, Grollman AP. Mutagenic potency of exocyclic DNA adducts: marked differences between Escherichia coli and simian kidney cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11899–11903. doi: 10.1073/pnas.91.25.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olinski R, Gackowski D, Rozalski R, Foksinski M, Bialkowski K. Oxidative DNA damage in cancer patients: a cause or a consequence of the disease development? Mutat. Res., Fundam. Mol. Mech. Mutagen. 2003;531:177–190. doi: 10.1016/j.mrfmmm.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- 54.Akasaka S, Guengerich FP. Mutagenicity of site-specifically located 1,N2-ethenoguanine in Chinese hamster ovary cell chromosomal DNA. Chem. Res. Toxicol. 1999;12:501–507. doi: 10.1021/tx980259j. [DOI] [PubMed] [Google Scholar]
- 55.Klein JC, Bleeker MJ, Saris CP, Roelen HC, Brugghe HF, van den Elst H, van der Marel GA, van Boom JH, Westra JG, Kriek E, Berns AJM. Repair and replication of plasmids with site-specific 8-oxodG and 8-AAFdG residues in normal and repair-deficient human cells. Nucleic Acids Res. 1992;20:4437–4443. doi: 10.1093/nar/20.17.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cajelli E, Ferraris A, Brambilla G. Mutagenicity of 4-hydroxynonenal in V79 Chinese hamster cells. Mutat. Res. Lett. 1987;190:169–171. doi: 10.1016/0165-7992(87)90050-9. [DOI] [PubMed] [Google Scholar]
- 57.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 58.Pang B, Zhou X, Yu H, Dong M, Taghizadeh K, Wishnok JS, Tannenbaum SR, Dedon PC. Lipid peroxidation dominates the chemistry of DNA adduct formation in a mouse model of inflammation. Carcinogenesis. 2007;28:1807–1813. doi: 10.1093/carcin/bgm037. [DOI] [PubMed] [Google Scholar]
- 59.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 60.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radical Biol. Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Munnia A, Bonassi S, Verna A, Quaglia R, Pelucco D, Ceppi M, Neri M, Buratti M, Taioli E, Garte S, Peluso M. Bronchial malondialdehyde DNA adducts, tobacco smoking, and lung cancer. Free Radical Biol. Med. 2006;41:1499–1505. doi: 10.1016/j.freeradbiomed.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Ham AJ, Engelward BP, Koc H, Sangaiah R, Meira LB, Samson LD, Swenberg JA. New immunoaffinity-LCMS/MS methodology reveals that Aag null mice are deficient in their ability to clear 1,N6-etheno-deoxyadenosine DNA lesions from lung and liver in vivo. DNA Repair. 2004;3:257–265. doi: 10.1016/j.dnarep.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Kusmierek JT, Singer B. 1,N2-ethenodeoxyguanosine: properties and formation in chloroacetaldehyde-treated polynucleotides and DNA. Chem. Res. Toxicol. 1992;5:634–638. doi: 10.1021/tx00029a007. [DOI] [PubMed] [Google Scholar]
- 64.Jeong YC, Sangaiah R, Nakamura J, Pachkowski BF, Ranasinghe A, Gold A, Ball LM, Swenberg JA. Analysis of M1G-dR in DNA by aldehyde reactive probe labeling and liquid chromatography tandem mass spectrometry. Chem. Res. Toxicol. 2005;18:51–60. doi: 10.1021/tx049853l. [DOI] [PubMed] [Google Scholar]
- 65.Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, Hecht SS. Identification of paraldol-deoxyguanosine adducts in DNA reacted with crotonaldehyde. Chem. Res. Toxicol. 2000;13:1065–1074. doi: 10.1021/tx000095i. [DOI] [PubMed] [Google Scholar]
- 66.Chung FL, Young R, Hecht SS. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 67.Douki T, Odin F, Caillat S, Favier A, Cadet J. Predominance of the 1,N2-propano 2′-deoxyguanosine adduct among 4-hydroxy-2-nonenal-induced DNA lesions. Free Radical Biol. Med. 2004;37:62–70. doi: 10.1016/j.freeradbiomed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 68.NTP . NTP technical report on the toxicology and carcinogenesis studies of 2,2′,4,4′,5,5′,-Hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in female Harlan Sprague-Dawley rats (Gavage Studies) US Department of Health and Human Services; Washington, DC.: 2006. pp. 4–168. [PubMed] [Google Scholar]
- 69.NTP . NTP technical report on the toxicology and carcinogenesis studies of a binary mixture of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) (Cas No. 57465-28-8) and 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in female Harlan Sprague-Dawley rats (gavage studies) US Department of Health and Human Services; Washington, DC.: 2006. pp. 1–258. [Google Scholar]
- 70.Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CN, Barnard SD, Hummel RA, Humiston CG. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol. Appl. Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- 71.Van den Berg M, Birnbaum L, Bosveld AT, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FX, Liem AK, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mutlu E, Collins LB, Stout MD, Upton PB, Daye LR, Winsett D, Hatch G, Evansky P, Swenberg JA. Development and application of an LC-MS/MS method for the detection of the vinyl chloride-induced DNA adduct N(2),3-ethenoguanine in tissues of adult and weanling rats following exposure to [(13)C(2)]-VC. Chem. Res. Toxicol. 2010;23:1485–1491. doi: 10.1021/tx1001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamartiniere CA, Dieringer CS, Lucier GW. Altered ontogeny of glutathione S-transferases by 2,4,5–2′,4′,5′-hexachlorobiphenyl. Toxicol. Appl. Pharmacol. 1979;51:233–238. doi: 10.1016/0041-008x(79)90465-4. [DOI] [PubMed] [Google Scholar]
- 74.Chu I, Villeneuve DC, Yagminas A, Lecavalier P, Poon R, Hakansson H, Ahlborg UG, Valli VE, Kennedy SW, Bergman A, Seegal RF, Feeley M. Toxicity of 2,4,4′-trichlorobiphenyl in rats following 90-day dietary exposure. J. Toxicol. Environ. Health. 1996;49:301–318. doi: 10.1080/00984108.1996.11667603. [DOI] [PubMed] [Google Scholar]
- 75.van Birgelen AP, Fase KM, van der Kolk J, Poiger H, Brouwer A, Seinen W, van den Berg M. Synergistic effect of 2,2′,4,4′,5,5′-hexachlorobiphenyl and 2,3,7,8-tetrachlorodibenzo-p-dioxin on hepatic porphyrin levels in the rat. Environ. Health Perspect. 1996;104:550–557. doi: 10.1289/ehp.96104550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aqil F, Shen H, Jeyabalan J, Xin X, Lehmler HJ, Ludewig G, Robertson LW, Gupta RC. Sustained expression of CYPs and DNA adduct accumulation with continuous exposure to PCB126 and PCB153 through a new delivery method: Polymeric implants. Toxicol. Rep. 2014;1:820–833. doi: 10.1016/j.toxrep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wacker M, Wanek P, Eder E. Detection of 1,N2-propanodeoxyguanosine adducts of trans-4-hydroxy-2-nonenal after gavage of trans-4-hydroxy-2-nonenal or induction of lipid peroxidation with carbon tetrachloride in F344 rats. Chem.-Biol. Interact. 2001;137:269–283. doi: 10.1016/s0009-2797(01)00259-9. [DOI] [PubMed] [Google Scholar]
- 78.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radical Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 79.Singh R, Teichert F, Verschoyle RD, Kaur B, Vives M, Sharma RA, Steward WP, Gescher AJ, Farmer PB. Simultaneous determination of 8-oxo-2′-deoxyguanosine and 8-oxo-2′-deoxyadenosine in DNA using online column-switching liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009;23:151–160. doi: 10.1002/rcm.3866. [DOI] [PubMed] [Google Scholar]
- 80.Shen J, Wanibuchi H, Salim EI, Wei M, Kinoshita A, Yoshida K, Endo G, Fukushima S. Liver tumorigenicity of trimethylarsine oxide in male Fischer 344 rats–association with oxidative DNA damage and enhanced cell proliferation. Carcinogenesis. 2003;24:1827–1835. doi: 10.1093/carcin/bgg143. [DOI] [PubMed] [Google Scholar]
- 81.Kolachana P, Subrahmanyam VV, Meyer KB, Zhang L, Smith MT. Benzene and its phenolic metabolites produce oxidative DNA damage in HL60 cells in vitro and in the bone marrow in vivo. Cancer Res. 1993;53:1023–1026. [PubMed] [Google Scholar]
- 82.Wyde ME, Wong VA, Kim AH, Lucier GW, Walker NJ. Induction of hepatic 8-oxo-deoxyguanosine adducts by 2,3,7,8-tetrachlorodibenzo-p-dioxin in Sprague-Dawley rats is female-specific and estrogen-dependent. Chem. Res. Toxicol. 2001;14:849–855. doi: 10.1021/tx000266j. [DOI] [PubMed] [Google Scholar]
- 83.Wyde ME, Eldridge SR, Lucier GW, Walker NJ. Regulation of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced tumor promotion by 17 beta-estradiol in female Sprague–Dawley rats. Toxicol. Appl. Pharmacol. 2001;173:7–17. doi: 10.1006/taap.2001.9166. [DOI] [PubMed] [Google Scholar]
- 84.Lehmann L, Esch HL, Kirby PA, Robertson LW, Ludewig G. 4-monochlorobiphenyl (PCB3) induces mutations in the livers of transgenic Fisher 344 rats. Carcinogenesis. 2007;28:471–478. doi: 10.1093/carcin/bgl157. [DOI] [PubMed] [Google Scholar]
- 85.Nair J, Sinitsina O, Vasunina EA, Nevinsky GA, Laval J, Bartsch H. Age-dependent increase of etheno-DNA-adducts in liver and brain of ROS overproducing OXYS rats. Biochem. Biophys. Res. Commun. 2005;336:478–482. doi: 10.1016/j.bbrc.2005.08.114. [DOI] [PubMed] [Google Scholar]
- 86.Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN. Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barnett YA, King CM. An investigation of antioxidant status, DNA repair capacity and mutation as a function of age in humans. Mutat. Res., DNAging: Genet. Instab. Aging. 1995;338:115–128. doi: 10.1016/0921-8734(95)00017-z. [DOI] [PubMed] [Google Scholar]
- 88.Intano GW, Cho EJ, McMahan CA, Walter CA. Age-related base excision repair activity in mouse brain and liver nuclear extracts. J. Gerontol. Ser. A. 2003;58:205–211. doi: 10.1093/gerona/58.3.b205. [DOI] [PubMed] [Google Scholar]
- 89.Chung FL, Zhang L, Ocando JE, Nath RG. Role of 1,N2-propanodeoxyguanosine adducts as endogenous DNA lesions in rodents and humans. IARC Sci. Publ. 1999:45–54. [PubMed] [Google Scholar]