Abstract
Objective
The objective of this case–control study was to quantify the immune responsiveness in individuals with type 2 diabetes (T2D) as compared with patients without diabetes (NT2D) diagnosed with periodontitis.
Research Design and Methods
Peripheral blood was collected from 20 patients with moderate-to-severe chronic periodontitis (10 T2D, 10 NT2D). Blood samples were stimulated with ultrapure Porphyromonas gingivalis and Escherichia coli lipopolysaccharide (LPS) for 24 hours. 14 cytokines/chemokines were quantified in culture supernatants using multiplex technology.
Results
T2D individuals demonstrated higher unstimulated levels of interleukin 6 (IL-6), IL-1β, tumor necrosis factor α, interferon γ, IL-10, IL-8, macrophage inflammatory protein 1α (MIP1α), and 1β (MIP1β), and higher stimulated levels of IL-6, IL-8, IL-10, MIP1α and MIP1β, along with lower unstimulated and stimulated levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) when compared with NT2D (p<0.05). Importantly, the LPS-induced levels of IL-6, IL-8, IL-10 and MIP1α strongly correlated with severity of disease, measured by pocket depths (PD), within the T2D group (r2≥0.7, p<0.05), but not within NT2D.
Conclusions
Among patients with chronic periodontitis, patients with T2D seem to have an enhanced LPS-induced immune responsiveness than individuals without diabetes, which correlates with periodontal disease severity, concomitant with a less robust GM-CSF response. This data may in part explain the higher predisposition to periodontitis in this population.
Keywords: Chronic Diabetic Complications, Chronic Inflammation, Cytokine(s), Immunology
Key messages.
Among patients with chronic periodontitis, patients with type 2 diabetes have an enhanced lipopolysaccharide-induced immune responsiveness than individuals without diabetes.
The enhanced immune responsiveness found in patients with type 2 diabetes correlates with their periodontal disease severity.
Our results may in part explain the higher predisposition to periodontitis in patients with type 2 diabetes.
Introduction
Periodontitis is a multifactorial/polymicrobial infection of the supporting tissues around teeth, known as the periodontium. Periodontitis is considered the result of an inflammatory response to the bacterial biofilm around teeth in a susceptible host. Periodontal tissue destruction is mediated by locally produced proinflammatory cytokines in response to the bacterial flora and its products. Lipopolysaccharides (LPS), an outer membrane of Gram-negative bacteria, often found in gingivitis and periodontitis lesions, are recognized by host receptors such as toll-like receptors (TLRs). TLR2 and TLR4 can recognize a variety of bacterial components and their interactions with LPS initiate an inflammatory response, which when unbalanced or excessive, may result in periodontal tissue destruction.1
There is clear evidence that the LPS of Gram-negative bacteria in periodontitis such as Porphyromonas gingivalis (Pg), Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Fusobacterium nucleatum and Tannerella forsythensis stimulate, via TLR2 and TLR4, the production of proinflammatory cytokines from the host, such as interleukin 1β (IL-1β) and tumor necrosis factor (TNF)α, as well as the production of matrix metalloproteases which induces alveolar bone resorption,2 resulting in progressing periodontal tissue destruction. It is possible that the production of local cytokines/chemokines and/or low-level asymptomatic bacteremia affects the plasma concentrations of these mediators,3 perpetuating a local as well as a systemic inflammatory state.
Research has shown that periodontal disease is closely associated with diabetes mellitus.4–10 For instance, individuals with type 2 diabetes (T2D) have been shown to be 2.81 times more likely to have clinical attachment loss and 3.43 times more likely to have radiographic bone loss than normoglycemic controls.4 8 In addition, glycemic control also has an impact on periodontal disease severity, whereby poorly controlled T2D individuals (presenting glycated hemoglobin (HbA1c) values of 9% or greater, as defined by authors) present increased progression of bone loss compared with controlled T2D (HbA1c<9%; OR 11.4 vs 2.2).11 Conversely, severe periodontitis may also impact the control of the diabetic state. Studies have shown that individuals with T2D and severe periodontitis experience greater incidence of diabetic complications, such as worsening of glycemic control (HbA1c ≥9% at a 2-year follow-up), nephropathy and ischemic heart disease, than individuals with diabetes with slight or no periodontitis.12–15 In fact, this strength of evidence on the two-way relationship between diabetes and periodontal disease have led some to suggest that periodontal disease should be listed among the ‘classic’ complications of diabetes,16 although the mechanisms in which this relationship occurs is still not completely understood. It is known, however, that individuals with T2D often have a shift in monocyte/macrophage function, which results in the overproduction of proinflammatory cytokines in response to periodontal pathogens.17 This overproduction of cytokines could then exacerbate the pathogenesis of periodontal disease destruction. Similarly, insulin resistance associated with T2D is strongly linked to the actions of IL-6 and TNFα.18 These data suggest several possible mechanisms to support the findings that severe periodontitis and its inflammatory component could increase the risk of poor glycemic control in individuals with T2D, as previously reported.12 Specifically, it seems that individuals with T2D could be more susceptible to periodontal diseases due to both a hyperinflammatory component leading to a predisposition for tissue destruction as well as an impaired immune response, which could delay/impair host's natural healing/regenerative capabilities during the disease course. On the other hand, periodontitis may aggravate the diabetes-associated host inflammatory component both locally and systemically, leading to worsening of diabetes status/control.
Thus, the aim of this study was to compare the LPS-induced immune responsiveness of individuals with T2D and periodontal disease, as compared with diabetes-free individuals who also have periodontitis. We hypothesized that the condition of T2D will exacerbate the LPS-induced immune responsiveness in patients with chronic periodontitis.
Materials and methods
Patient selection
Two populations diagnosed with moderate-to-severe chronic periodontitis were recruited for this case–control study: individuals with T2D and without diabetes (NT2D). Patients were recruited from the Endocrinology clinic, at the Shands Medical Plaza and from the Graduate Periodontology clinic at the University of Florida College of Dentistry, from August 2007 to November 2009. All subjects signed an informed consent in order to participate in the study according to the UF institutional Review Board approval (protocol #70-2007). Inclusion criteria for all individuals were as follows: Subjects aged 45–75 years; presence of at least 20 teeth; diagnosis of moderate-to-severe chronic periodontitis, as defined by the presence of at least four sites with probing depth of 5 mm or more and attachment loss of 3 mm or more with bleeding on probing. Inclusion criteria for the individuals with T2D: diagnosis of T2D (HbA1c levels of ≥6.5%); currently under standard treatment and physicians care for diabetes control; have not altered their diabetes medication in the past 3 months. Subjects without diabetes had seen their primary care physician within a year prior to study inclusion and were in good general health. Exclusion criteria: diagnosed with any forms of aggressive or necrotizing periodontal disease; presence of any other systemic diseases or conditions that could influence the course of periodontal disease and/or glucose control (such as kidney/liver failure, immunosuppressive diseases); under any medications that could influence the characteristics of either diabetes and/or periodontal disease (such as immunosuppressive drugs; antibiotics, long-term use of steroids); pregnant or lactating women.
Periodontal examination
All patients received a full mouth periodontal evaluation for the diagnosis of periodontal disease. Full mouth periodontal parameters evaluation and diagnosis was performed and determined by two calibrated examiners (LMS and RM). Intracalibration and intercalibration was performed on a separate set of periodontitis subjects. Calibration was attained when at least 80% of duplicate periodontal measurements of pocket depth and clinical attachment levels were within 1 mm. Subject diagnosis was given according to the 1999 Classification of Periodontal Disease established by the American Academy of Periodontology.19
LPS stimulation and detection of cytokines/chemokines
Approximately 4 mL of peripheral blood was collected by venipuncture and processed as described previously.20 Briefly, blood was diluted 1:4 in RPMI1640 (Invitrogen, Carlsbad, California, USA) then stimulated or left unstimulated with 1 µg/mL of ultrapure Escherichia coli (Ec) or Pg LPS (InvivoGen, San Diego, California, USA). After 24 hours, 14 cytokines/chemokines; eotaxin, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon γ (INFy), IL-1β, IL-6, IL-8, IL-10, IL-2, IL-12p40, IL-12p70, IP10, macrophage inflammatory protein 1α (MIP1α), granulocyte colony-stimulating factor (GMCSF) and tumor necrosis factor alpha TNFα, were detected and quantified in the supernatant using multiplex fluorescence detection kits (Millipore, St. Charles, Missouri, USA). Culture supernatants and cytokine capture-bead cocktails were incubated for 2 hours. The samples were then incubated for 1.5 hours with biotin-labeled anticytokine and for 30 min in a 1:12.5 dilution of streptavidin-phycoerythrin. Data were obtained by Luminex 200 and analyzed with Milliplex Analyst software (Millipore) with five parameter logistics and standard curves. Unstimulated data were subtracted from the stimulated values.
Statistical analysis
Analysis of cytokines/chemokine means from T2D and non-T2D groups were compared by non-paired t-tests, with a significance level of α=0.05. Mann-Whitney tests were used data was not normally distributed. Spearman correlation was run among stimulated/non-stimulated levels of systemic markers and clinical parameters of periodontal disease. According to our preliminary data on cytokine levels before and after LPS stimulation, a sample size of 10 per group would give us at least 80% power to detect a minimum 20% difference in the levels of cytokines between the groups, with a 15% SD. p Values were not adjusted at this initial evaluation as the independent versus grouped role of cytokines/chemokines here has not been defined in this disease/population.21
Results
The demographics and clinical results of these two populations are as follows (table 1): 10 subjects per group participated in the study whereby the age range of those with T2D and those without was not statistically different (51–69, mean 61.3±5.4; and 45–64, mean 53.3±6.4, respectively, p>0.05). Similarly, the ratios of female/male gender between the groups were also similar 4/6 (T2D) 5/5 (T2D-free). There was only one smoker among T2D group and two in the NT2D group (p>0.05). The mean HbA1c values for subjects with T2D was 8.13±1.23 and all subjects were diagnosed with moderate-to-severe chronic periodontitis (mean pocket depth of 5.22±0.52 and 4.5±0.23 mm and mean clinical attachment levels of 5.64±0.93 and 4.5±1.2 mm for NT2D and T2D, respectively, p>0.05). Patients with T2D had higher body mass index (BMI) than NT2D (p=0.0178).
Table 1.
Demographics of the population
| Age | F/M | PD (mm) | CAL (mm) | HbA1c | BMI | Smoking | |
|---|---|---|---|---|---|---|---|
| T2D (10) | 61.3±5.4 | 4/6 | 4.5±0.23 | 4.5±1.2 | 8.13±1.23 | 36.4±10.95* | 1 |
| NT2D (10) | 53.3±6.4 | 5/5 | 5.22±0.52 | 5.64±0.93 | n/a | 25.7±3.52 | 2 |
*p<0.05 by t-test between groups. BMI, body mass index; CAL, clinical attachment level; F, females; HbA1c, glycated hemoglobin; M, males; NT2D, patients without type 2 diabetes; PD, pocket depth; T2D, patients with type 2 diabetes.
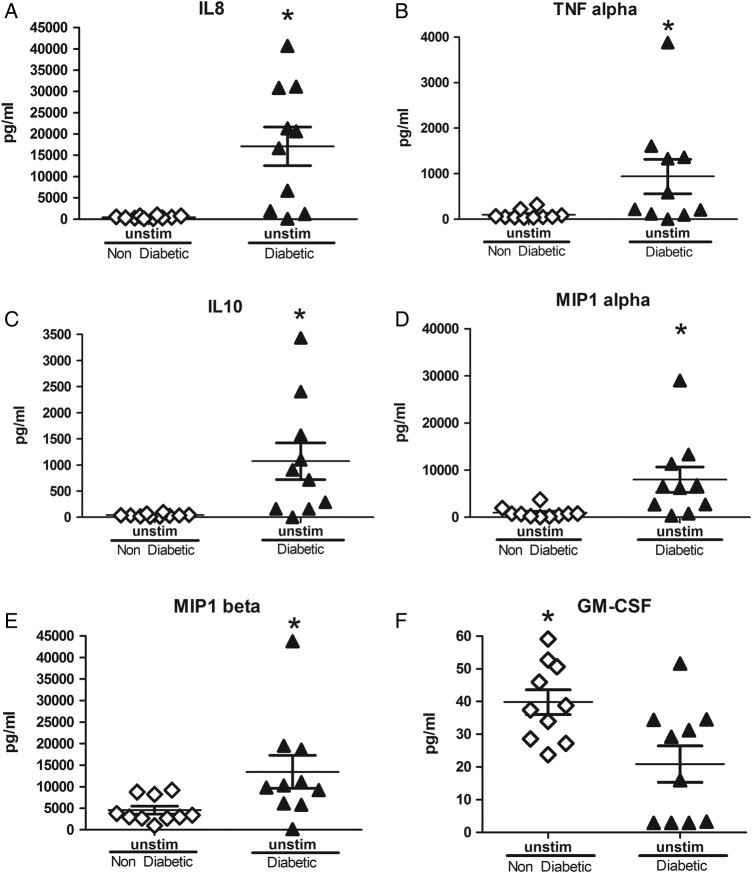
In the absence of LPS stimulation, peripheral blood cultures of individuals with T2D and chronic periodontitis presented with higher levels of IL-6, IL-1β, TNFα, INFy, IL-10, IL-8, MIP1α, and MIP1β compared with those from individuals without T2D but who still had chronic periodontitis (p<0.05, figure 1A–J, p<0.05). Interestingly, these same cultures from individuals without T2D presented with higher levels of GM-CSF compared with those from individuals with T2D (figure 1J, p<0.05).
Figure 1.
Unstimulated levels of soluble mediators from in cultures of individuals with and without T2D with periodontitis. Cultures from individuals with T2D presented with higher levels IL-6, IL-1β, TNFα, INFy, IL-10, IL-8, MIP1α, and MIP1β, while those from NT2D individuals presented with higher values of GM-CSF. *p<0.05, **p<0.01 between T2D and NT2D groups by Mann-Whitney test. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; MIP1, macrophage inflammatory protein 1; NT2D, non-type 2 diabetes; T2D, type 2 diabetes; TNF, tumor necrosis factor.
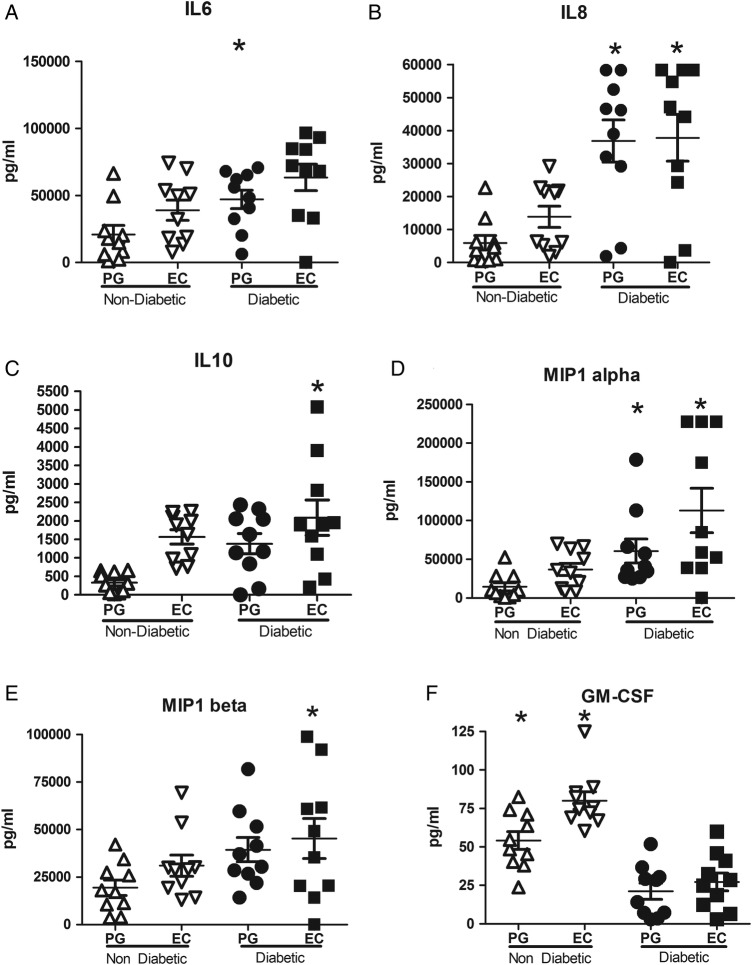
Following LPS stimulation with either Ec or Pg LPS, cultures from T2D individuals also presented with higher levels of IL-6, IL-8, IL-10, MIP1α and MIP1β (p<0.05, figure 2A–E) concomitant with lower levels of GM-CSF (figure 2F, p<0.05) when compared with NT2D individuals. All other markers evaluated did not demonstrate statistically significant differences between the two cohorts.
Figure 2.
Pg and Ec LPS induced levels of soluble mediators from in cultures of individuals with and without T2D with periodontitis. LPS stimulated cultures from individuals with T2D presented with higher levels of IL-6, IL-8, IL-10, MIP1α, and MIP1β, while those from NT2D individuals showed higher values of GM-CSF. *p<0.05, **p<0.01, ***p<0.001, between T2D and NT2D groups by Mann-Whitney test. Ec LPS, Escherichia coli lipopolysaccharide; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; MIP1, macrophage inflammatory protein 1; NT2D, non-type 2 diabetes; T2D, type 2 diabetes.
Importantly, periodontal pocket depth, a measurement of periodontal disease severity, correlated with Ec LPS-stimulated levels of IL-6, IL-8, IL-10 and MIP1α, as well as Pg LPS-stimulated levels of IL-8 only within the T2D participant cohort (table 2). No other significant correlations were found.
Table 2.
Correlation values (R2) for the different cytokines/chemokines with pocket depth within individuals with and without T2D with chronic periodontitis
| IL-6 | IL-8 | IL-10 | MIP1a | MIP1b | |
|---|---|---|---|---|---|
| T2D | |||||
| Ec LPS | 0.693* | 0.751* | 0.772* | 0.732* | 0.468 |
| Pg LPS | 0.48 | 0.707* | 0.413 | −0.024 | −0.073 |
| NT2D | |||||
| Ec LPS | 0.188 | 0.103 | 0.273 | 0.382 | 0.05 |
| Pg LPS | 0.442 | 0.552 | 0.127 | 0.479 | 0.345 |
*p<0.05 by Spearman correlation.
Ec LPS, Escherichia coli lipopolysaccharide; NT2D, patients without type 2 diabetes; Pg LPS, Porphyromonas gingivalis lipopolysaccharide; T2D, patients with type 2 diabetes.
Discussion
The influence of T2D on periodontal disease is well accepted, whereby there is also substantial evidence indicating that diabetes is a risk factor for periodontal disease.4 9 Previous investigations have demonstrated that chronic periodontitis severity is significantly associated with elevated plasma levels of many proinflammatory cytokines in individuals with T2D.18 22–24 For instance, a recent study evaluated plasma levels of TNFα in subjects with T2D and chronic periodontitis and found that TNFα had a significant positive association with periodontitis, raising the hypothesis that high circulating levels of TNFα in these individuals could influence both diabetes and periodontitis severity.24 Conversely, another study did not find a correlation between the severity of periodontal disease and serum TNFα levels in T2D individuals.25 However, these authors did find a positive correlation between C reactive protein (CRP) levels and periodontal parameters in these patients, which could also indicate a more proinflammatory state.25 Indeed, periodontal disease, even in the absence of T2D, has been associated with higher levels of IL-6 and CRP when compared with periodontally healthy individuals.3 26 While the current study did not directly evaluate the plasma or serum concentration of soluble mediators, it did demonstrate higher levels of proinflammatory markers, such as IL-1β, IL-6 and TNFα, in unstimulated cultures from T2D subjects with periodontal disease, compared with NT2D individuals with periodontal disease. This exaggerated expression may be reflective of a higher inflammatory status observed in T2D, and also reflective of a higher BMI encountered in these patients, and thus, may exacerbate the inflammatory state within the periodontium of T2D individuals in this population.
The current study also demonstrates among patients diagnosed with chronic periodontitis, those with T2D have an exacerbated inflammatory response to bacterial LPS. Other studies have demonstrated a correlation between high levels of proinflammatory cytokines with active progressive periodontal lesions in diabetes-free individuals.3 27 Thus, we propose that the increased LPS-induced inflammatory response reported here could at least in part contribute to the higher susceptibility of individuals with T2D to periodontal disease. Similar to our findings, Salvi et al23 also found a significantly higher monocytic expression of TNFα (4.6-fold) in T2D individuals when compared with diabetes-free controls. Note stimulations levels here accounts for LPS stimulation alone as unstimulated levels were subtracted from the stimulated ones.
According to the present findings, IL-6 levels, a major mediator of the host response to tissue injury, infection and bone resorption, were expressed at higher levels in cultures from T2D individuals with or without LPS stimulation. Loos et al3 reported that IL-6 can be detected in plasma of >50% of patients with severe periodontitis. In addition, several studies demonstrate elevated levels of serum IL-6 in individuals with T2D.22 28 To this end, Ross et al29 found that expression of IL-6 within the periodontal tissues increases when disease status changes from ‘no disease’ to ‘one disease’ (periodontal disease) to ‘two diseases’ (periodontitis + diabetes). In the present study, IL-8 was also found to be elevated in cultures from T2D individuals in the absence and presence of LPS stimulation. IL-8 is known to be involved in the recruitment of PMNs and is highly expressed in the junctional epithelium adjacent to infected periodontal defects, where PMNs infiltrate. Similarly, MIP1α, which was also found in the current study to be elevated in cultures from T2D individuals, has been correlated to high levels of IL-8 in early mucositis lesions.30 Therefore, the exacerbated induction of these cytokines in patients with T2D may contribute to susceptibility of this patient population to the initial stages of periodontal breakdown.27 Interestingly, cultures (with and without LPS stimulation) from T2D individuals also presented with higher levels of IL-10 concomitant with lower levels of GM-CSF when compared with those of diabetes-free individuals. IL-10 is a pleiotropic cytokine which can be involved in the inhibition of cytokine synthesis due to its inhibitory effect on macrophage-monocytes31 as well as the differentiation and chemotaxis of cytotoxic T cells. GM-CSF is mainly responsible for the differentiation of innate immune cells such as monocytes and dendritic cells. Thus, high levels of IL-10 and low levels of GM-CSF along with a substantial proinflammatory milieu could indicate an environment in which clearance of pathogen by innate responses is inhibited leading to a sustained inflammatory stimulus resulting in host-mediated periodontal breakdown. Together these data suggest that T2D has the potential to aggravate local tissue inflammation observed in periodontal disease. Further studies need to be conducted to elucidate the specifics of these mechanisms.
One limitation of the present study is the absence of a periodontally healthy control group. The inclusion of a healthy control group would enable us to evaluate the role of periodontal disease alone in LPS-induced inflammatory responses. However, other studies have already shown a heightened inflammatory state in patients with periodontitis when compared with a periodontally healthy cohort.3 20 Thus, the objective of the present study was to evaluate the additive role of T2D on the inflammatory response observed in periodontal disease. Finally, it is clear that a larger study population and a longitudinal evaluation (postperiodontal treatment) would be crucial to draw further conclusions regarding the inflammatory response in individuals with T2D and different severities of periodontitis, response to treatment, and diabetes control.
In summary, the present study describes a significantly more robust LPS-induced inflammatory response in T2D patients with periodontitis, which could explain the observed increased susceptibility to periodontal disease in T2D patients. In support of this, the present study observed a positive and strong correlation between LPS-stimulated levels of multiple inflammatory markers, including IL-6, IL-8 and MIP1α, with pocket depth. Importantly, this correlation was only observed within the individuals with T2D, but not diabetes-free individuals. These data strengthen the evidence that an exaggerated response to LPS by individuals with T2D may contribute to the exaggerated breakdown and/or progression of periodontal disease in these patients (T2D).
Acknowledgments
The authors would like to thank the department of Periodontology and the University of Florida Clinical Research Center and all its staff for the outstanding support that enabled the completion of this study.
Footnotes
Contributors: All authors of this paper have contributed significantly to the paper as stated below: RM was involved in research conduct, data acquisition and analysis, paper write-up. FG was involved in paper write-up. HH was involved in methods-assays, data analysis. MC-S was involved in research methods oversight/expertise, patient referrals, paper review. IA was involved in research methods oversight/expertise, paper review. SMW was involved in research methods and data analysis oversight/expertise, paper review. LMS was involved in research conduct and oversight, data acquisition and analysis, final paper write-up.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: University of Florida Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Authors are open to share any of the original data that resulted from this study.
References
- 1.Yoshioka H, Yoshimura A, Kaneko T et al. . Analysis of the activity to induce toll-like receptor (TLR)2- and TLR4-mediated stimulation of supragingival plaque. J Periodontol 2008;79:920–8. 10.1902/jop.2008.070516 [DOI] [PubMed] [Google Scholar]
- 2.Kikkert R, Laine ML, Aarden LA et al. . Activation of toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiol Immunol 2007;22:145–51. 10.1111/j.1399-302X.2007.00335.x [DOI] [PubMed] [Google Scholar]
- 3.Loos BG, Craandijk J, Hoek FJ et al. . Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol 2000;71:1528–34. 10.1902/jop.2000.71.10.1528 [DOI] [PubMed] [Google Scholar]
- 4.Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol 1991;62:123–31. 10.1902/jop.1991.62.2.123 [DOI] [PubMed] [Google Scholar]
- 5.Novaes AB Jr, Pereira AL, de Moraes N et al. . Manifestations of insulin-dependent diabetes mellitus in the periodontium of young Brazilian patients. J Periodontol 1991;62:116–22. 10.1902/jop.1991.62.2.116 [DOI] [PubMed] [Google Scholar]
- 6.Rylander H, Ramberg P, Blohme G et al. . Prevalence of periodontal disease in young diabetics. J Clin Periodontol 1987;14:38–43. 10.1111/j.1600-051X.1987.tb01511.x [DOI] [PubMed] [Google Scholar]
- 7.Seppälä B, Seppälä M, Ainamo J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol 1993;20:161–5. 10.1111/j.1600-051X.1993.tb00338.x [DOI] [PubMed] [Google Scholar]
- 8.Shlossman M, Knowler WC, Pettitt DJ et al. . Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc 1990;121:532–6. 10.14219/jada.archive.1990.0211 [DOI] [PubMed] [Google Scholar]
- 9.Taylor GW, Burt BA, Becker MP et al. . Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol 1998;69:76–83. 10.1902/jop.1998.69.1.76 [DOI] [PubMed] [Google Scholar]
- 10.Nishimura F, Takahashi K, Kurihara M et al. . Periodontal disease as a complication of diabetes mellitus. Ann Periodontol 1998;3:20–9. 10.1902/annals.1998.3.1.20 [DOI] [PubMed] [Google Scholar]
- 11.Taylor GW, Burt BA, Becker MP et al. . Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol 1998;3:30–9. 10.1902/annals.1998.3.1.30 [DOI] [PubMed] [Google Scholar]
- 12.Taylor GW, Burt BA, Becker MP et al. . Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol 1996;67(Suppl 10):1085–93. 10.1902/jop.1996.67.10s.1085 [DOI] [PubMed] [Google Scholar]
- 13.Saremi A, Nelson RG, Tulloch-Reid M et al. . Periodontal disease and mortality in type 2 diabetes. Diabetes Care 2005;28:27–32. 10.2337/diacare.28.1.27 [DOI] [PubMed] [Google Scholar]
- 14.Thorstensson H, Kuylenstierna J, Hugoson A. Medical status and complications in relation to periodontal disease experience in insulin-dependent diabetics. J Clin Periodontol 1996;23(3 Pt 1):194–202. 10.1111/j.1600-051X.1996.tb02076.x [DOI] [PubMed] [Google Scholar]
- 15.Demmer RT, Desvarieux M, Holtfreter B et al. . Periodontal status and A1C change: longitudinal results from the study of health in Pomerania (SHIP). Diabetes Care 2010;33:1037–43. 10.2337/dc09-1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993;16:329–34. 10.2337/diacare.16.1.329 [DOI] [PubMed] [Google Scholar]
- 17.Salvi GE, Yalda B, Collins JG et al. . Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol 1997;68:127–35. 10.1902/jop.1997.68.2.127 [DOI] [PubMed] [Google Scholar]
- 18.Crook M. Type 2 diabetes mellitus: a disease of the innate immune system? An update. Diabet Med 2004;21:203–7. 10.1046/j.1464-5491.2003.01030.x [DOI] [PubMed] [Google Scholar]
- 19.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999;4:1–6. 10.1902/annals.1999.4.1.1 [DOI] [PubMed] [Google Scholar]
- 20.Shaddox L, Wiedey J, Bimstein E et al. . Hyper-responsive phenotype in localized aggressive periodontitis. J Dent Res 2010;89:143–8. 10.1177/0022034509353397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 22.Correa FO, Gonçalves D, Figueredo CM et al. . Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol 2010;37:53–8. 10.1111/j.1600-051X.2009.01498.x [DOI] [PubMed] [Google Scholar]
- 23.Salvi GE, Beck JD, Offenbacher S. PGE2, IL-1 beta, and TNF-alpha responses in diabetics as modifiers of periodontal disease expression. Ann Periodontol 1998;3:40–50. 10.1902/annals.1998.3.1.40 [DOI] [PubMed] [Google Scholar]
- 24.Engebretson S, Chertog R, Nichols A et al. . Plasma levels of tumour necrosis factor-alpha in patients with chronic periodontitis and type 2 diabetes. J Clin Periodontol 2007;34:18–24. 10.1111/j.1600-051X.2006.01017.x [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Wei B, Li J et al. . Association of periodontal parameters with metabolic level and systemic inflammatory markers in patients with type 2 diabetes. J Periodontol 2010;81:364–71. 10.1902/jop.2009.090544 [DOI] [PubMed] [Google Scholar]
- 26.Noack B, Genco RJ, Trevisan M et al. . Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol 2001;72:1221–7. 10.1902/jop.2000.72.9.1221 [DOI] [PubMed] [Google Scholar]
- 27.Tonetti MS, Imboden MA, Gerber L et al. . Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun 1994;62:4005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kardes¸ler L, Buduneli N, Cetinkalp S et al. . Adipokines and inflammatory mediators after initial periodontal treatment in patients with type 2 diabetes and chronic periodontitis. J Periodontol 2010;81:24–33. 10.1902/jop.2009.090267 [DOI] [PubMed] [Google Scholar]
- 29.Ross JH, Hardy DC, Schuyler CA et al. . Expression of periodontal interleukin-6 protein is increased across patients with neither periodontal disease nor diabetes, patients with periodontal disease alone and patients with both diseases. J Periodont Res 2010;45:688–94. 10.1111/j.1600-0765.2010.01286.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petković AB, Matić SM, Stamatović NV et al. . Proinflammatory cytokines (IL-1beta and TNF-alpha) and chemokines (IL-8 and MIP-1alpha) as markers of peri-implant tissue condition. Int J Oral Maxillofac Surg 2010;39:478–85. 10.1016/j.ijom.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 31.Fiorentino DF, Zlotnik A, Mosmann TR et al. . IL-10 inhibits cytokine production by activated macrophages. J Immunol 1991;147:3815–22. [PubMed] [Google Scholar]