Abstract
Objectives
Data on acute geriatric patients' nutritional status are lacking, and the associations among physical function, sarcopenia, health status and nutritional status are not sufficiently investigated in this population. The aims of this study are to investigate (1) nutritional status and sarcopenia in a group of acute geriatric patients, (2) the association between nutritional status, physical function and sarcopenia in acute geriatric patients, controlling for health status.
Design
A cross-sectional study.
Setting
Two acute geriatric hospital wards in Norway.
Participants
This study included 120 patients with a mean age of 82.6±8 years. The following inclusion criteria were used: age ≥65 years and admitted to an acute geriatric ward. The exclusion criteria included terminal illness, Mini-Mental State Examination <23, language difficulties or severe aphasia.
Main outcome measures
Nutritional status was assessed using the Mini Nutritional Assessment (MNA). Physical function was measured using the Barthel activities of daily life index and the Short Physical Performance Battery (SPPB). Sarcopenia was diagnosed using the mid-arm muscle circumference, gait speed and grip strength, in accordance with the EWGSOP algorithm. Diseases are organised by organ system classification.
Results
On the basis of the MNA classification, nearly one in two patients were at risk of malnutrition, while one in four were malnourished. Sarcopenia was present in 30% of the patients. A multivariate linear regression model was estimated and showed significant independent associations between SPPB score (β 0.64, 95% CI 0.38 to 0.90), sarcopenia (β −3.3, 95% CI −4.9 to −1.7), pulmonary disease (β −2.1, 95% CI −3.7 to −0.46), cancer (β −1.7, 95% CI −3.4 to −0.033) and nutritional status.
Conclusions
Our study shows a high prevalence of risk of malnutrition, malnutrition and sarcopenia. Further, the results indicate that a low total SPPB score, sarcopenia, cancer and pulmonary disease are significantly associated with declines in nutritional status, as measured by the MNA, in acute geriatric patients.
Keywords: MNA, Nutritional status, Sarcopenia, Physical function, Acute geriatric
Strengths and limitations of this study.
The main outcome measures employed in this study are validated for the hospitalised elderly.
All data are complete and there are no missing values.
All data were collected in face-to-face interviews with the same experienced clinician.
The study includes a relatively small sample size which may increase the risk of type II error (rejecting a true alternative hypothesis).
All analyses are correlational and no causality can be demonstrated.
Introduction
Malnutrition and risk of malnutrition are reported to be highly prevalent among acutely sick elderly individuals.1 2 In Norway, it is suggested that between 45% and 75% of hospitalised patients are malnourished or at risk of malnutrition.3–6 Malnutrition increases the risk of morbidity, mortality and impaired physical, mental and social function, all of which may result in reduced quality of life as well as having fiscal consequences.1 2 7 8 Unintentional weight loss is a major sign of malnutrition, which includes a loss of skeletal muscle mass9 leading to gradual, progressive muscle wasting, a common feature of ageing.10 11 Acute or chronic illness, and physical inactivity alone or in concert with malnutrition or inadequate protein intake can hasten the loss of lean body mass and increase the risk of functional impairment.12 13 Moreover, malnutrition is associated with geriatric syndromes such as frailty and sarcopenia.14 15 Sarcopenia refers to the loss of skeletal muscle mass and decline in associated muscular function with ageing.16 17 In summary, malnutrition, physical impairment and sarcopenia are amendable as well as indicators for people being at risk of adverse health outcomes.18
There is a lack of evidence concerning the prevalence of risk of malnutrition, malnutrition and sarcopenia in acute geriatric patients, in Norway and internationally. Furthermore, a thorough investigation of the association between physical function and nutritional status, controlling for health status (ie, medical conditions, medications and sarcopenia), seems to be missing in the field. Only two studies have explored the association between nutritional status and physical function using performance-based assessments, that is, grip strength7 and ‘Timed Up and Go’ (TUG), in acute geriatric patients.19 However, neither study controlled for medication use or sarcopenia, which are both considered to influence physical function substantially. Thus, our aims were to investigate nutritional status and sarcopenia in a group of acute geriatric patients. Furthermore, we wanted to assess which physical function and health status variables had significant, independent associations with nutritional status. Our goal is to contribute to the knowledge of preventing malnutrition and supplementing recent research, as well as to tailor interventions for enhancing good health.
Material and methods
Study design and sample
A cross-sectional study was designed and conducted at two different hospitals in the eastern part of Norway. Both hospitals operate mainly as local hospitals, with some regional and national medical responsibilities. Enrolment and assessment began in May 2014 and ended in May 2015. The data were collected from two internal medicine wards, which were divided into multiple subdivisions, namely, cardiology, stroke and acute geriatrics. Acute geriatric wards were defined according to Baztán et al20 as a ward with an independent ‘physical location and structure and run by a specialised multidisciplinary team with direct responsibility for the care of elderly people with acute medical disorders, including acute exacerbations of chronic diseases’.20 The following inclusion criteria were used: age ≥65 years and admitted as an acute geriatric ward. All included patients were admitted from home or an institution, that is, nursing home. The exclusion criteria included terminal illness, Mini Mental State Examination<23, language difficulties or severe aphasia. Patients were approached while being admitted to the hospital. In total, 234 patients were screened, 93 (39.7%) of whom did not meet the inclusion criteria (57 women and 36 men) and 21 (9.0%) of whom did not wish to participate (5 men and 16 women) (see figure 1). Thus, 120 persons (51.3%) agreed to participate and were included. All patients were approached shortly after admission, and median time from admission to screening was 2 days (IQR: 5.0).
Figure 1.
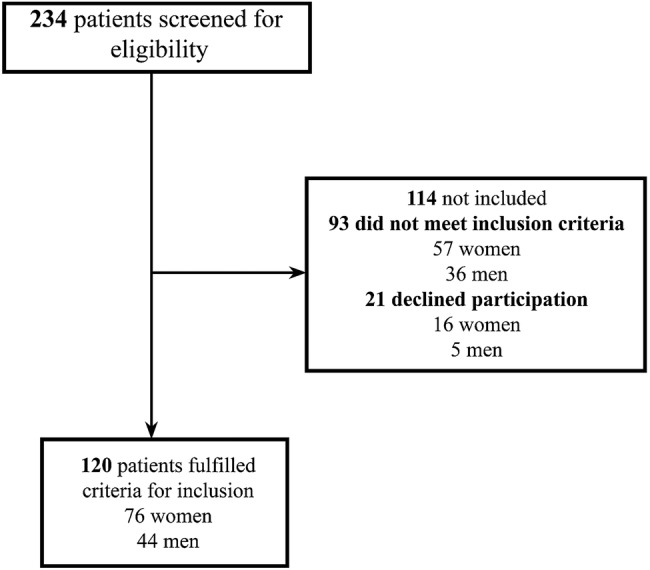
Flow chart over inclusion process of patients.
Ethics
The patients received written and oral information about the study, and written consent was required before inclusion. One registered nurse (ELJ) collected all the data. The study was performed in accordance with the Helsinki Declaration on human studies. To comply with ethical standards, all patients received information about their nutritional status after participating in the study.
Measurement of nutritional status
Nutritional status was assessed using the Norwegian version of the full Mini Nutritional Assessment (MNA). The MNA is a validated screening and assessment tool for the elderly, that is, those aged ≥65 years.21 Briefly, the MNA consists of 18 items (5 questions in the screening part and 13 in the assessment part) capturing anthropometric measures, such as body mass index (BMI) and calf and arm circumference; dietary intake (protein, fruit and vegetable, and fluid); appetite; general health (eg, use of prescribed medications; self-reported health; presence of acute illness, psychological stress or dementia); and mobility. The total score ranges from 0 to 30, where a score of ≤17 points indicates malnutrition, 17–23.5 indicates risk of malnutrition, and ≥24 points indicates normal nutrition.
For the patients capable of standing upright, a Tanita BC-418 MA Body Composition Analyzer (Tanita Corp, Tokyo, Japan) was used to measure weight. For patients with pacemakers, the wards' regular floor scales were used. Patients incapable of standing were weighed using the wards' in-bed scales. Participants were asked to stand against the wall, and a wall-mounted height rod with an accuracy of 1 cm was used to measure height. For patients who were unable to stand, height was estimated using the demi-span and the formulas in the MNA guidelines for women and men, respectively: (1.35×demi-span in cm)+60.1 and (1.40×demi-span in cm)+57.8. BMI was defined as weight (kilograms) divided by height×height in metres.
Physical function
To measure the patients' functioning in activities of daily life (ADL), we employed the Norwegian Barthel Index (BI). The BI is frequently used to measure ADL functioning among the elderly.22 23 It is scored on a scale (0–20), where a higher score indicates a high degree of independence in performing ADL, and vice versa. The BI was used as a self-reported questionnaire.
The SPPB was used to evaluate balance, mobility and muscle strength by examining the patient's ability to stand with their feet in side-by side, semitandem and tandem positions, the time needed to walk 4 m and the time needed to rise from a chair and return to a seated position five times.24 Performance on each of these three tests was scored from 0 to 4, leaving a maximum score of 12 for those individuals performing at the highest levels. Patients who tried, but were unable to complete either test, that is, gait speed, chair stands and/or balance, received a score of 0 on the given test. Only individuals who refused to complete a test were considered missing data. This test battery has proved a valid and reliable measure of lower extremity performance.24 25
Sarcopenia
For this study, we adopted the diagnostic criteria for sarcopenia from the European Working Group on Sarcopenia in Older People (EWGSOP; table 1). The EWGSOP26 refers to different stages of sarcopenia: presarcopenia, sarcopenia and severe sarcopenia. Presarcopenia is characterised as low muscle mass without low muscle strength or low physical function. Sarcopenia is characterised by low muscle mass in combination with low muscle strength or low physical function. The last stage, severe sarcopenia, is characterised by low muscle mass, low strength and low physical function.26
Table 1.
Diagnosis of sarcopenia, variables and cut-off values
| Criterion | Measurement methods | Cut-off points |
|---|---|---|
| Muscle mass | MAMC | Women: <19.2 cm, men <21.1 cm |
| Muscle strength | Handgrip strength | Women: ≤20 kg, men: ≤30 kg |
| Physical performance | Gait speed | ≤0.8 m/s |
MAMC, mid-arm muscle circumference.
The EWGSOP algorithm suggests using muscle mass and muscle strength (gait speed and/or grip strength) as criteria for sarcopenia.26
Muscle mass
Skinfold thickness at the triceps muscle, that is, TSF, was measured with a Baseline
Skinfold Caliper (range from 0.0 to 67 mm; min graduation, 1.0 mm). TSF was measured three times for each patient to compute the average. The mid-arm circumference (MAC) was measured using a flexible measuring tape on the patient's right arm, unless it was affected by a disability or other conditions. Based on MAC and the average of TSF, mid-arm muscle circumference (MAMC) was calculated using the following formula:27 28
Owing to a lack of reliable cut-off points, the MAMC tertiles calculated in the ilSIRENTE study29 were used to identify patients with reduced muscle mass. Hence, in this study, MAMC <21.1 cm in men and <19.2 cm in women was considered low muscle mass.29
Muscle strength
Muscle strength was measured by grip strength using a Sammons Preston Jamar hand dynamometer. All patients performed three measurements per hand for a total of six measurements. The measurements were performed in a seated position with the elbows flexed at 90°, not supported by the armrests and with the forearm and wrist in a neutral position.30 For bedridden patients, grip strength was assessed while lying at 30° in bed with elbows supported as described by Hillman et al.31 To avoid muscle fatigue, the patients paused 10–20 s between measurements and alternated between the left and right hands for every measurement. According to the EWGSOP algorithm, measurements <20 kg for women and <30 kg for men are considered low muscle strength.26 In this study, the highest of the six measurements was used in the statistical analysis.
Gait speed
Gait speed was measured by having the patient walk 4 m to a marker using their regular walking pace. The test was performed in the patient's room or in a hallway of the respective hospital. The Norwegian version of the Short Physical Performance Battery (SPPB)24 32 was performed twice as per the standards, and the fastest gait speed was used in the statistical analysis. According to the EWGSOP algorithm, a gait speed <0.8 m/s is considered poor physical function.26
Health status
The patients provided information about their basic characteristics, such as education, relationship and housing situation. Medical histories, including number of diseases, medications and current cause of admission, were retrieved from the patient charts. Diseases were organised by organ system classification and Charlson Comorbidity Index (CCI), while medications were recorded as number of prescribed medications. In the present study, CCI is employed as a method of weighting comorbidities in the sample. The CCI used in this study consists of 19 conditions, each assigned a score between 1 and 6, depending on the severity of the condition. The total score of the index is calculated by adding the individual score of each condition. In addition, one extra point is added for every 10 years above the age of 40.33
Data analysis
Continuous variables are presented as the mean value±SD, while categorical and non-normally distributed variables are reported as the median and IQR. The internal consistency of the MNA, BI and SPPB was measured by Cronbach's α coefficients, which were found to be satisfactory at 0.71, 0.85 and 0.81, respectively.34
Differences between women and men at baseline were assessed using independent t-tests and Mann-Whitney U tests (for continuous variables) or χ2 tests (for categorical variables). Univariate linear regressions were fitted to evaluate the associations of different variables with nutritional status. All independent variables with p values ≤0.15 in the univariate regression models were included in a multivariate model. One multiple linear regression model was fitted for physical function (SPPB and BI), sarcopenia and health status while controlling for age and gender. Sarcopenia is used as a dummy variable in the model (0=no sarcopenia or presarcopenia, 1=sarcopenia or severe sarcopenia).
To determine the robustness of the multivariate model, both backward and forward regression was conducted, with the same results. The multivariate model was carefully examined, and criteria for linear regression, that is, linearity, homoscedasticity, multicollinearity and normally distributed residuals, were shown to be met. Statistical analyses were performed using IBM SPSS Statistics for Windows, V.22.0 (IBM Corp, Released 2013, Armonk, New York, USA). p Values ≤0.05 were considered statistically significant, and all tests were two tailed. Floor and ceiling effects were said to be present if more than 20% of respondents achieved the lowest or highest possible score.35
Results
Nutritional status, sarcopenia and physical function
Of 120 patients, 44 men and 76 women, with a mean age of 82.5 (±8.0) years were included (table 2). Almost one in two patients were at risk of malnutrition, while one in four were actually malnourished according to the MNA classification (table 2).
Table 2.
Description of the study population.
| Variables | Total (N=120) | Male (N=44) | Female (N=76) |
|---|---|---|---|
| Patients characteristics | |||
| Age: mean (±SD) | 82.5 (±8.0) | 82.5 (±8.6) | 81.8 (±7.4) |
| Living situation:* | |||
| Alone, N (%) | 88 (73.3) | 24 (54.5) | 64 (84.2) |
| With other, N (%) | 32 (26.7) | 20 (45.5) | 12 (15.8) |
| In-home care before admission:* | |||
| Yes, N (%) | 101 (84.2) | 41 (93.2) | 60 (78.9) |
| No, N (%) | 19 (15.8) | 3 (6.8) | 16 (21.1) |
| Education: | |||
| Low (≤10 years), N (%) | 49 (40.8) | 20 (45.5) | 29 (38.2) |
| High (≥11 years), N (%) | 71 (59.2) | 24 (54.5) | 47 (61.8) |
| Nutritional status | |||
| Weight in kg: mean (±SD) | 65.4 (±15.6) | 74.3 (±14.0) | 59.5 (±13.2) |
| BMI: mean (±SD) | 22.8 (±5.0) | 23.6 (±4.2) | 21.9 (±4.5) |
| MNA: mean (±SD)** | 19.8 (±4.8) | 22.0 (±3.8) | 20.1 (±4.7) |
| MNA classification: | |||
| Malnutrition, N (%) | 32 (26.7) | 8 (18.2) | 24 (31.6) |
| Risk of malnutrition, N (%) | 58 (48.3) | 21 (47.7) | 37 (48.7) |
| Normal nutritional status, N (%) | 30 (25.0) | 15 (34.1) | 15 (19.7) |
| Physical function | |||
| SPPB: mean (±SD) | 3.6 (±3.1) | 4.8 (±2.8) | 4.6 (±2.8) |
| BI: mean (±SD) | 14.0 (±4.3) | 15.0 (±3.3) | 14.8 (±4.2) |
| Sarcopenia | |||
| Sarcopenia** | |||
| Presarcopenia, N (%) | 2 (1.7) | 0 | 2 (2.6) |
| Sarcopenia, N (%) | 15 (12.5) | 3 (6.8) | 12 (15.8) |
| Severe sarcopenia, N (%) | 21 (17.5) | 0 | 21 (27.6) |
| Uncertain diagnosis, N (%) | 2 (1.7) | 2 (4.5) | 0 |
| HGS kg: mean (±SD)**† | 15.6 (±8.0) | 20.3 kg (±6.5) | 12.7 kg (±6.0) |
| GS m/s: mean (±SD)‡ | 0.54 (±.21) | 0.52 (±.21) | 0.55 (±.21) |
| MAMC: mean (±SD)** | 22.0 (±4.0) | 24.2 (±3.1) | 20.7 (±3.9) |
| Health status | |||
| Diseases and medication | |||
| CCI: mean (±SD) | 6.0 (±2.2) | 6.3 (±2.6) | 5.7 (±2.0) |
| Pulmonary, N (%) | 38 (31.7) | 10 (22.7) | 28 (36.8) |
| Cancer, N (%) | 29 (24.2) | 9 (20.5) | 20 (26.3) |
| Neurological diseases, N (%) | 60 (50.0) | 20 (45.4) | 40 (52.6) |
| Digestive, N (%) | 52 (43.3) | 17 (38.6) | 35 (46.1) |
| Hypertensive and ischaemic heart diseases, N (%) | 90 (75.0) | 34 (77.3) | 56 (73.7) |
| Mental and behavioural disorders, N (%) | 27 (24.2) | 11 (25.0) | 16 (21.1) |
| Genitourinary, N (%)* | 31 (25.8) | 17 (38.6) | 14 (18.4) |
| Endocrine, N (%) | 29 (24.2) | 14 (31.8) | 15 (19.7) |
| Musculoskeletal system and connective tissue, N (%)* | 66 (55.0) | 18 (40.9) | 48 (63.2) |
| Eye, N (%) | 26 (21.7) | 6 (13.6) | 20 (26.3) |
| Arteries, arterioles, capillaries, vein and lymphatic vessels, N (%) | 33 (27.5) | 11 (25) | 22 (28.9) |
| Other diseases, N (%) | 92 (76.7) | 32 (72.7) | 60 (78.9) |
| Number of prescribed medications: mean (±SD) | 7.3 (±3.8) | 6.7 (±3.8) | 7.6 (±3.8) |
Patient's characteristics, nutritional status, physical function, sarcopenia and health status.
*p value <0.05, **p Value <0.001.
†N=118.
‡N=98.
BI, Barthel activities of daily life Index; BMI, body mass index; CCI, Charlson Comorbidity Index; GS, gait speed; HGS, handgrip strength; MAMC, mid-arm muscle circumference; MNA, Mini Nutritional Assessment; NO, number of; SPPB, Short Physical performance battery.
One in three patients had sarcopenia. Severe sarcopenia was seen in 17.5% of patients. Women had a higher rate and more severe sarcopenia (p<0.05) than men, whereas presarcopenia was equally distributed in both genders. Two patients were not able to complete the required tests (ie, grip strength and gait speed) and thus did not receive a sarcopenia diagnosis. No significant differences in physical function (BI and SPPB) were found between women and men (table 2). However, the results of the SPPB showed that 20.0% received a total score of zero (n=24), 0.8% (n=1) scored 11 and none of the patients obtained the maximum score of 12 points.
Associations among nutritional status, physical function, sarcopenia and health status
The results indicate that men had higher MNA scores than women (p=0.04; table 3). All diseases had a significant inverse association with nutritional status as measured by the MNA. Both SPPB and BI were positively and significantly associated with nutritional status, with p<0.001 and p=0.03, respectively (table 3).
Table 3.
Univariate linear regression models, dependent variable MNA score.
| Variables (N=120) | 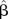 |
95% CI | p Value |
|---|---|---|---|
| Patients characteristics | |||
| Age | 0.007 | −0.10 to 0.12 | 0.894 |
| Gender | 1.8 | 0.022 to 3.6 | 0.047 |
| Physical function | |||
| SPPB (range: 0–11) | 0.74 | 0.49 to 0.98 | <0.001 |
| BI (range: 0–20) | 0.21 | 0.015 to 0.41 | 0.035 |
| Health status | |||
| Sarcopenia: (no=0, yes=1)* | −3.2 | −5.0 to −1.4 | <0.001 |
| Pulmonary (no=0, yes=1) | −3.0 | −4.8 to −1.2 | 0.001 |
| Cancer (no=0, yes=1) | −2.6 | −4.5 to −0.56 | 0.012 |
| Neurological (no=0, yes=1) | −2.0 | −3.7 to −0.29 | 0.022 |
| Digestive (no=0, yes=1) | −1.8 | −3.5 to −0.031 | 0.046 |
| Medication per day | −0.17 | −0.40 to 0.056 | 0.138 |
Unadjusted models.
*N=118.
BI, Barthel activities of daily life Index; MNA, Mini Nutritional Assessment; SPPB, short physical performance battery.
The total SPPB score had the highest standardised coefficient and was the most important covariate for nutritional status, as measured by the MNA (table 4). Sarcopenia, pulmonary disease and cancer had the second, third and fourth highest standardised coefficients, while neurological disease, BI, number of prescribed medications and digestive disease had lower standardised coefficients and were thus less important for predicting the MNA score.
Table 4.
Multivariate linear regression models, dependent variable MNA score. Adjusted model
| Model 1 (R2=38.8%)* (N=118) |
|||||
|---|---|---|---|---|---|
| Variables | Covariates | 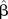 |
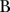 |
95% CI | p Value |
| Physical function | SPPB | 0.64 | 0.42 | 0.38 to 0.90 | <0.001 |
| BI | 0.088 | 0.07 | −0.10 to 0.28 | 0.356 | |
| Health status | Sarcopenia | −3.3 | −0.32 | −4.9 to −1.7 | <0.001 |
| Pulmonary | −2.1 | −0.20 | −3.7 to −0.46 | 0.012 | |
| Cancer | −1.7 | −0.15 | −3.4 to −0.033 | 0.046 | |
| Neurological | −1.0 | −0.10 | −2.5 to 0.49 | 0.185 | |
| Digestive | 0.29 | 0.03 | −1.2 to 1.8 | 0.706 | |
| Number of medications per day | 0.087 | 0.07 | −0.11 to 0.28 | 0.383 | |
*Adjusted for gender and age.
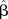 , Unstandardised coefficient; B, Standardised β-coefficient; BI, Barthel activities of daily life Index; MNA, Mini Nutritional Assessment; SPPB, Short physical performance battery.
, Unstandardised coefficient; B, Standardised β-coefficient; BI, Barthel activities of daily life Index; MNA, Mini Nutritional Assessment; SPPB, Short physical performance battery.
The results indicate that physical function, as measured by the SPPB and indicated by sarcopenia, remained highly significantly (p<0.001) associated with MNA after adjusting for potential confounders. Pulmonary disease and cancer still showed inverse associations with nutritional status, indicating that patients with pulmonary disease and cancer had a decline in their MNA scores of 2.0 and 1.7 points, respectively, compared to patients without pulmonary disease (p=0.012) or cancer (p=0.046). This model explained 38.8% of the variance in nutritional status and was significant (p<0.001; table 4).
Discussion
This is the first Norwegian cross-sectional study to assess the associations between physical function, health status and nutritional status. The results demonstrate that malnutrition is a highly prevalent problem in acute geriatric patients because 75% were at risk of malnutrition or already malnourished. Two previous international studies conducted in acute geriatric wards reported an even higher prevalence of malnutrition.19 36 Studies that employed the full MNA to assess the risk of malnutrition in acute geriatric wards reported that 90.1% (n=205)19 and 100% of the patients (n=148) were at risk of malnutrition or malnourished.36 The higher prevalence reported in these two studies may be explained by the fact that these studies included patients with dementia, which is known to increase the risk of malnutrition.37
In this study, pulmonary diseases and cancer showed inverse associations with nutritional status. These findings are consistent with previous results suggesting that patients with cancer have high rates of malnutrition due to appetite loss and side effects of medical treatment.38 Patients suffering from pulmonary diseases are also at high risk of developing malnutrition. Chronic diseases, such as chronic obstructive pulmonary disease, are considered energy intensive, with weight loss and malnutrition being common consequences.39 Surprisingly, no significant associations were detected between neurological disorders and nutritional status in our study. In contrast, Saka et al40 found a highly significant association between MNA scores ≤23 and neurological disorders in elderly patients admitted to an outpatient clinic (n=413). These conflicting findings may be related to the comparatively small sample size of this study, as the lack of statistical power may result in non-significant associations.41
Surprisingly, we found no associations between age or gender and nutritional status. Advanced age was reported as an important risk factor for malnutrition, and the prevalence of malnutrition tends to increase with ageing.37 Females aged 65 years or above were also at higher risk of malnutrition than men of the same age.42 Our discordant findings may be related to the advanced age (a mean of 82 years) and predominance of women (females=63.3%) in the sample.
This study indicates that one in three patients were sarcopenic according to the EWGSOP criteria. Only three previous studies have estimated the prevalence of sarcopenia in hospitalised elderly persons.15 43 44 The estimated prevalence in these studies varies from 10% (n=432)43 to 21.4% (n=103)15 and 26% (n=103).44 Our results were slightly higher, which may be explained by a different classification approach. Both Cerri et al15 and Rossi et al44 adopted the diagnostic approach suggested by the EWGSOP, but muscle mass was measured by bioelectrical impedance analysis (BIA). Gariballa et al43 assessed muscle mass using MAMC but did not include gait speed. In addition, Cerri et al15 included only patients with MNA scores ≤23, and a significant proportion of the patients (22.3%, n=23) were not able to complete handgrip or gait speed tests. This may clarify why our results indicate a higher proportion of sarcopenia than similar studies.
We found that sarcopenia was associated with poor nutritional status, and malnutrition is often recognised as a risk factor for developing sarcopenia; hence, these syndromes frequently coexist.18 Malnutrition and sarcopenia are geriatric syndromes, which often play an essential role in the frailty process.18 A previous study that included only acute geriatric patients (N=103) at risk of malnutrition or who were malnourished, according to the MNA-SF, investigated the association between nutritional status and sarcopenia.15 They found no significant association between sarcopenia and nutritional status. The fact that they only included patients at risk of malnutrition or with malnutrition may have increased the difficulty of identifying a significant relationship between the variables due to low variation in the data. Another interesting finding in our study was that nutritional status, as measured by the MNA, was strongly associated with physical function, as measured by the SPPB, after controlling for health status and other potential confounders. This finding is in line with previous studies of hospitalised elderly patients.8 19
Strengths and limitations
To the best of our knowledge, this study is the first to simultaneously assess physical function using the SPPB and nutritional status using the MNA in acute geriatric patients. Previous studies have generally employed TUG, which assesses only the gait speed and the ability to rise from and sit down in a chair. In contrast, the SPPB evaluates participants' balance, mobility and muscle strength in lower extremities, and including the overall SPPB score may provide a more accurate picture of a patient’s overall mobility than gait speed. In summary, the SPPB may provide a more thorough assessment of physical function than TUG measures. Moreover, owing to the fact that similar results concerning nutritional status and physical function have been reported in previous studies, the external validity of this study is strengthened. The limitations of this study are the small sample size, which may increase the risk of type II error (rejecting a true alternative hypothesis). In addition, a small sample size requires that the results are interpreted carefully. Furthermore, we assessed muscle mass using MAMC, although there is debate about whether anthropometric measures are adequate and reliable markers of muscle mass. One study suggests that BIA may be a better marker than MAMC for predicting adverse outcomes and malnutrition in some patient populations.45 However, the burden of frailty in our sample limited the use of BIA because 28.3% (n=34) of the included patients could not perform the test. Specifically, 73.5% (n=25) of patients were incapable of standing, and 26.5% (n=9) of patients had medical conditions that contraindicated using BIA. MAMC allowed the quick assessment of muscle mass without unnecessary exertion from the patients. Another weakness of this study is that a relatively large portion of the patients received a score of zero on the SPPB (20%, n=24). While not considered to be causing a floor effect (>20%) in this study, these results suggest that BIA and SPPB are not appropriate tests for patients with the most severe illnesses or those with the greatest functional declines. As in all cross-sectional studies, there are some methodological limitations. For example, we cannot presume causal relationships among nutritional status, physical function and health status. Furthermore, along with physical function and health status, ageing may be associated with profound psychosocial and environmental changes, such as isolation, loneliness, depression and inadequate finances, which may also have significant effects on nutritional status.46 We did not assess these variables, which may be considered a limitation of this study. These aspects should be included in future studies to clarify the associations among physical function, health status and nutritional status.
Conclusion
A significant proportion of acute geriatric patients are malnourished or are at risk of malnutrition. One in three patients suffer from sarcopenia. Our results indicate that a low total score on SPPB, sarcopenia, cancer and pulmonary disease are associated with a decline in nutritional status, as measured by the MNA, in acute geriatric patients. Future studies should assess whether nutritional and exercise interventions improve acute geriatric patients' physical function and reduce the risk of developing sarcopenia.
Acknowledgments
The authors thank the patients who participated in the study, the health professionals for assisting in the data collection and Professor Dr Are Hugo Pripp for kind help with the statistical analysis.
Footnotes
Contributors: ABe and ABy had the original idea for the study and developed the study design with ELJ and TB. ELJ performed the data collection, analysed the data and wrote the first draft of the paper. All authors contributed to further drafts.
Funding: This research is fully funded by Oslo and Akershus University College of Applied Sciences.
Competing interests: None declared.
Ethics approval: Regional Committee for Medical and Health Research Ethics in Norway and the ethical committees of the respective hospitals approved the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Stratton RJ, Green CJ, Elia M. Disease-related malnutrition: an evidence-based approach to treatment. 1st edn Wallingford: CABI, 2003. [Google Scholar]
- 2.Abd-El-Gawad WM, Abou-Hashem RM, El Maraghy MO et al. . The validity of Geriatric Nutrition Risk Index: simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with Mini Nutritional Assessment. Clin Nutr 2014;33:1108–16. 10.1016/j.clnu.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Ranhoff AH, Gjoen AU, Mowe M. Screening for malnutrition in elderly acute medical patients: the usefulness of MNA-SF. J Nutr Health Ageing 2005;9:221–5. [PubMed] [Google Scholar]
- 4.Eide HK, Šaltytė Benth J, Sortland K et al. . Prevalence of nutritional risk in the non-demented hospitalised elderly: a cross-sectional study from Norway using stratified sampling. J Nutr Sci 2015;4:e18 10.1017/jns.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowé M, Bøhmer T. The prevalence of undiagnosed protein-calorie undernutrition in a population of hospitalized elderly patients. J Am Geriatr Soc 1991;39:1089–92. 10.1111/j.1532-5415.1991.tb02874.x [DOI] [PubMed] [Google Scholar]
- 6.Mowé M, Bøhmer T, Kindt E. Reduced nutritional status in an elderly population (>70 y) is probable before disease and possibly contributes to the development of disease. Am J Clin Nutr 1994;59:317–24. [DOI] [PubMed] [Google Scholar]
- 7.Persson MD, Brismar KE, Katzarski KS et al. . Nutritional status using mini nutritional assessment and subjective global assessment predict mortality in geriatric patients. J Am Geriatr Soc 2002;50:1996–2002. 10.1046/j.1532-5415.2002.50611.x [DOI] [PubMed] [Google Scholar]
- 8.Vivanti A, Ward N, Haines T. Nutritional status and associations with falls, balance, mobility and functionality during hospital admission. J Nutr Health Ageing 2011;15:388–91. 10.1007/s12603-010-0302-8 [DOI] [PubMed] [Google Scholar]
- 9.Heymsfield SB, McManus C, Stevens V et al. . Muscle mass: reliable indicator of protein-energy malnutrition severity and outcome. Am J Clin Nutr 1982;35:1192–9. [DOI] [PubMed] [Google Scholar]
- 10.Morley JE, Abbatecola AM, Argiles JM et al. . Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 2011;12:403–9. 10.1016/j.jamda.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennie MJ, Selby A, Atherton P et al. . Facts, noise and wishful thinking: muscle protein turnover in ageing and human disuse atrophy. Scand J Med Sci Sports 2010;20:5–9. 10.1111/j.1600-0838.2009.00967.x [DOI] [PubMed] [Google Scholar]
- 12.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010;13:34–9. 10.1097/MCO.0b013e328333aa66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paddon-Jones D. Interplay of stress and physical inactivity on muscle loss: nutritional countermeasures. J Nutr 2006;136:2123–6. [DOI] [PubMed] [Google Scholar]
- 14.da Silva Alexandre T, de Oliveira Duarte YA, Ferreira Santos JL et al. . Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus Dynapenia as a risk factor for disability in the elderly. J Nutr Health Ageing 2014;18:547–53. 10.1007/s12603-013-0424-x [DOI] [PubMed] [Google Scholar]
- 15.Cerri AP, Bellelli G, Mazzone A et al. . Sarcopenia and malnutrition in acutely ill hospitalized elderly: prevalence and outcomes. Clin Nutr 2015;34:745–51. 10.1016/j.clnu.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 16.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci 2000;55:M716–24. 10.1093/gerona/55.12.M716 [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990S–1S. [DOI] [PubMed] [Google Scholar]
- 18.Vandewoude MF, Alish CJ, Sauer AC et al. . Malnutrition-sarcopenia syndrome: is this the future of nutrition screening and assessment for older adults? J Ageing Res 2012;2012:651570 10.1155/2012/651570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrader E, Baumgartel C, Gueldenzoph H et al. . Nutritional status according to Mini Nutritional Assessment is related to functional status in geriatric patients—independent of health status. J Nutr Health Ageing 2014;18:257–63. 10.1007/s12603-013-0394-z [DOI] [PubMed] [Google Scholar]
- 20.Baztán JJ, Suárez-García FM, López-Arrieta J et al. . Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BMJ 2009;338:b50 10.1136/bmj.b50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vellas B, Guigoz Y, Garry PJ et al. . The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999;15:116–22. 10.1016/S0899-9007(98)00171-3 [DOI] [PubMed] [Google Scholar]
- 22.Mahoney FI. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 23.Collin C, Wade DT, Davies S et al. . The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61–3. 10.3109/09638288809164103 [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L et al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 25.Studenski S, Perera S, Wallace D et al. . Physical performance measures in the clinical setting. J Am Geriatr Soc 2003;51:314–22. 10.1046/j.1532-5415.2003.51104.x [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonelli Incalzi R, Landi F, Cipriani L et al. . Nutritional assessment: a primary component of multidimensional geriatric assessment in the acute care setting. J Am Geriatr Soc 1996;44:166–74. 10.1111/j.1532-5415.1996.tb02434.x [DOI] [PubMed] [Google Scholar]
- 28.Gibson R. Principles of nutritional assessment. 2nd edn New York: Oxford University Press, 2005. [Google Scholar]
- 29.Landi F, Russo A, Liperoti R et al. . Midarm muscle circumference, physical performance and mortality: results from the ageing and longevity study in the Sirente geographic area (ilSIRENTE study). Clin Nutr 2010;29:441–7. 10.1016/j.clnu.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 30.Fess EE, Moran C. Clinical assessment recommendations. Indianapolis, IN: American Society of Hand Therapists Monograph, 1981. [Google Scholar]
- 31.Hillman TE, Nunes QM, Hornby ST et al. . A practical posture for hand grip dynamometry in the clinical setting. Clin Nutr 2005;24:224–8. 10.1016/j.clnu.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 32.Pernille T. Screeningtest for fysisk funksjon hos eldre: Norsk oversettelse av Short Physical Performance Battery (SPPB) [Screening Test for physical function in elderly : Norwegian translation of the Short Physical Performance Battery (SPPB)]. Fysioterapeuten [Physiotherapist] 2013;5. [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 34.Field A. Discovering statistics using IBM SPSS statistics: and sex and drugs and rock ‘n’ roll. 4th edn Los Angeles: Sage, 2013. [Google Scholar]
- 35.Di Carlo A, Lamassa M, Baldereschi M et al. . Risk factors and outcome of subtypes of ischemic stroke. Data from a multicenter multinational hospital-based registry. The European Community Stroke Project. J Neurol Sci 2006;244:143–50. [DOI] [PubMed] [Google Scholar]
- 36.Wakabayashi H, Sakuma K. Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle 2014;5:269–77. 10.1007/s13539-014-0162-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morley JE. Anorexia of ageing: physiologic and pathologic. Am J Clin Nutr 1997;66:760–73. [DOI] [PubMed] [Google Scholar]
- 38.Ravasco P, Monteiro-Grillo I, Camilo ME. Does nutrition influence quality of life in cancer patients undergoing radiotherapy? Radiother Oncol 2003;67:213–20. 10.1016/S0167-8140(03)00040-9 [DOI] [PubMed] [Google Scholar]
- 39.Schols AM. Nutrition in chronic obstructive pulmonary disease. Curr Opin Pulm Med 2000;6:110–15. 10.1097/00063198-200003000-00005 [DOI] [PubMed] [Google Scholar]
- 40.Saka B, Kaya O, Ozturk GB et al. . Malnutrition in the elderly and its relationship with other geriatric syndromes. Clin Nutr 2010;29:745–8. 10.1016/j.clnu.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 41.Altman DG. Practical statistics for medical research. London: Chapman and Hall, 1991. [Google Scholar]
- 42.Castel H, Shahar D, Harman-Boehm I. Gender differences in factors associated with nutritional status of older medical patients. J Am Coll Nutr 2006;25:128–34. 10.1080/07315724.2006.10719523 [DOI] [PubMed] [Google Scholar]
- 43.Gariballa S, Alessa A. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr 2013;32:772–6. 10.1016/j.clnu.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 44.Rossi AP, Fantin F, Micciolo R et al. . Identifying sarcopenia in acute care setting patients. J Am Med Dir Assoc 2014;15:303.e7–12. 10.1016/j.jamda.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 45.Faisy C, Rabbat A, Kouchakji B et al. . Bioelectrical impedance analysis in estimating nutritional status and outcome of patients with chronic obstructive pulmonary disease and acute respiratory failure. Intensive Care Med 2000;26:518–25. 10.1007/s001340051198 [DOI] [PubMed] [Google Scholar]
- 46.World Health Ogranization. World report on ageing and health. Luxembourg: World Health Organization; 2015. [Google Scholar]


