Sir,
Therapy resistance and relapse are the major issues associated with the current management of cancer.[1] Cancer stem cells (CSCs) have inbuilt mechanisms to develop resistance. The most commonly overexpressed markers of CSCs are aldehyde dehydrogenase (ALDH) enzymes and ATP-binding cassette transporters (e.g., ABCG2).[2] Moreover, cells have the capability to induce checkpoint responses when exposed to genotoxic agents (i.e., chemotherapy or radiation therapy), causing arrest of cells cycle. Resistance followed by chemotherapy administration as observed clinically can be divided into two phases. The first phase involves conversion of actively dividing cells to a completely dormant stage where cells do not divide though remain metabolically active. This stage can be defined as therapy-induced senescence.[3] At this stage, chemotherapeutic agents fail to produce desired effect because cells are not dividing. The second phase is conversion of these dormant cells to actively dividing cells after longer duration. These cells may be even more resistant than parent cells and hence, require higher concentration of chemotherapeutic agents to arrest cell division.[4] Disulfiram, an antabuse drug, is reported to inhibit ALDH,[5] efflux pump,[6] and some other potential cellular targets in cancer cells. Caffeine is reported to inhibit the checkpoint responses that are responsible for development of senescence.[7] Hence, both disulfiram and caffeine simultaneously can be used to target CSCs and senescent cells, which are major contributors of therapy resistance.
A549 cell line was procured from the National Centre for Cell Science (Pune, India). It was cultured in Dulbecco's modified Eagle's media supplemented with 10% fetal bovine serum and antibiotics (100 μg/ml streptomycin and ampicillin). When the culture became 70–80% confluent, media were discarded and replaced with fresh media containing cisplatin and incubated for 3 days. After that, the media were replaced with cisplatin-free media, and survived cells were allowed for recovery for 4 days. These procedures were repeated with increasing concentrations of cisplatin (1.66, 3.33, 6.66, and 16.66 μM). The final cell line obtained was labeled as A549-R. Further, both of cell types were pretreated with caffeine (515 μM) and/or disulfiram (0.101 μM), followed by cisplatin exposure. The cytotoxic effect in each case was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. As the analysis of single-dose point (i.e., only IC50) may be ambiguous, we decided to compare whole curves. Nonlinear regression (curve fitting) analysis was carried out; parameters were measured, and the curves were compared by extra sum of squares F-test (P < 0.05) using GraphPad Prism® version 5.01 (GraphPad Software, Inc. La Jolla, California, USA).
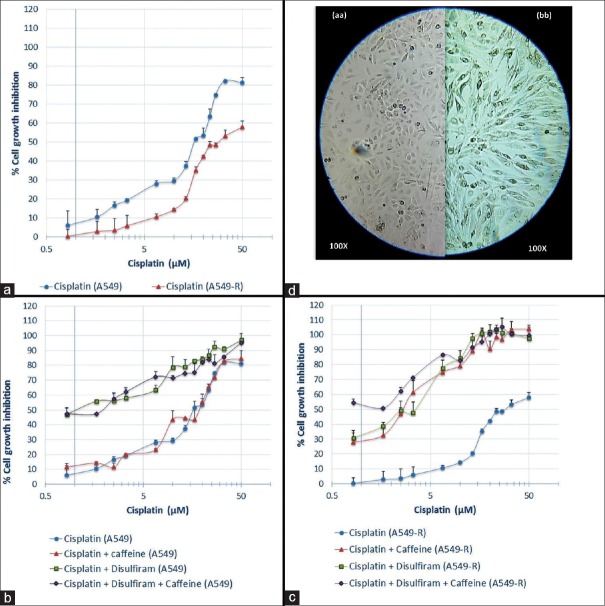
After exposure to cisplatin, the cells appeared bigger (2–4 times), flattened, and elongated with bigger and clearly visible nuclei [Figure 1d]. Vacuoles were seen in the cytoplasm of some cells. The cisplatin IC50 got almost doubled on A549-R cells. Both the curves presented in [Figure 1a] were significantly different (P < 0.0001) from each other. Both caffeine and disulfiram pretreatment reduced the IC50 of cisplatin in A549-R cells [Figure 1b]. Both when combined, a further reduction was observed [Figure 1c]. A similar study on A549 cells showed reduced IC50 in only disulfiram pretreated cells. Further, how A549 and A549-R cells responded to given therapies can be depicted from graphs. The outcome of statistical treatment that was given to the different curves is shown in Table 1.
Figure 1.
Graphs represents (a) effect of increasing concentration of cisplatin on A549 and A549-R cells, and effect of treatment of various drug combinations followed by exposure to various concentrations of cisplatin on (b) A549 cell line and (c) A549-R cell line, respectively. Each point in the graphs represents mean ± standard error of the mean of two observations (d) Represents morphology of (aa) A549 cells and (bb) A549-R cells
Table 1.
Curve fitting analysis for different curves of Graph b and Graph c
Both CSC-related markers expressing cells[1] and senescent cells[4] have been individually reported to be the major cause of resistance. Targeting both of them together can be expected to improve disease prognosis. The present study was conducted to determine benefits of such combined targeting. We observed a reduced toxicity of disulfiram in senescent cells than that observed in naive A549 cells. However, its pretreatment reversed the cisplatin resistance. Checkpoint responses can be inhibited by caffeine but at higher concentrations.[7] In this study, we used relatively lower concentration of caffeine to improve cisplatin cytotoxicity. Cisplatin efficacy was not altered by caffeine pretreatment to A549 cells. However, against senescent cells, it completely reversed the cisplatin resistance. The results indicated that caffeine might be acting by inhibiting the checkpoint responses in the senescent cells and forcing the cells forward in the cell cycle. The presence of cisplatin at this juncture becomes more meaningful in producing cytotoxicity. Exposure of A549-R cells to combination of caffeine and disulfiram caused reduction in IC50 of cisplatin even more than that observed in naive A549 cells.
Thus, simultaneous use of caffeine and disulfiram as adjuvant therapy to chemotherapeutic agent is likely to improve the prognosis of the disease. However, a clinical study in this direction can throw more light.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Disulfiram powder, for the research purpose, was provided as a gift sample by Tripada Healthcare Pvt. Ltd., Ahmedabad, India.
REFERENCES
- 1.Lee HE, Kim JH, Kim YJ, Choi SY, Kim SW, Kang E, et al. An increase in cancer stem cell population after primary systemic therapy is a poor prognostic factor in breast cancer. Br J Cancer. 2011;104:1730–8. doi: 10.1038/bjc.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cojoc M, Mäbert K, Muders MH, Dubrovska A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin Cancer Biol. 2015;31:16–27. doi: 10.1016/j.semcancer.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: An emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76:947–57. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Achuthan S, Santhoshkumar TR, Prabhakar J, Nair SA, Pillai MR. Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J Biol Chem. 2011;286:37813–29. doi: 10.1074/jbc.M110.200675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SK, Kim H, Lee DH, Kim TS, Kim T, Chung C, et al. Reversing the intractable nature of pancreatic cancer by selectively targeting ALDH-high, therapy-resistant cancer cells. PLoS One. 2013;8:e78130. doi: 10.1371/journal.pone.0078130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauna ZE, Peng XH, Nandigama K, Tekle S, Ambudkar SV. The molecular basis of the action of disulfiram as a modulator of the multidrug resistance-linked ATP binding cassette transporters MDR1 (ABCB1) and MRP1 (ABCC1) Mol Pharmacol. 2004;65:675–84. doi: 10.1124/mol.65.3.675. [DOI] [PubMed] [Google Scholar]
- 7.Crescenzi E, Palumbo G, de Boer J, Brady HJ. Ataxia telangiectasia mutated and p21CIP1 modulate cell survival of drug-induced senescent tumor cells: Implications for chemotherapy. Clin Cancer Res. 2008;14:1877–87. doi: 10.1158/1078-0432.CCR-07-4298. [DOI] [PubMed] [Google Scholar]