Abstract
The current National Comprehensive Cancer Network (NCCN) Guidelines consider the role of 2-deoxy-2-18F-fluoro-d-glucose positron emission tomography/computer tomography (FDG PET/CT) in the evaluation of cholangiocarcinoma (CCA) as "uncertain," and have recommended contrast enhanced computed tomography (CECT) but not FDG PET/CT as a routine imaging test for CCA workup. We set out to compare the diagnostic performance of FDG PET/CT and CECT in patients with CCA. The retrospective study included patients with CCA who underwent FDG PET/CT and CECT within 2-month interval between 2011 and 2013 in our hospital. Lesion-based comparison was conducted. Final diagnoses were made based on the composite clinical and imaging data with minimal 6-month follow-up. A total of 18 patients with 28-paired tests were included. There is a total of 142 true malignant lesions as revealed by the 6-paired pre-treatment and 22-paired post-treatment tests. On a lesion-based analysis, the sensitivities, specificities, positive predictive values (PPVs), negative predictive values (NPVs), and accuracies of PET/CT and CECT for detection of CCA were 96.5%, 55.5%, 97.2%, 50.0%, 94.1% and 62.2%, 66.7%, 96.7%, 10.0%, 62.5%, respectively. FDG PET/CT detected more intrahepatic malignant and extrahepatic metastases; and had significant higher sensitivity, NPV, and accuracy than CECT, while similar in specificity and PPV. No true positive lesion detected on CECT that was missed on PET/CT, and none of the false negative lesions on PET/CT were detected on CECT. Six patients had paired pretreatment tests, and FDG PET/CT results changed planned management in three patients. Our data suggest that FDG PET/CT detect more primary and metastatic lesions and lead to considerable changes in treatment plan in comparison with CECT.
Keywords: Cholangiocarcinoma, contrast enhanced computed tomography, diagnostic performance, 2-deoxy-2-18F-fluoro-d-glucose positron emission tomography/computer tomography, lesion-based comparison
Introduction
Cholangiocarcinoma (CCA) is an aggressive malignant neoplasm that arises from the cancerous biliary duct epithelium. It accounts for 3% gastrointestinal malignancies.[1,2,3] There have been various classifications based on the pathologic and radiologic appearance of cholangiocarcinoma. Based on their location, cholangiocarcinoma can be classified into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC), which include perihilar and distal bile duct cancers. According to the American Cancer Society,[4] about 2,000–3,000 people develop cholangiocarcinoma each year in the United States. Though extrahepatic is more common than ICC, the incidence of ICC has increased.[3] In the United States, the age-adjusted incidence of ICC has increased by 165% from 0.32/100,000 in 1975 to 1979 to 0.85/100,000 in 1995 to 1999.[5,6] A 2002 analysis based on the mortality data from 22 countries in the World Health Organization databank, reported an increasing trend for mortality from ICC in most countries.[7]
Cholangiocarcinoma poses a diagnostic and therapeutic challenge as it is both rare and nonspecific in clinical presentation. And cholangiocarcinoma often remain symptomatically indolent until later stages. Treatment options dependent on tumor size and location, extent of bile duct involvement, invasion of adjacent critical vasculatures, and presence of distant metastases. To date, surgical resection continues to be the most effective treatment of CCA. Negative resection margins achieved with major hepatic and regional lymph nodes resections are associated with improved outcome.[1,2,3,4] For patients with proximal CCA, which includes hilar and ICC, who underwent surgical resection with R0 margins (defined as absence of microscopic disease involvement in any resection margin) have a 5-year survival rate of 20–45%.[5,6,7,8,9] For patients with ECC who underwent resection with R0 margins, 5-year survival rates ranged 20–54%.[6,10,11,12,13] These relatively low 5-year survival rates further underscore the aggressive nature of cholangiocarcinoma and the importance of early detection and proper staging.
Imaging plays a role in accurate diagnosis, characterization, localization and staging of CCA, and assessment of tumor therapy response. An ideal imaging test for CCA should help in both detection and staging disease, thus helping in preparation for advanced surgical procedures and other nonsurgical treatment planning. Routine clinical workup in diagnosing CCA include contrast enhanced computed tomography (CECT) and magnetic resonance imaging (MRI) to assess the involvement of the liver, major vessels, and regional lymph nodes, to help determine tumor respectability. Other imaging modalities, including 2-deoxy-2-18F-fluoro-D-glucose positron emission tomography/computer tomography (FDG PET/CT), have been increasingly used in the clinical practice. Although the National Comprehensive Cancer Network (NCCN) acknowledged that there have been emerging evidence for FDG PET/CT in diagnosing and staging of cholangiocarcinoma patients, it only recommend CECT or MRI, but not FDG PET/CT, in the routine clinical workup in the latest NCCN Guideline.[8]
The aim of this study is to compare the lesion-based efficacy and accuracy of FDG PET/CT and CECT in identifying primary cholangiocarcinoma lesions and distant metastatic lesions.
Patients and Methods
Patient selection
This institutional review board approved retrospective study included all patients with biopsy proven cholangiocarcinoma who underwent FDG PET/CT and CECT between January 2011 and December 2013 at our institution. The patients with gallbladder cancer were excluded from our study, whereas those who underwent FDG PET/CT and CECT within a the last 2 months were included. All patients had a follow-up for at least 6 months after imaging.
Imaging protocols
FDG PET/CT imaging
The FDG PET examinations were performed on a GE Discovery 690 FDG PET/CT scanner (GE Medical Systems, Milwaukee, WI) using a standard protocol. Patients fasted at least 4 h before scanning and had a blood glucose level <200 mg/dL at the time of FDG injection. Dedicated PET/CT scans from the skull base to the upper thighs were obtained 60-90 min after intravenous (IV) injection of 0.37-0.481 MBq of FDG. CT parameters were as follows: 120 kV, 120 mAs, pitch 0.813, 16 × 1.5 mm collimation. The PET parameter: Was as follows 3 min bed/position.
CECT imaging
All scans were obtained by using a GE CT scanner (GE Healthcare, Wisconsin) with 16 or 64 detector rows. Patients were scanned in the supine position with acquisition parameters at 120 kVp and 250-500 mAs. A standard collimation of 16 × 0.75 mm was used, with a gantry rotation speed of 0.5 s and a pitch factor of 1.15. Patients received IV injection of 80 mL of Omnipaque-350 contrast at 3.5–4.5 mL/s via an IV access, followed by 40 mL saline flush. CT images in the portal venous phase were acquired 70 s post injection and 15 min for the delayed phase. Coronal and sagittal reformats were performed at 2.5-mm slice thickness from the original acquired data. Images were interpreted on a picture archiving and communication system (PACS) terminal.
Imaging evaluation
The acquired FDG PET/CT and CECT images of each patient were independently assessed on a dedicated AW PACS workstation (GE Healthcare, Wisconsin). Independent review results were compared with the original reports. A lesion was considered malignant by CECT if it demonstrated any combination of two or more of the following characteristics: The lesion was hypoattenuating, demonstrated enhancement on portal venous or delayed phase scans, caused distortion of normal anatomic architecture, caused obstruction or dilatation of the biliary tree, or was associated with adenopathy FDG. FDG PET/CT images were retrospectively analyzed using a combined qualitative and quantitative method: Focal FDG avid soft tissue and osseous lesions with obvious higher-than-liver background FDG avidity in at least two consecutive slices were determined as positive lesions, and the lesions' SUVmax were recorded. FDG avidity was correlated with corresponding CT abnormality. Distinction between postoperative changes and malignant lesions was based on clinical context, the length time interval after surgery, and follow-up imaging studies.
All charts were reviewed and at least 6 months of clinical follow-up were tracked to determine the presence or absence of cholangiocarcinoma. The lesion-based final diagnosis of cholangiocarcinoma was determined by consensus of all imaging physicians using a composite of all clinical, pathological, and imaging information during the follow-up period. For each eligible patient, the positive lesions on CECT and FDG FDG PET/CT were scrutinized and counted.
Lesion-based analysis
To investigate how FDG PET/CT vies against CECT in identifying primary cholangiocarcinoma tumors and regional and distant metastasis, a lesion-based analysis was conducted. In this method, each paired CECT and FDG PET/CT study was independently analyzed and all lesions consistent with cholangiocarcinoma or metastasis were counted. A true positive lesion represented any primary cholangiocarcinoma tumor or metastatic lesion that met the imaging criteria at the follow-up examination and/or was confirmed by surgery or biopsy. A false positive lesion corresponded to a lesion that met the imaging criteria but subsequently proved to represent a nonneoplastic process either by tissue sampling or by resolution on subsequent imaging. True negative lesions were lesions that met the imaging criteria for cholangiocarcinoma but were predicted to be caused by another etiology, while false negative lesions were lesions missed by either FDG PET/CT or CECT and identified by the other modality and subsequently shown to represent cholangiocarcinoma or related metastasis.
Statistical analysis
Bayesian statistical analysis was performed to determine the overall sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated as the evaluation of diagnostic performance. Differences in assessment between PET-CT and CECT were tested for significance using MeNemar's test or Fisher's exact test. Statistical analyses were performed using Statistical Analysis System (SAS, North Carolina) software, version 9.2. A P value of <0.05 was considered statistically significant.
Results
During the study period (January 2011-December 2013), a total of 18 patients diagnosed with cholangiocarcinoma underwent FDG PET/CT and CECT within a 2-month interval. This cohort was comprised of 10 males and 8 females (56% and 44%, respectively) with a median age of 57 years (range 28–78 years). Fifteen of the eighteen patients underwent image guided biopsy at our institution that yielded a diagnosis of cholangiocarcinoma, while the remaining three patients were diagnosed with cholangiocarcinoma at an outside facility and were transferred to our institution for further management. Nine patients had ICC, another nine had ECC; out of these nine patients, three presented with perihilar (Klatskin tumor). Of the 18 total patients were included in our study, a total of 28 paired FDG PET/CT and CECT studies were obtained between January 2011 and December 2013. Among these 28 paired studies, 6 were prior to initiation of treatment, remaining 22 were after initiation of treatment. Five patients had two paired studies; one patient had three paired studies, one patient had four paired studies, for the remaining eleven patients, each had a single paired FDG PET/CT and CECT study. Treatment for these 18 patients included liver transplant, chemotherapy, radiation, endoscopic and percutaneous biliary stent placement, transarterial chemoembolization, and catheter directed Yttrium-90 radioembolization (Y-90 RE) treatment and a combination of these treatments. Posttreatment FDG PET/CT studies were performed for the purpose of restaging and follow-up.
Lesion-based analysis
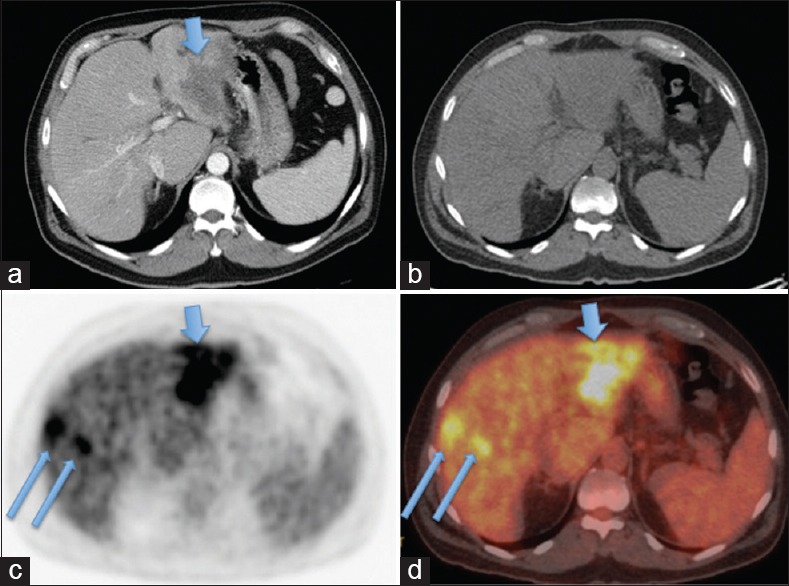
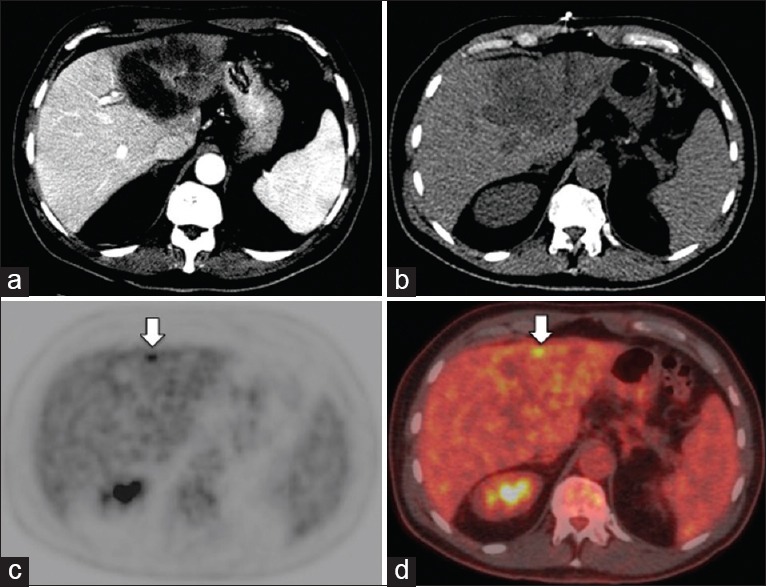
Lesion-based analysis [Table 1] of the 28 paired studies yielded 138 true positive, 4 false positive, 5 true negative, and 5 false negative lesions identified by FDT PET/CT. For CECT, 89 true positive, 3 false positive, 6 true negative, and 54 false negative lesions were identified. FDG PET/CT identified a total of 50 hypermetabolic lesions that were undetected by CECT. Of these 50 lesions, 31 were intrahepatic lesions [Figures 1 and 2], 1/31 was deemed as false positive lesion from focal hypermetabolic activity related to inflammation after the biliary catheter placement; 10 were regional and distant lymph nodes but 3/10 were deemed as inflammatory change; and 9 osseous metastasis [Figure 3]. There were three low attenuation liver lesions on posttreatment CECT was not detected on PET/CT, and were deemed as posttreatment necrosis based on the follow-up images. There were five lesions not detected by FDG PET/CT (false negative lesions) that proved to be metastatic lesions. None of these five lesions were detected on CECT. Three of these were non-FDG-avid mesenteric subcentimeter to borderline sized lymph nodes that were shown to be positive for metastasis after surgical resection. The other two lesions were subcentimeter osseous metastasis in vertebral bodies that were indistinct due to background FDG-uptake within the bone marrow, and missed on CT component of PET/CT and CEACT. Upon retrospective analyses, these two sclerotic lesions slightly progressed on both CECT and CT component of PET/CT, but didn't show any higher-than-background FDG avidity. The two lesions were retrospectively deemed as bone metastases, with resolution beyond PET resolution. Taken altogether, none of the true positive lesions identified on CECT was missed on FDG PET/CT, and none of the false negative lesions on FDG PET/CT were detected on CECT.
Table 1.
2×2 Tables of the lesion-based analysis of imaging modalities
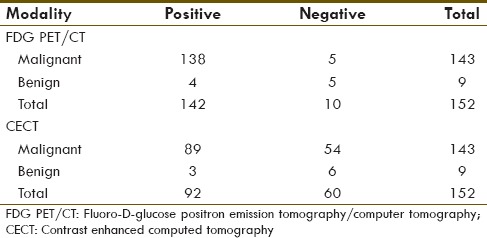
Figure 1.
FDG PET/CT detected more intrahepatic lesions than CECT (a) CECT only detected one conglomerate mass in the left lobe liver (arrow), (b–d) FDG PET/CT (B: CT, C: PET, D: Fused PET/CT) detected the same left lobe lesion (short arrows) and more lesions within the right lobe liver (long arrows)
Figure 2.
Patient with early recurrent cholangiocarcinoma detected on FDG PET/CT but not on contrast enhanced CT(CECT) (a) Follow-up CECT performed 3-months after the Y-90 radioembolization (Y90-RE) showed low attenuation, central necrotic left lobe lesions in the region of Y-90 RE, deemed as post-treatment change, (b–d) PET/CT (B: CT; C: PET; D: Fused PET/CT) obtained within 1 week after CECT detected a small focal FDG avid lesion at the peripheral edge of Y-90 RE (arrows), suggestive of early recurrence and was confirmed on follow-up images
Figure 3.
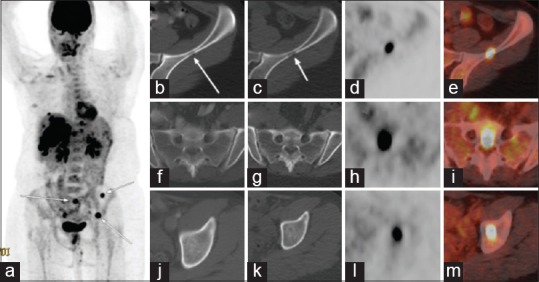
Patient with metastatic cholangiocarcinoma detected on FDG PET/CT but not on contrast enhanced CT (CECT) (a) PET/CT MIP image showed multiple hypermetabolic intrahepatic foci and several bone metastases (arrows) (b) CECT only detected the conglomerate right lobe primary lesion in the liver (arrow) (c-e) PET/CT (C: CT; D: PET; E: Fused PET/CT) detected both the primary right lobe lesion (long arrows), and 3 additional small intrahepatic malignant lesions (short arrows). Furthermore, CECT (F, J) failed to detect the bone metastases in the sacrum (F) and left acetabulum (J); while PET/CT (G,K: CT; H,L: PET; I,M: Fused PET/CT) clearly demonstrated the FDG-avid bone metastases (arrows)
Of the six patients from the pretreatment CECT and FDG PET/CT studies, three had a change in management plan based on the results of the FDG PET/CT. All the three patients had CECT before FDG PET/CT, one with Klatskin tumor, the other two with ICC. Based on CECT findings, these three patients were initially planned to have either transplantation (for the two patients with ICC), or adjuvant chemoradiation postsurgical resection (for the patient with Klatskin tumor). As FDG PET/CT identified more intrahepatic malignant lesions [Figure 1], and lymph node and osseous metastases [Figure 3], these patients became nonsurgical candidates and received chemotherapy trial instead.
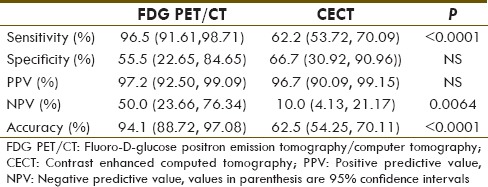
In terms of lesion-based diagnostic performance [Table 2], FDG PET/CT has significantly higher sensitivity of 96.5% (138/143) compared to that of CECT (62.2%) (89/92) (P < 0.0001). Although CECT had greater specificity of 66.7% (6/9) as compared to 55.5% (5/9) for FDG PET/CT, there is no statistical significant difference between the two modalities. The PPV for FDG PET/CT and CECT were very similar (97.2% and 96.7%, respectively). The NPV and accuracy of FDG PET/CT and CECT were 50.0% and 94.1%, versus 10.0% and 62.5%, respectively. The FDG PET/CT had significant higher NPV and accuracy over CT, with P < 0.01 [Table 2].
Table 2.
Diagnostic performance of the imaging modalities
Discussion
For patients with CCA, accurate identification of all primary and metastatic lesions is critical for optimal clinical management, such as candidacy for liver transplantation based on the Mayo Clinic protocol,[14,15,16] comprehensive resection of the disease,[5,17,18] and adjuvant chemo- and radiotherapy to regional metastases. Imaging plays an important role in the accurate detection and characterization the tumor extent, assessment of tumor resectability, and evaluation of treatment response.
Though not intensively published, data have been accumulating showing that FDG PET/CT has similar accuracy as CECT in diagnosing primary tumor in patients with cholangiocarcinoma, and may be advantageous in detecting regional lymph node and distant metastases, thus aiding in the multidisciplinary management of cholangiocarcinomas.[9,10,11,12,13] However, the latest NCCN guidelines still think "the role of PET imaging has not been established in the evaluation of patients with cholangiocarcinoma."[19] As NCCN Guidelines are widely recognized and used as the standard for clinical policy in oncology by clinicians and insurance payers, we set to add the database of FDG PET/CT in evaluation of CCA, through summarizing our institutional experience in a lesion-based comparison between FDG PET/CT and NCCN-recommended CECT.
In this restrospective study, we compared the lesion-based efficacy and accuracy of CECT and FDG PET/CT in staging and follow-up of patients with CCA. For the 18 consecutive patients with biopsy proven cholangiocarcinoma included in our study, we found that FDG PET/CT detected more disease than CECT, which directly affected the management of three out of six patients who had pretreatment FDG PET/CT. In comparison to CECT, FDG PET/CT identified more intrahepatic, lymph node and distant metastatic lesions [Figures 1 and 3]. There were no positive lesions detected on CECT that were missed on FDG PET/CT, and none of the false negative lesions on FDG PET/CT was detected on CECT.
Several studies have shown the effectiveness of FDG PET and PET/CT in confirming the diagnosis and staging CCA, particularly for detecting regional lymph node and distant metastases in patients with CCA compared to CECT and MRI.[18,19,20,21,22,23,24] In a patient-based comparison study, Petrowsky et al.[25] reported that FDG PET/CT and CECT provided a comparable accuracy for the primary ICC (N = 14) and ECC (N = 33). All distant metastases (12/12) were detected by PET/CT, but only 3/12 by CECT (P < 0.001). FDG PET/CT findings resulted in a change of management in 17% of patients deemed resectable after CECT workup. Slightly different to their report, our lesion-based comparison study indicates that FDG PET-CT showed no significant difference in specificity and PPV compared to CECT for diagnosing malignant lesions. However, FDG PET-CT revealed significantly higher sensitivity, NPV and accuracy over CECT in determining the extent of malignant disease. Thus, FDG PET/CT has an overall significant better diagnostic performance than CECT in our patient cohort with cholangiocarcinoma. In our study, 12 out of the 18 patients underwent treatment prior to being evaluated with FDG PET/CT, which correlates with a more advanced disease status. It is possible that our patient population, as a cohort, had a greater tumor burden than that evaluated by Petrowsky et al. Our data also suggested that, as shown in Figure 2, FDG PET/CT is advantageous in detecting early recurrence/metastasis in posttreatment patients.
As FDG PET/CT could detect occult metastasis and characterize indeterminate lesions,[26] it can have a major influence on clinical decision-making, usually resulting in changing of management plan in 10–30% of the patients.[17,21,22,25,27] Although our study is limited by the small number of patients with FDG PET/CT prior to treatment, our data indicate that FDG PET/CT directly influenced management decisions in three out of six patients who were evaluated prior to initiation of treatment. In all three of these patients, distant regional and distant metastasis were identified by FDG PET/CT that were not detected by CECT.
Conclusion
Our study adds to the growing body of data supporting the advantageous utility of FDG PET/CT in pretreatment planning and staging, as well as in restaging and detecting subtle, occult recurrent and metastatic lesions, in patients with CCA.
Footnotes
Source of Support: Nil.
Conflict of Interest: All the authors declare that they have no conflict of interests.
References
- 1.Serrablo A, Tejedor L. Outcome of surgical resection in Klatskin tumors. World J Gastrointest Oncol. 2013;5:147–58. doi: 10.4251/wjgo.v5.i7.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robles R, Sanchez-Bueno F, Ramirez P, Brusadin R, Parrilla P. Liver transplantation for hilar cholangiocarcinoma. World J Gastroenterol. 2013;19:9209–15. doi: 10.3748/wjg.v19.i48.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vern-Gross TZ, Shivnani AT, Chen K, Lee CM, Tward JD, MacDonald OK, et al. Survival outcomes in resected extrahepatic cholangiocarcinoma: Effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys. 2011;81:189–98. doi: 10.1016/j.ijrobp.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 4.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–14. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 6.Marsh Rde W, Alonzo M, Bajaj S, Baker M, Elton E, Farrell TA, et al. Comprehensive review of the diagnosis and treatment of biliary tract cancer 2012. Part II: Multidisciplinary management. J Surg Oncol. 2012;106:339–45. doi: 10.1002/jso.23027. [DOI] [PubMed] [Google Scholar]
- 7.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–71. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akoad M, Jenkins R. Proximal biliary malignancy. Surg Clin North Am. 2008;88:1409. doi: 10.1016/j.suc.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–475. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Society AC. Bile Duct Cancer (Cholangiocarcinoma) American Cancer Society. 2014:51. [Google Scholar]
- 12.Veillette G, Castillo CF. Distal biliary malignancy. Surg Clin North Am. 2008;88:1429–47, xi. doi: 10.1016/j.suc.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Warren KW, Choe DS, Plaza J, Relihan M. Results of radical resection for periampullary cancer. Ann Surg. 1975;181:534–40. doi: 10.1097/00000658-197505000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen CB, Heimbach JK, Gores GJ. Surgery for cholangiocarcinoma: The role of liver transplantation. HPB (Oxford) 2008;10:186–9. doi: 10.1080/13651820801992542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Pedersen R, Kremers W, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–7. doi: 10.1097/01.tp.0000253551.43583.d1. [DOI] [PubMed] [Google Scholar]
- 16.Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–61. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–7. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Ito H, Allen PJ, Gonen M, Klimstra D, D’Angelica MI, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251:675–81. doi: 10.1097/SLA.0b013e3181d3d2b2. [DOI] [PubMed] [Google Scholar]
- 19.Members NG. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers. 2015. [Last accessed on 2015 Jul 01]. Available from: http://www.nccn.org .
- 20.Breitenstein S, Apestegui C, Clavien PA. Positron emission tomography (PET) for cholangiocarcinoma. HPB (Oxford) 2008;10:120–1. doi: 10.1080/13651820801992583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D’Angelica M, Fong Y, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57–65. doi: 10.1016/j.jamcollsurg.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Kim MH, Lee TY, Hwang CY, Kim JS, Yun SC, et al. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: A prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145–51. doi: 10.1111/j.1572-0241.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee SW, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J Gastroenterol. 2010;45:560–6. doi: 10.1007/s00535-009-0188-6. [DOI] [PubMed] [Google Scholar]
- 24.Moon CM, Bang S, Chung JB, Park SW, Song SY, Yun M, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography in differential diagnosis and staging of cholangiocarcinomas. J Gastroenterol Hepatol. 2008;23:759–65. doi: 10.1111/j.1440-1746.2007.05173.x. [DOI] [PubMed] [Google Scholar]
- 25.Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Albazaz R, Patel CN, Chowdhury FU, Scarsbrook AF. Clinical impact of FDG PET-CT on management decisions for patients with primary biliary tumours. Insights Imaging. 2013;4:691–700. doi: 10.1007/s13244-013-0268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruys AT, Bennink RJ, van Westreenen HL, Engelbrecht MR, Busch OR, Gouma DJ, et al. FDG-positron emission tomography/computed tomography and standardized uptake value in the primary diagnosis and staging of hilar cholangiocarcinoma. HPB (Oxford) 2011;13:256–62. doi: 10.1111/j.1477-2574.2010.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


