Spinal cord injury (SCI) is a devastating trauma that leaves approximately 10,000 to 20,000 people paralyzed every year in the United States. The majority of these cases are young people that will live to almost a full life expectancy, however, their quality of life is significantly reduced. After SCI there is loss of both sensory and motor function below the level of injury. Due to this loss of function SCI patients are often unable to care for themselves and rely on friends and family to serve as their primary caregivers. The Christopher Reeve Foundation found that more than 50 million people each year provide this care in the U.S., the value of which is estimated to be $306 billion annually. Along with the financial burden, SCI patients have emotional stress and continued health care problems throughout their lives most predominately renal complications and pressure sores. These facts demonstrate the overwhelming need to restore function after SCI.
After SCI, there is immediate insult followed by secondary inflammatory damage, which is a cascade of cellular and molecular responses that result in a dense astroglial scar (Fawcett and Asher, 1999). This inflammatory process leads to neuronal and oligodendrocyte apoptosis, increased astroglial reactivity, increased lesion volume, and diminished functional recovery. Neural tissue is complex and most likely treating SCI will involve a combination treatment targeting different cell types at specific time points. There are several therapeutic proteins that have been shown to improve function after SCI by targeting specific aspects of the problem. Anti-inflammatory cytokines such as interleukin-10 (IL-10) have been shown to reduce the amount of secondary damage and reduce the number of cells apoptosing (Thompson et al., 2013), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3) have been shown to increase axonal sprouting and growth (Hanna et al., 2016), and chondroitinase ABC has been shown to break down glycosaminoglycans in the glial scar which inhibit axonal growth (Hanna et al., 2013). An ideal drug delivery platform would have the ability to locally deliver several different therapeutic proteins in a sustained manner with the adaptability to tailor the time frame of protein delivery.
Calcium phosphate coatings: Calcium phosphate (CaP) minerals have a high affinity for proteins, which is well known from standard chromatography methods (Morrison et al., 2010). CaP minerals have also been used recently as carriers for growth factors that influence tissue formation, including transforming growth factor beta 1 (TGF-β1), insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), bone morphogenetic protein 2 (BMP2), and NT-3 (Suarez-Gonzalez et al., 2010, 2012; Yu et al., 2014; Hanna et al., 2016). The incorporation of therapeutic proteins into CaP coatings grown in simulated body fluids is particularly advantageous in tissue engineering, as it allows for a high level of control over coating properties and resultant protein release kinetics. In particular, physicochemical properties of CaP coatings can be readily controlled, resulting in controllable protein binding and subsequent protein release kinetics that are dictated by the protein-CaP affinity and CaP dissolution rate.
Previous work has demonstrated that CaP coatings can be created on various biomaterials, ranging from bioceramics to bioresorbable polymerz (Lu et al., 2009; Lee et al., 2010, 2011; Suarez-Gonzalez et al., 2010, 2012; Yu et al., 2014). Incubation of biomaterials in modified simulated body fluids (mSBF) results in rapid heterogeneous nucleation and growth of a continuous CaP coating, which are plate-like and porous on the nanometer scale creating a high surface area for the binding of therapeutic proteins (Figure 1A–E). The nanoporous structure and dissolution rate of the CaP coatings can be systematically altered by varying the calcium, phosphate, and carbonate contents in the mSBF solution. Importantly, altering the structure and dissolution rate of the coating leads to control over protein binding and release kinetics. Moreover, multiple proteins can be incorporated into the same CaP coating, either in the same region or in distinct locations, or CaP coatings can be layered with different proteins incorporated into different layers (Lee et al., 2010, 2011; Yu et al., 2014).
Figure 1.
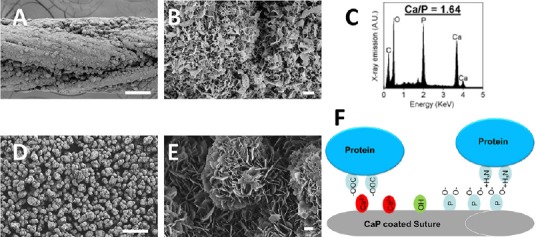
Calcium phosphate (CaP) coatings can be grown on a variety of materials in modified simulated body fluids (mSBF).
(A) Scanning electron microscopy micrographs of CaP coated Vicryl sutures show a continuous layer of CaP coating. (B) Higher magnification shows the nanoporous plate like structure creating a large surface area for protein binding. (C) Energy dispersive spectroscopygraph obtained from a CaP coated suture reveals peaks of calcium and phosphate crystals. (D) Scanning electron microscopy micrographs of CaP coated β-tricalcium phosphate microparticles shows a continuous layer of CaP coating and (E) higher magnification reveals a nano-porous structure similar to the CaP coated sutures. (F) The CaP coatings are a mosaic of positively charged crystal calcium ions and clusters of negatively charged oxygen atoms associated with crystal phosphates and the majority of protein binding to the CaP coating is a combination of metal affinity and phosphoryl cation exchange. The illustration shows proteins binding via metal chelation between carboxyl clusters and crystal calcium ions and proteins binding via cation exchange between negatively charged oxygen atoms associated with crystal phosphates and positive amines on the protein. Scale bars: A and D: 100 μm, B and E: 1 μm.
Although protein-CaP affinity is a parameter that can influence protein release rate, CaP properties can be varied widely to dictate protein release kinetics, regardless of the protein-mineral affinity. The CaP coatings are a mosaic of positively charged crystal calcium ions and clusters of negatively charged oxygen atoms associated with crystal phosphates (Figure 1F). The majority of protein binding to the CaP coating is a combination of metal affinity and phosphoryl cation exchange. The affinity interaction of protein carboxyl clusters with crystal calcium ions represents a classic metal chelating mechanism, which is much stronger than cation exchange (Figure 1F) (Morrison et al., 2010). These different binding mechanisms allow for efficient binding of both acidic and basic proteins (Jae et al., 2010). It has also been shown that proteins bound to CaP coatings remain biologically active for an extended time period in vivo.
Treatment of SCI via protein delivery from CaP coatings: Dr. Amgad Hanna's group recently published a manuscript in which they treated SCI in a rat model by implanting sciatic nerve grafts (SNGs) to serve as scaffolds for new axon growth and incorporated CaP coated sutures releasing NT-3 (Hanna et al., 2016). Two promising results from this study are that the sutures did not show any negative effects in vivo and that the NT-3 released from the CaP coatings was biologically active. At two months post implantation into the spinal cord, the CaP coatings and sutures were completely dissolved and the sciatic nerve grafts appeared healthy and well apposed to the spinal cord. The SNGs that had the CaP coated sutures releasing NT-3 had significantly more axons growing into them when compared to just a bolus injection of NT-3. Thus, the NT-3 released from the coating was active and had a biological effect on the growing axons. Although these results are promising, the CaP coated sutures releasing NT-3 did not significantly improve the hindlimb function of the rats after SCI compared to rats that were only treated with SNGs. Both groups, the rats treated with only SNGs and the rats treated with SNGs plus CaP coated sutures releasing NT-3, had significant hindlimb functional improvement compared to untreated controls. However, there was not a significant difference in hindlimb function in the rats treated with only SNGs compared to the rats treated with SNGs plus CaP coated sutures releasing NT-3. One plausible reason for the lack of functional improvement could be that the continued release of NT-3 on the SNGs is promoting the axons to grow in the SNGs and there is a lack of signal for them to grow into the spinal cord at the distal end of the SNGs. Another possibility is that more axons did grow into the spinal cord at the distal end, but then failed to make functional connections. In either scenario the lack of functional improvement was not a limitation of the CaP coated sutures, because the CaP coated sutures significantly improved axon growth. In this study, the limitation in the level of functional improvement is probably an indication of the importance of the location of the CaP coated sutures and the timeframe of NT-3 release. The ideal timeframe may be having the NT-3 completely released when the axons grow to the distal end of the graft. Moreover, the regenerating axons will almost certainly need more guidance cues in order to grow to the correct location and make functional connections. Future studies would need to be carried to reveal optimal release time and location of NT-3 release.
Since SCI is very complex, it will probably require a combination treatment. Thus CaP coatings may be a uniquely enabling technology for the treatment of SCI, because they have the ability to incorporate several different therapeutic proteins at different time frames. CaP coatings could be used to deliver an anti-inflammatory during the acute stage after SCI to reduce secondary damage and apoptosis, followed by the release of chondroitinase ABC to aid in new axon growth by digesting the glycosaminoglycans in the glial scar. Along with proteins adjusting the environment after SCI, it would be beneficial to release growth factors to promote axon sprouting, growth, and provide guidance cues to the regenerating axons. Based on previous data the placement and timeframe of the growth factor release will be critical. Another benefit of CaP coatings is their adaptability to be grown on many different materials, shapes, and sizes. In areas where sutures are used, CaP coatings can be grown on standard surgical sutures and in areas where minimal disruption is preferred biodegradable CaP coated microparticles can be injected (Figure 1A–E). This could prove advantageous especially for growing axons through the injured spinal cord because CaP coated microparticles with specific timeframe release of growth factors could be injected at different levels of the spinal cord.
Conclusions: Due to the different stages after SCI, the interplay of several different cell types, and the molecular responses after SCI, the preferred treatment will probably need to be a combination treatment with drugs targeted at specific timelines. For several reasons, CaP coatings may prove to be superior when compared to other drug delivery platforms. The CaP coatings can be grown on a large variety of materials including sutures and resorbable polymers. Both acidic and basic proteins bind to the CaP coating and the binding aids in retaining protein stability and biological activity. The structure and dissolution rate of the CaP coatings can be altered, which adjusted the timeframe of drug delivery. Multiple types of therapeutic proteins can be bound onto a layer of CaP coating or the CaP coating can be layered with different proteins for specific timelines of protein delivery. Thus, CaP coatings may prove to be a unique tool for treating SCI.
The authors would like to thank the Bryon Riesch Paralysis Foundation for their generous financial support of this study (#133-PRJ57YV).
References
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Hanna A, Kaeppler KE, Ehlers ME, Dadsetan M, Yaszemski MJ, Toigo RD, Kim J, Hwang E, Bogarin-Miranda E, Buchholz MM, Springer AR, Hellenbrand DJ. Peripheral nerve grafts and chondroitinase abc application improves functional recovery after complete spinal cord transection. J Neurol Res. 2013;3:85–95. [Google Scholar]
- Hanna A, Thompson DL, Hellenbrand DJ, Lee JS, Madura CJ, Wesley MG, Dillon NJ, Sharma T, Enright CJ, Murphy WL. Sustained release of neurotrophin-3 via calcium phosphate-coated sutures promotes axonal regeneration after spinal cord injury. J Neurosci Res. 2016;94:645–652. doi: 10.1002/jnr.23730. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lu Y, Baer GS, Markel MD, Murphy WL. Controllable protein delivery from coated surgical sutures. J Mater Chem. 2010;20:8894–8903. [Google Scholar]
- Lee JS, Suarez-Gonzalez D, Murphy WL. Mineral coatings for temporally controlled delivery of multiple proteins. Adv Mater. 2011;23:4279–4284. doi: 10.1002/adma.201100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Markel MD, Nemke B, Lee JS, Graf BK, Murphy WL. Influence of hydroxyapatite-coated and growth factor-releasing interference screws on tendon-bone healing in an ovine model. Arthroscopy. 2009;25:1427–1434. doi: 10.1016/j.arthro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CJ, Gagnon P, Cramer SM. Unique selectivity windows using selective displacers/eluents and mobile phase modifiers on hydroxyapatite. J Chromatogr A. 2010;1217:6484–6495. doi: 10.1016/j.chroma.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Suarez-Gonzalez D, Barnhart K, Migneco F, Flanagan C, Hollister SJ, Murphy WL. Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials. 2012;33:713–721. doi: 10.1016/j.biomaterials.2011.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Gonzalez D, Barnhart K, Saito E, Vanderby R, Jr, Hollister SJ, Murphy WL. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. J Biomed Mater Res A. 2010;95:222–234. doi: 10.1002/jbm.a.32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CD, Zurko JC, Hanna BF, Hellenbrand DJ, Hanna A. The therapeutic role of interleukin-10 after spinal cord injury. J Neurotrauma. 2013;30:1311–1324. doi: 10.1089/neu.2012.2651. [DOI] [PubMed] [Google Scholar]
- Yu X, Khalil A, Dang PN, Alsberg E, Murphy WL. Multilayered inorganic microparticles for tunable dual growth factor delivery. Adv Funct Mater. 2014;24:3082–3093. doi: 10.1002/adfm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]


