Neuropathic pain, a type of pain arising after direct damage or disease of the nervous system, is often intractable and challenges the search of effective therapeutic strategies. In particular, neuropathic pain is a very frequent sequel of spinal cord injury (SCI) and a decisive contributor to decreased quality of life.
Several and intricate mechanisms appear to be involved in the onset and maintenance of neuropathic pain after SCI. However, it is only within the last years that neuroinflammatory consequences of SCI have been linked to the stubborn pain that these patients may suffer (Walters, 2014).
Neuroinflammation occurs in the nervous system as a response to pathogens, toxins, trauma or neurodegeneration, and contemplates the active participation of glial cells. While a controlled neuroinflammatory response after injury has been shown to play a neuroprotective role, dysregulation of glial cells - mainly microglia and astrocytes - may result detrimental to neurons and other glial cells, via the production of neurotoxic factors that exacerbate the damage. In particular, reactive gliosis along with the release of pro-inflammatory cytokines is a signature of human and experimental central nervous system (CNS) injury and is critically placed at the crossroads of neuroinflammation and neuropathic pain (Walters, 2014).
However, a main concern after SCI is that general suppression of inflammation may impair regeneration and repair in the spinal cord. These concepts challenge the development of novel strategies aimed at reducing pain without interfering with functional outcomes.
Based upon an active research over the past decades, it is now a well-consolidated concept that progesterone, beyond its recognized role in reproduction, acts as a neurosteroid/neuroactive steroid with multiple and diverse functions in the nervous system (De Nicola et al., 2013). These functions, comprising neuroprotection, myelin formation, control of inflammation, regulation of glial cell function and neurotransmission, have been recently extended to the modulation of pain sensitivity (Coronel et al., 2011). In fact, progesterone, a well-known suppressor of the inflammatory response following CNS injury, stands as a promising candidate to block the multiple cellular and molecular events leading to damage and chronic pain after SCI (Garcia-Ovejero et al., 2014; Coronel et al., 2016).
SCI, neuroinflammation and pain: SCI is a devastating condition that results not only in the loss of function below the level of the lesion, but also in the development of intractable chronic pain in up to 40% of patients (Finnerup, 2008). SCI leads to increased excitability of the neuronal pain processing circuitry, which is symptomatically expressed as allodynia, pain induced by normally innocuous stimuli, and hyperalgesia, increased pain response elicited by noxious stimuli. The maladaptive mechanisms underlying this type of pain are complex and not completely understood.
As indicated, spinal glial cells are central players in the onset of neuroinflammation after SCI. Among the glial mediators released within the CNS, special emphasis has been placed on pro-inflammatory cytokines. Cytokines, like interleukin 1β (IL-1β) and tumor necrosis factor α (TNFα), comprise a large family of soluble peptides and proteins which not only coordinate the innate and adaptive responses to infection but also display specific neuromodulatory functions in a variety of CNS diseases and neurotoxic conditions (Walters, 2014).
In a recent outstanding review, Walters (2014) has shed light on this central question: why spinal neuroinflammation should produce neuropathic pain after injury? To satisfy this inquiry, this state-of-the-art review offers a new perspective on the several interconnected neuroinflammatory pathways after SCI and expands our view of the complex consequences of SCI-induced neuroinflammation. In addition, it recapitulates the large body of evidence indicating that interactions among astrocytes, microglia and neurons are critical for the onset and maintenance of neuropathic pain (Ji et al., 2013; Walters, 2014).
The widespread release of pro-inflammatory cytokines during the inflammatory response after SCI can regulate synaptic transmission and plasticity in the dorsal horn of the spinal cord, a critical site for nociceptive modulation (Ji et al., 2013; Walters, 2014). In fact, these mediators, such as IL-1β and TNFα, have been implicated in the sensitization of nociceptors, their central synaptic targets or both, promoting long-term maladaptive plasticity and unrelenting neuropathic pain (Walters, 2014).
Particularly, IL-1β may facilitate pain via neural-glial interactions (Ji et al., 2013). These actions involve the participation of a functional receptor (IL-1RI), a decoy receptor (IL-1RII), and a specific endogenous antagonist (IL-1ra). As IL-1RI is not only found in glial cells but also distributed in neurons (Gardoni et al., 2011), IL-1β can act directly on neurons to enhance synaptic transmission and neuronal activity in the superficial dorsal horn. In fact, IL-1RI has been pointed out as a coordinating factor for the functional interaction between IL-1β and N-methyl-D-aspartate receptor (NMDAR) (Gardoni et al., 2011), a crucial player in pain transmission. In fact, the local release of IL-1β has been involved in the phosphorylation of NMDAR subunits during pain conditions.
Accordingly, targeting these processes in order to reduce the excitability of dorsal horn neurons and prevent the development of chronic pain appears as a tempting idea.
Progesterone and chronic pain after SCI: Using experimental models of spinal injury, studies from our laboratory and others have shown that progesterone restores the expression of neuronal enzymes and trophic factors, induces white matter tissue preservation, increases oligodendrocyte numbers and the expression of myelin basic protein and improves motor outcomes (De Nicola et al., 2013; Garcia-Ovejero et al., 2014). What is more, progesterone has been shown to reduce neuropathic pain after SCI (Coronel et al., 2011, 2014, 2016).
In the last years, preclinical studies from our laboratory have explored the impact of progesterone administration on neuropathic conditions. We have found that early progesterone treatment prevents mechanical allodynia and significantly reduces the number of painful responses to cold stimulation in male rats subjected to SCI. In good correlation with the observed attenuation of pain behaviors, progesterone administration prevents the injury-induced increase in the expression of NMDAR subunits (NR1, NR2A and NR2B) and the gamma isoform of protein kinase C, both key players for chronic pain generation (Coronel et al., 2011). Progesterone also results in lower number of neuronal profiles exhibiting the phosphorylated form of NR1 (pNR1) (Coronel et al., 2011), a post-translational modification that is essential to induce enhanced NMDAR activity, increased neuronal responsiveness and pain.
More recently, we have also shown that progesterone administration is able to attenuate the SCI-induced increase in the number of immunoreactive astrocytes and microglial cells, and to regulate the expression of pro-inflammatory enzymes, such as the inducible isoform of nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) (Coronel et al., 2014). Additionally, early progesterone administration results in lower IL-1β, IL-6 and TNFα mRNA levels and causes a further increase in IL-1RII expression, sustaining the SCI-induced high levels of IL-1ra expression in the acute phase after injury (Coronel et al., 2016).
However, in the chronic allodynic phase after SCI, all injured animals either receiving progesterone or not showed a basal cytokine expression profile. This pattern appeared to be in divergence with the frequently described high expression levels of these pro-inflammatory mediators involved in neuropathic conditions.
Here, it is important to note that a key point concerning the glial control of pain is how the mediators released by these cells can regulate synaptic transmission (Viviani et al., 2014). As recently emphasized (Viviani et al., 2014), the final consequences of the neuroinflammatory process in the CNS may rely on a delicate balance between the production of cytokines and the ability of neurons to sense them through the expression of specific receptors.
For this reason, we decided to take a closer look to the neuronal expression of IL-1RI. Interestingly, in the chronic phase after SCI we found an increased number of IL-1RI/NR1 positive neurons in the dorsal horn (Coronel et al., 2016), which resulted coincident with the establishment of allodynic behaviors. These observations were in line with our previous results showing increased NR1 mRNA levels and higher number of pNR1 immunoreactive neurons in the dorsal horn of allodynic animals (Coronel et al., 2011), and supports a potential interaction between IL-1β signaling and NMDAR activation. Thus, it is possible that, in these conditions, even basal IL-1β levels could be maintaining the hyperexcitability of local neurons and contributing to neuropathic pain.
Notably, our results also show that at the chronic time-point after SCI, animals receiving progesterone exhibited a significantly lower number of IL-1RI/NR1 positive neurons, and did not display aversive responses to mechanical and cold stimuli (Coronel et al., 2016). Thus, sustained progesterone administration might maintain neuronal IL-1RI expression and/or its membrane localization at control basal levels, diminishing the responsiveness of dorsal neurons to IL-1β and contributing to attenuate pain behaviors. Remarkably, progesterone anti-allodynic effects are maintained even after the treatment has stopped (Coronel et al., 2014), supporting the relevance of targeting several key components of the central injury cascade occurring after SCI.
The spinal cord displays a wide variety of progesterone receptors in both neurons and glial cells, including the classic nuclear receptor (PR), that can act as a ligand-activated transcription factor regulating the expression of target genes or may be delivered to the cell membrane where it interacts with Src/Ras/MAPK signaling pathway (De Nicola et al., 2013)(Figure 1). New membrane progesterone receptors (mPRs) and a membrane progesterone binding protein (PGRMC1) have been also described in spinal cord neurons and glial cells, opening exciting perspectives for pain modulation (Figure 1). Thus, similarly to what has been observed in other nervous system structures, the spinal cord offers multiple sites for progesterone actions.
Figure 1.
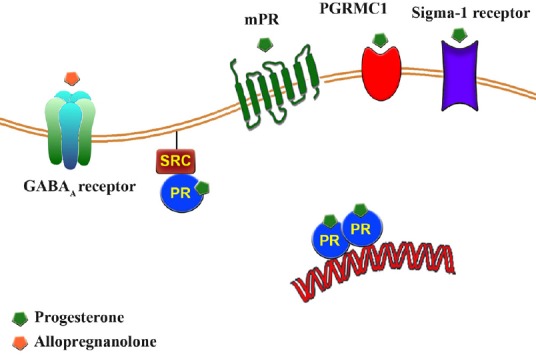
Schematic cartoon showing the multiple receptors and signaling pathways involved in progesterone and allopregnanolone actions.
Progesterone effects are mediated via interaction with an array of progesterone receptors including the classic nuclear receptor (PR), progesterone membrane receptors (mPR) and the membrane-associated protein progesterone receptor-membrane component 1 (PGRMC1). In addition, progesterone acts as a competitive antagonist of sigma-1 receptor. Allopregnanolone does not bind to PR and acts as a potent endogenous positive allosteric modulators of GABAA receptor. Reprinted from Steroids, 110, M.F. Coronel, F. Labombarda and S.L. Gonzalez, “Neuroactive steroids, nociception and neuropathic pain: a flashback to go forward”, 77-87, 2016 Elsevier Inc., with permission from Elsevier (license number 3912030467638).
What is more, progesterone regulation of pro-inflammatory cytokines and enzymes could be mediated through PR modulation of NF-κB transactivation potential. Since NF-κB regulates glia activation and the production of a varied range of pro-inflammatory mediators, these mechanisms could help to explain the anti-inflammatory actions of progesterone in different animal models of pain. Notably, our recent work has shown that a functional PR is required for progesterone modulation of cytokine expression after SCI (Labombarda et al., 2015). Moreover, progesterone has been shown to modulate toll-like receptor/NF-κB signaling pathway, strongly involved in inflammatory events and gliosis after SCI and during demyelinating diseases and pain conditions.
Finally, progesterone can be converted to its reduced metabolite allopregnanolone, a potent modulator of the GABA type A receptor (Figure 1), that is rising as a safe therapy for neuropathic pain. Such many cross-road signaling pathways may be relevant in the control of pain processing and warrant further exploration.
Therefore, should progesterone be considered a promising molecule to prevent chronic pain after SCI? Notwithstanding the recent disappointing results of two large phase 3 clinical trials aimed at testing the neuroprotective effects of progesterone after traumatic brain injury, a recent and more balanced view of the strengths and limitations of these translational studies presumes that this steroid still offers a great promise for the treatment of CNS injuries (Schumacher et al., 2015).
In fact, the overwhelming amount of preclinical evidences bring hope for the use of progesterone as a protective agent after CNS injuries and for the treatment of several neuropathologies such as amyotrophic lateral sclerosis, multiple sclerosis, ischemic stroke (De Nicola et al., 2013; Schumacher et al., 2015) and pain conditions (Coronel et al., 2011, 2014, 2016).
Since a variety of standard therapies usually block individual mechanisms and exhibit reduced effectiveness, progesterone, by targeting simultaneous and major pain-related processes, might represent a valuable tool to prevent chronic pain in the clinical setting. Moreover, several additional attributes make progesterone a potential drug for the treatment of injuries or diseases of the nervous system leading to chronic pain, such as its ability to cross the blood brain/spinal barrier-allowing systemic administration- its low cost and limited adverse effects.
In conclusion, a better understanding of the neuroinflammatory dynamics during progesterone administration might help to improve the design of steroid-based therapies to prevent pain after SCI. However, major challenges for progesterone-based therapy, including the appraisal of pharmacokinetics, bioavailability and sex-related differences in the levels or in the action of progesterone and its metabolites, should be confronted for a useful translational approach intended to prevent the severe neuropathic pain condition afflicting spinal cord injured patients.
All these unanswered questions will certainly open the way to novel research in the field of chronic pain after SCI.
We apologize to all authors whose work could not be cited here due to paper length limitation. We also thank Dr. Florencia Labombarda for the design of the original cartoon reprinted here.
This work was partly supported by grants from CONICET (PIP201-101-00576) and Fundación René Barón.
References
- Coronel MF, Labombarda F, De Nicola AF, Gonzalez SL. Progesterone reduces the expression of spinal cycloxygenase-2 and inducible nitric oxide synthase and prevents allodynia in a rat model of central neuropathic pain. Eur J Pain. 2014;18:348–359. doi: 10.1002/j.1532-2149.2013.00376.x. [DOI] [PubMed] [Google Scholar]
- Coronel MF, Labombarda F, Villar MJ, De Nicola AF, González SL. Progesterone prevents allodynia after experimental spinal cord injury. J Pain. 2011;12:71–83. doi: 10.1016/j.jpain.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Coronel MF, Labombarda F, González SL. Neuroactive steroids, nociception and neuropathic pain: A flashback to go forward. Steroids. 2016;110:77–87. doi: 10.1016/j.steroids.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Coronel MF, Raggio MC, Adler NS, De Nicola AF, Labombarda F, Gonzalez SL. Progesterone modulates pro-inflammatory cytokine expression profile after spinal cord injury: implications for neuropathic pain. J Neuroimmunol. 2016;292:85–92. doi: 10.1016/j.jneuroim.2016.01.011. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Coronel MF, Garay LI, Gargiulo-Monachelli G, Gonzalez Deniselle MC, Gonzalez SL, Labombarda F, Meyer M, Guennoun R, Schumacher M. Therapeutic effects of progesterone in animal models of neurological disorders. CNS Neurol Disord Drug Targets. 2013:12. [PubMed] [Google Scholar]
- Finnerup NB. A review of central neuropathic pain states. Curr Opin Anaesthesiol. 2008;21:586–589. doi: 10.1097/ACO.0b013e32830a4c11. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, González S, Paniagua-Torija B, Lima A, Molina-Holgado E, De Nicola AF, Labombarda F. Progesterone reduces secondary damage, preserves white matter and improves locomotor outcome after spinal cord contusion. J Neurotrauma. 2014;31:857–871. doi: 10.1089/neu.2013.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Boraso M, Zianni E, Corsini E, Galli CL, Cattabeni F, Marinovich M, Di Luca M, Viviani B. Distribution of interleukin-1 receptor complex at the synaptic membrane driven by interleukin-1β and NMDA stimulation. J Neuroinflammation. 2011;18:14. doi: 10.1186/1742-2094-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013;154:S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labombarda F, Jure I, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF. A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J Steroid Biochem Mol Biol. 2015:154. doi: 10.1016/j.jsbmb.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Denier C, Oudinet JP, Adams D, Guennoun R. Progesterone neuroprotection: The background of clinical trial failure. J Steroid Biochem Mol Biol. 2015;160:53–66. doi: 10.1016/j.jsbmb.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Viviani B, Boraso M, Marchetti N, Marinovich M. Perspectives on neuroinflammation and excitotoxicity: a neurotoxic conspiracy? Neurotoxicology. 2014;43:10–20. doi: 10.1016/j.neuro.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Walters ET. Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense. Exp Neurol. 2014:258. doi: 10.1016/j.expneurol.2014.02.001. [DOI] [PubMed] [Google Scholar]


