Light is an electromagnetic stimulus that in mammals is sensed by specialized neurons in the retina. The physiological response to light encompasses two fundamental and different functional outputs: image-forming and non-image forming. The image-forming response is classically known as vision, while the non-image forming responses include the circadian photo-entrainment and the pupillary reflex. Each one is processed by different neurons.
Visual information starts in the outer retina where the classical photoreceptors, cones and rods sense photons thanks to their visual pigments formed by a protein (opsin or rhodopsin) and the chromophore cis-retinal. Photoreceptors transmit the signal to intermediate neurons in the retina and these in turn to the general population of retinal ganglion cells (RGCs). RGCs then convey this signal through their axons to the retinorecipient areas in the brain where the luminous information is used to form the image.
In rats and mice, RGCs can be identified by retrograde tracing from the superior colliculi (SCi), where the majority of RGCs (~98%) project, or from the optic nerve thus tracing their whole population. The tracers of choice for rodents are the fluorescent molecules fluorogold (FG) or hydroxystilbamidine methanesulfonate (OHSt). Another strategy to identify RGCs consists of the immunodetection of proteins that are only expressed by RGCs. Out of the presently known RGC markers, Brn3a is of special interest because is only expressed by those RGCs in charge of transmitting the image-forming information. Brn3a is a member of the Brn3 family of transcription factors, and in the rodent retina is expressed by all RGCs except those that express melanopsin and one half of the ipsilateral projection (Nadal-Nicolas et al., 2012, 2014).
Non-image forming information is conveyed out of the retina by a subtype of RGC that is intrinsically-photosensitive (ipRGC). ipRGCs have the capacity of sensing photons because they express a third photopigment, melanopsin (Provencio et al., 2000). There are several subtypes of ipRGCs, M1 to M5, and recently it has been reported the presence of melanopsin expressing interneurons (Valiente-Soriano et al., 2014). This classification is based on soma size, dendritic stratification in the retina, and projection nuclei in the brain (reviewed in Sand et al., 2012). Using transgenic mice it has been shown that 90% of ipRGCs express immunodetectable amounts of melanopsin (Brown et al., 2010), thus melanopsin immunodetection has become presently the tool of choice to identify ipRGCs in mice and rats. We will refer to ipRGCs identified with melanopsin immunodetection as melanopsin positive RGCs (m+RGCs).
m+RGCs are subject of intensive research, not only because they are involved in basic physiological functions, but because they are idiosyncratic RGCs in their response to injury and neuroprotection (Vidal-Sanz et al., 2015). In adult rats and mice, we have recently reported their total numbers and topographical distribution (Galindo-Romero et al., 2013; Valiente-Soriano et al., 2014), and studied their survival in comparison with the rest of RGCs after different types of insults (Nadal-Nicolas et al., 2015a, b). We showed for the first time that Brn3a and melanopsin expression in RGCs are mutually exclusive (Galindo-Romero et al., 2013; Nadal-Nicolas et al., 2012, 2014, 2015a; Valiente-Soriano et al., 2014). Therefore, Brn3a and melanopsin immunodetectecion offers a unique tool to study in the same retinas but independently both RGC subtypes, image-forming RGCs (Brn3a+RGCs), and non-image forming RGCs (m+RGCs) (Figure 1A). Thus, using this methodology combined with automated routines for quantification and topographical visualization, we have described that m+RGCs represent ~2.4% of the total RGC population in rats (Galindo-Romero et al., 2013; Nadal-Nicolas et al., 2014, 2015b; Valiente-Soriano et al., 2014) and mice (Valiente-Soriano et al., 2014), and that their topography is complementary to the distribution of Brn3a+RGCs (Figure 1B).
Figure 1.
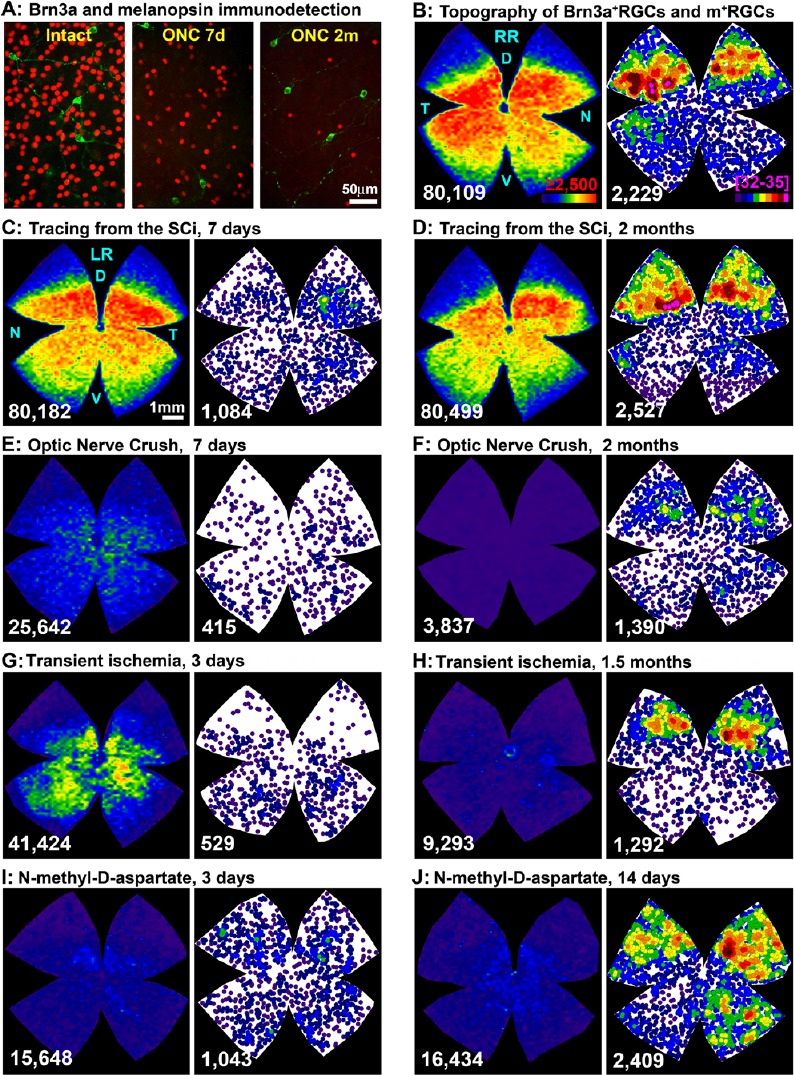
Transient down-regulation of melanopsin expression induced by retrograde tracing or retinal insults.
(A) Magnifications from flat mounted retinas showing the double immunodetection of Brn3a (red) and melanopsin (green) in intact retinas and retinas analyzed 7 days or 2 months after optic nerve crush (ONC). (B) Topography of Brn3a+retinal ganglion cells (RGCs, left, isodensity map) and m+RGCs (right, neighbour map) in the same intact retina. (C–J) Brn3a+RGCs isodensity maps and m+RGCs neighbour maps from the same representative retinas within each experiment: 7 days (C) or 2 months (D) after tracing from the superior colliculi (SCi), 7 days (E) or 2 months after ONC (F), 3 days (G) or 1.5 months (H) after transient ischemia of the retina, and 3 days (I) or 14 days (J) after intravitreal injection of NMDA (100 nM). Isodensity maps colour scale (B, left panel) goes from 0 (purple) to ≥ 2.500 (red) RGCs/mm2, and for neighbour maps (B, right panel) goes from 0 (purple) to 32–35 (bright pink) m+RGC neighbours in a radius of 0.276 mm. At the bottom of each map is shown the number of Brn3a or melanopsin positive RGCs counted in their respective retinas. Bar scales in A and C. RR: Right retina (B); LR: left retina (from C–J). D: Dorsal; V: ventral; N: nasal; T: temporal.
When we first started to characterize the population of m+RGCs in rats, we observed that in traced-retinas fewer m+RGCs were detected when compared to un-traced retinas (Figure 1C). In addition, in traced-retinas melanopsin signal was circumscribed to the somas but missing from the dendrites (see Figure 2 in Nadal-Nicolas et al., 2015a). We thought that this was most probably an immunodetection artefact, an impairment caused by the accumulation of the tracer in the RGC bodies, but several facts argued against this explanation: i) m+RGC immunodetection was worse when the tracer was applied around the optic nerve (60% of total m+RGCs were not detected) than when applied on the surface of the superior colliculi (45%) (see Figure 3 and 4 in Nadal-Nicolas et al., 2015a); ii) the decrease of melanopsin signal was more marked in the dorsal (56% or 75% of m+RGC loss after tracing from the SC or optic nerve, respectively) than in the ventral retina (40% or 60%, respectively) (Figure 1C here, and Figure 5 in Nadal-Nicolas et al., 2015a); iii) the mean soma diameter of the immunoidentified m+RGCs was significantly smaller in traced vs. untraced retinas, indicating either neuronal shrinking or that only small m+RGCs were stained (see Figure 6 in Nadal-Nicolas et al., 2015a); iv) in pigmented rats the loss of melanopsin detection was softer than in albinos (see Figures 3 and 4 in Nadal-Nicolas et al., 2015a); v) both tracers, FG and OHSt, elicited the same response (Nadal-Nicolas et al., 2015a). Furthermore, we have recently analyzed a third tracer, dextran tetramethylrhodamine (DTMR), and the effect on melanopsin immunodetection is the same (unpublished data); vi) in the sham tracing controls, where the same procedure was carried out but only vehicle was applied, melanopsin immunodetection was normal, indicating that the tracer itself was the reason of the impaired melanopsin detection (see Figure 4 in Nadal-Nicolas et al., 2015a), and finally; vii) Brn3a immunodetection is not impaired by tracing (Figure 1C, D). Then, if this was not an artefact and having in mind that most tracers do not persist in the retina, could it be reverted with time? Or, would it be a permanent effect meaning that tracing kills m+RGCs? We focused the subsequent experiments on albino rats because the tracing effect was stronger and albino strains are the most used in research. We analyzed retinas at 2 months after tracing from the SCi instead of the 7 standard days, and we found that effectively the topography, number (Figure 1D), and soma size of m+RGCs was back to that observed in intact un-traced retinas (Nadal-Nicolas et al., 2015a). Thus, we interpret these results as an indication that retrograde tracing does not kill m+RGCs but triggers a transient down-regulation of melanopsin expression in the rat retina that recovers fully with time.
As pointed above, m+RGCs and Brn3a+RGCs behave differently to retinal insults: m+RGCs are more resilient to damage (Nadal-Nicolas et al., 2015b; Vidal-Sanz et al., 2015). Therefore, the next step was to compare their temporal loss after axotomy. Optic nerve crush (ONC) causes the exponential loss of Brn3a+RGCs, and by 7 days only survive ~50% (Nadal-Nicolas et al., 2012, 2014, 2015a, b). This loss was apparently greater for m+RGCs, since at 7 days we could only detect approximately 21% (Nadal-Nicolas et al., 2015a, b). However, to our surprise, while the percent of surviving Brn3a+RGCs 2 months after ONC further decreased to 8%, the percent of detected m+RGCs was higher than at 7 days, close to 50% (Figure 1A, E, F).
To fully exclude an immunodetection artefact we decided to analyse by real-time quantitative PCR the level of the melanopsin mRNA in intact, traced, and axotomized retinas processed at 7 days and 2 months after ONC. Our results showed that indeed, melanopsin mRNA followed the same transient down-regulation than that observed in the anatomical experiments (see Figure 8 in Nadal-Nicolas et al., 2015a).
We next wondered whether this transient down-regulation of melanopsin was a general response to retinal injury and thus, we have also evaluated the response of Brn3a+RGCs and m+RGCs to transient ischemia of the retina (TIR), and to excitotoxicity. The transient ischemic insult was elicited by elevation of the intraocular pressure above normal levels (increase of 65 mmHg above basal) and retinas were analyzed 3 days or 1.5 months later. The excitotoxicity insult was induced by a single intravitreal injection of 100 nM N-methyl-D-aspartate (NMDA), and retinas were analysed 3 or 14 days later. Both studies are yet unpublished, but here we show part of these results (Figure 1G–J). In both models, already at day 3 after the insult there is a significant loss of Brn3a+RGCs (40% of RGCs survival after TIR, and 25% after NMDA) that progresses in the TIR model (20% survival at 1.5 months) but not after NMDA injection. Regarding m+RGCs, 12% or 60% of these cells are detected 3 days after TIR or NMDA, respectively. At later time points, their number increases: 1.5 months after TIR 40% of m+RGCS are detected (Figure 1G–H) and, interestingly, 14 days after NMDA treatment the expression of melanopsin is fully recovered, back to normal values (Figure 1I, J). These results are very exciting because they indicate that melanopsin regulation is associated with the intensity of the insult and/or the course of damage. After TIR, RGC death progresses with time and the recovery of melanopsin signal occurs in a slow and incomplete way. A partial death of m+RGCs might possibly be caused by the ischemic insult as well as by axotomy, and therefore only a partial recovery of the melanopsin signal is detected (Nadal-Nicolas et al., 2015a, b). On the other way, NMDA (at this concentration) produces a quick and simultaneous death of Brn3a+RGCs but not of m+RGCs that are able to recover fully in a quicker way.
In conclusion, our work demonstrates that tracing and melanopsin immunodetection is not a good method to study m+RGCs and the general RGC population, since the down-regulation of melanopsin by retrograde tracing could provide inaccurate results regarding actual numbers of m+RGCs. Brn3a and melanopsin double immunostaining is an excellent alternative to evaluate both cell populations in parallel. Finally, because melanopsin expression does not correlate with m+RGC survival short after retinal injury, we advice to carry out long-term experiments when using melanopsin detection to identify ipRGCs.
This work is supported by the Spanish Ministry of Education and Science SAF2015-67643-P; Spanish Ministry of Economy and Competitiveness ISCIII-FEDER “Una manera de hacer Europa” PI13/00643.
References
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Romero C, Jimenez-Lopez M, Garcia-Ayuso D, Salinas-Navarro M, Nadal-Nicolas FM, Agudo-Barriuso M, Villegas-Perez MP, Aviles-Trigueros M, Vidal-Sanz M. Number and spatial distribution of intrinsically photosensitive retinal ganglion cells in the adult albino rat. Exp Eye Res. 2013;108:84–93. doi: 10.1016/j.exer.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Jimenez-Lopez M, Salinas-Navarro M, Sobrado-Calvo P, Alburquerque-Bejar JJ, Vidal-Sanz M, Agudo-Barriuso M. Whole number distribution and co-expression of brn3 transcription factors in retinal ganglion cells of adult albino and pigmented rats. PLoS One. 2012;7:e49830. doi: 10.1371/journal.pone.0049830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Madeira MH, Salinas-Navarro M, Jimenez-Lopez M, Galindo-Romero C, Ortin-Martinez A, Santiago AR, Vidal-Sanz M, Agudo-Barriuso M. Transient downregulation of melanopsin expression after retrograde tracing or optic nerve injury in adult rats. Invest Ophthalmol Vis Sci. 2015a;56:4309–4323. doi: 10.1167/iovs.15-16963. [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Salinas-Navarro M, Jimenez-Lopez M, Sobrado-Calvo P, Villegas-Perez MP, Vidal-Sanz M, Agudo-Barriuso M. Displaced retinal ganglion cells in albino and pigmented rats. Front Neuroanat. 2014;8:99. doi: 10.3389/fnana.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Sobrado-Calvo P, Jimenez-Lopez M, Vidal-Sanz M, Agudo-Barriuso M. Long-Term Effect of Optic Nerve Axotomy on the Retinal Ganglion Cell Layer. Invest Ophthalmol Vis Sci. 2015b;56:6095–6112. doi: 10.1167/iovs.15-17195. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand A, Schmidt TM, Kofuji P. Diverse types of ganglion cell photoreceptors in the mammalian retina. Prog Retin Eye Res. 2012;31:287–302. doi: 10.1016/j.preteyeres.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente-Soriano FJ, Garcia-Ayuso D, Ortin-Martinez A, Jimenez-Lopez M, Galindo-Romero C, Villegas-Perez MP, Agudo-Barriuso M, Vugler AA, Vidal-Sanz M. Distribution of melanopsin positive neurons in pigmented and albino mice: evidence for melanopsin interneurons in the mouse retina. Front Neuroanat. 2014;8:131. doi: 10.3389/fnana.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Sanz M, Nadal-Nicolas FM, Valiente-Soriano FJ, Agudo-Barriuso M, Villegas-Perez MP. Identifying specific RGC types may shed light on their idiosyncratic responses to neuroprotection. Neural Regen Res. 2015;10:1228–1230. doi: 10.4103/1673-5374.162751. [DOI] [PMC free article] [PubMed] [Google Scholar]


