Keywords: nerve regeneration, electrophysiology, complete nerve amputation, spinal cord, myelin sheath, myelinated fiber, epineurium anatomosis, neural regeneration
Abstract
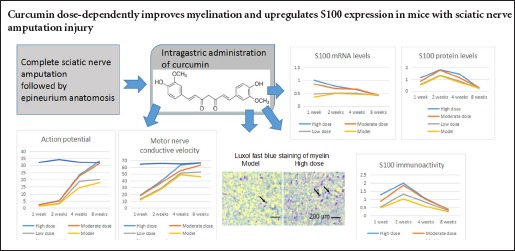
The repair of peripheral nerve injury after complete amputation is difficult, and even with anastomosis, the rapid recovery of nerve function remains challenging. Curcumin, extracted from plants of the genus Curcuma, has been shown to have anti-oxidant and anti-inflammatory properties and to improve sciatic nerve crush injury in rats. Here, we determined whether curcumin had neuroprotective effects following complete peripheral nerve amputation injury. BALB/c mice underwent complete sciatic nerve amputation, followed by an immediate epineurium anastomosis. Mice were intragastrically administered curcumin at doses of 40 (high), 20 (moderate), and 10 mg/kg/d (low) for 1 week. We found that myelin in the mice of the high- and moderate-dose curcumin groups appeared with regular shape, uniform thickness, clear boundary, and little hyperplasia surrounding the myelin. High and moderate doses of curcumin markedly improved both action potential amplitude of the sciatic nerves and the conduction velocity of the corresponding motor neurons, and upregulated mRNA and protein expression of S100, a marker for Schwann cell proliferation, in L4–6 spinal cord segments. These results suggest that curcumin is effective in promoting the repair of complete sciatic nerve amputation injury and that the underlying mechanism may be associated with upregulation of S100 expression.
Introduction
Curcumin, a yellow polyphenol extract derived from plants of the genus Curcuma, has anti-oxidant and anti-inflammatory properties (Menon and Sudheer, 2007; Sandur et al., 2007). Curcumin is most commonly used in Asian countries as a dietary supplement. Curcumin has been shown to be safe in patients with cancer when consumed at a daily dose of up to 8–12 g for 3 months, although mild nausea or diarrhea has been reported (Hsu and Cheng, 2007; Goel et al., 2008).
Curcumin has a wide range of targets in the central nervous system, and its neuroprotective effect involves a variety of mechanisms, including impacting neurotransmitters in the brain, regulating the hypothalamic-pituitary-adrenal axis, improving neural factors and nerve regeneration, and inhibiting neuronal apoptosis through multiple pathways (Liu et al., 2010; Wang et al., 2010, Zhang et al., 2010; Ahmed et al., 2011; Jaisin et al., 2011; Stankowska et al., 2015). Curcumin improves cognition and mood in healthy adults aged 60–85 years (Cox et al., 2015). Studies have shown that curcumin has neuroprotective effects in Alzheimer and Parkinson diseases through multiple pathways (Wang et al., 2010, 2014; Yang et al., 2014; Kuo and Lin, 2015; He, et al., 2016; Huang et al., 2016; Goozee et al., 2016; Song et al., 2016). Curcumin also improves neuropathic pain in rats and chemotherapeutic drug-induced neuropathy (Jeon et al., 2013; Moini Zanjani et al., 2014; Agthong et al., 2015; Babu et al., 2015).
In the periphery, nerve injury may result in neuronal death of the corresponding spinal cord region and dorsal root ganglion, and apoptosis is an important form of neuronal death (Yan et al., 1992). Curcumin has been shown to promote nerve regeneration after sciatic nerve crush injury in either normal or diabetic rats (Ma et al., 2013, 2016). However, the effects of curcumin on the functional recovery of peripheral nerves following complete amputation and the mechanism involved have not been fully elucidated.
S100 proteins, encoded by a family of S100 genes (Marenholz et al., 2004), contain two calcium-binding sites and have highly conserved amino acid sequences in vertebrates (Marenholz et al., 2004). S100 proteins have been implicated in a variety of intracellular and extracellular functions (Donato, 2003), and have been considered markers for traumatic brain injury (Uher and Bob, 2012; Shakeri et al., 2013; Fang et al., 2014; Lesko et al., 2014; Heidari et al., 2015; Krohn et al., 2015; Mazzone et al., 2015; Wolf et al., 2015). In neural regeneration research, S100 proteins indicate the proliferation of Schwann cells (Wang et al., 2015; Wu et al, 2015; Jiang et al., 2016). However, the relationship between curcumin and S100 proteins in the repair of peripheral nerve amputation has been under studied.
The current study explores the effects of curcumin on peripheral nerve regeneration in a BALB/c mouse model of complete sciatic nerve amputation injury and the mechanism involved.
Materials and Methods
Modeling and drug administration
Healthy male BALB/c mice (n = 200) weighing 25 ± 2 g were reproduced and housed in the Basic Medical College Laboratory Animal Center of Jilin University in China under routine conditions (Changchun, Jilin Province, China; Laboratory Animal Production License No. SCXK [Ji] 2007-0003). All experimental animal procedures were conducted in accordance with the ethical standards of the Animal Ethics Committee of Jilin University in China.
The mice were divided into five equal groups (n = 40 each): normal control; untreated model; high-dose curcumin, 40 mg/kg/d; moderate-dose curcumin, 20 mg/kg/d; low-dose curcumin, 10 mg/kg/d. Each group included four time periods (1, 2, 4, and 8 weeks), with 10 mice in each time period.
Generation of the mouse model was performed as described previously (Du et al., 2013; Jia et al., 2014). Briefly, mice were anesthetized and fixed in the prone position. A 2-cm longitudinal incision was made in the unilateral piriformis muscle. Sciatic nerves were separated and amputated completely 0.5 cm below the ischial tuberosity. The epineurium was immediately microsurgically anastomosed. The observation of a continuous epineurium under a stereoscopic microscope indicated the successful generation of the model. The muscle and skin were sutured.
Curcumin (molecular weight, 368.39 Da) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Its structure is shown in Figure 1. The curcumin doses used in the present study were based on that used in previous studies of 10 mg/kg/d in rats (Da Silva Morrone et al., 2016; Huang et al., 2016), which converts to 20 mg/kg/d in mice. Thus, the high dose of curcumin was set at 40 mg/kg/d, the moderate dose at 20 mg/kg/d, and the low dose at 10 mg/kg/d. The drug administration procedures were as follows. Curcumin powder was dissolved in dimethyl sulfoxide (DMSO) and subsequently mixed with 0.9% NaCl, forming a concentration series of 1, 0.5, 0.25 mg/mL for the high-dose, moderate-dose, low-dose curcumin groups, respectively. The final concentration of DMSO in 0.9% NaCl saline was 0.05%. Curcumin was intragastrically administered in a volume of 1 mL daily for 1 week. The model group received the operation and DMSO-saline, but no curcumin. Naïve mice, that underwent no operation and received no drug, served as the normal control.
Figure 1.
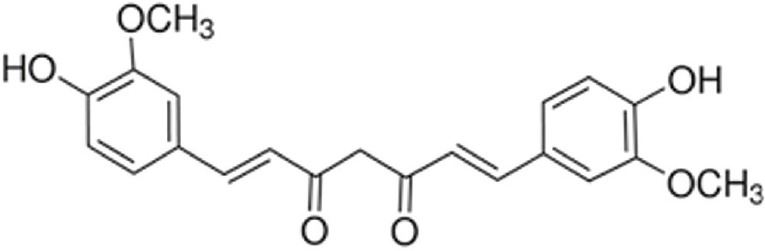
Structure of the curcumin molecule.
Electroneurophysiological testing
At 1, 2, 4, and 8 weeks following curcumin administration, affected sciatic nerves were examined using electromyography with a unit that measures evoked potential response (Keypoint, Medtronic A/C Inc., Skovlunde, Denmark) as described previously (Cao et al., 2012; Jia et al., 2013). Briefly, sciatic nerves were exposed at 23°C. Detection electrodes were placed for recording the action potential amplitude following a single 10 mA current stimulus. The corresponding motor nerve conductive velocity (MNCV) was calculated with the formula MNCV = distance between two stimuli electrodes/action potential incubation period difference.
Sampling of lumber spinal cord segments L4–6
Ten mice in each group were intraperitoneally anesthetized with 3% ketamine 100 mg/kg/d 1, 2, 4, and 8 weeks after curcumin administration. After the electrophysiological tests were completed, the central canal was opened via a midline incision of the posterior vertebral column. The lumbar segments of the spinal cord (L4–6) connecting the injured sciatic nerves were removed and immediately stored in liquid nitrogen for subsequent processing.
Luxol fast blue staining
Sciatic nerves obtained 1, 2, 4, and 8 weeks following curcumin administration were fixed for 3 days in 10% neutral formaldehyde for staining with Luxol fast blue. Briefly, sciatic nerve slices were stained for 12 hours in Luxol fast blue solution at 60°C. The slices were then incubated for 5 minutes in 95% ethanol and for 15 seconds in 0.05% lithium carbonate, and then washed with 70% ethanol and distilled water. After the slices were dehydrated and cleared, they were mounted and coverslipped for microscopic observation. In each slice, five fields at a magnification of 400× were randomly selected for image acquisition. The diameter and number of myelinated fibers were calculated using Image-Pro Plus 6.0 software (Media Cybernetics Inc., Silver Spring, MD, USA).
Immunohistochemical staining
Spinal cord segments L4–6 obtained 1, 2, 4, and 8 weeks after curcumin administration were fixed for 3 days in 10% neutral formaldehyde before being used for immunohistochemical staining of S100. Briefly, L4–6 spinal cord slices were placed in 3% H2O2 for 10 minutes to block endogenous peroxidase, and then boiled for 10 minutes in 0.01 M sodium citrate buffer (pH 6.0). Slices were then blocked for 15 minutes with anti-species serum and washed with 0.01 M phosphate-buffered saline with Tween 20 (pH 7.4). Rabbit anti-mouse S100 polyclonal antibody (1:500; Beyotime Institute of Biotechnology, Najing, Jiangsu Province, China) was added and incubated at 4°C overnight. Biotinylated goat anti-rabbit IgG (1:1,000; Boster, Wuhan, Hubei Province, China) was added for 20 minutes at 37°C. Streptavidin and biotinylated horseradish peroxidase were added for 20 minutes at 37°C. The labeled antigens were visualized using 3,3′-diaminobenzidine (Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China). Brown staining indicated positive protein labeling. Microscopic examination (PM-10A0, Olympus, Beijing, China) was conducted by two pathologists who were blinded to treatments and used Image-Pro Plus 6.0 software (Media Cybernetics Inc.) to analyze the integrated optical density by scanning the stained area.
Quantitative real-time polymerase chain reaction (PCR)
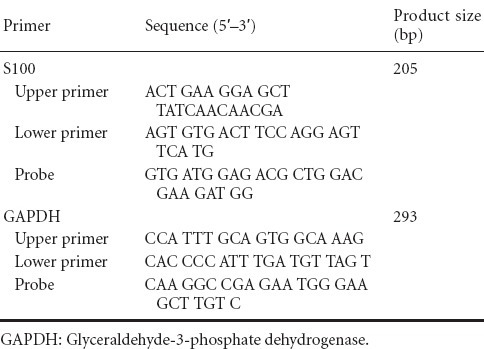
Total RNA was extracted from the L4−6 spinal cord samples obtained 1, 2, 4, and 8 weeks following curcumin administration using the TRIzol reagent method. The cDNA was synthesized using reverse transcriptase with total RNA templates. S100 mRNA was quantified by quantitative real-time PCR using cDNA templates. Primers of S100 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed by Beacon Designer 7 software (Premier Biosoft Inc., Palo Alto, CA, USA) and synthesized by Sangon Bioengineering (Shanghai, China; Table 1). A pair of GAPDH primers served as an internal reference in each reaction system. The reaction conditions were as follows: 95°C for 30 seconds; 58°C for 60 seconds; 72°C for 60 seconds, 40 cycles. The cycle threshold (Ct) values were obtained. The relative mRNA of interest/GAPDH mRNA expression was calculated using 2−ΔΔCt method.
Table 1.
Primers of S100 and GAPDH designed by Beacon Designer 7 software
Western blot assay
At 1, 2, 4, and 8 weeks following curcumin administration, L4–6 spinal cord samples were lysed in ice-cold radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 12% gels and electroblotted onto polyvinylidene fluoride membranes. The membranes were incubated in rabbit anti-mouse S100 polyclonal antibody (diluted 1:5,000 with PBS containing 1% bovine serum albumin; Beyotime Institute of Biotechnology) at 4°C overnight. They were then incubated with horseradish peroxidase-labeled goat anti-rabbit IgG for 1 hour at 37°C and visualized with 3,3′-diaminobenzidine using a western blotting 3,3′-diaminobenzidine kit (Beyotime Institute of Biotechnology). X-ray film was exposed, scanned, and analyzed. Protein levels were represented as the ratio of the relative grayscale value for the protein of interest to that of GAPDH, and analyzed using Image-Pro Plus 6.0 software (Media Cybernetics Inc.).
Statistical analysis
Data, stated as the mean ± SD, were analyzed with SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA). One-way analysis of variance and Dunnett's test were used to compare differences among groups. P < 0.05 was considered statistically significant.
Results
Effects of curcumin on electrophysiological function of the sciatic nerve
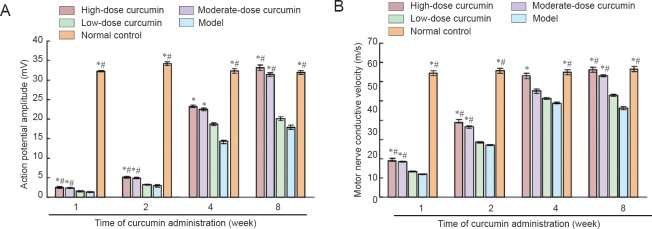
The action potential amplitude and MNCV were examined to determine neural function (Figure 2). After curcumin administration, the action potential amplitude and MNCV of each dose group increased from 1 to 8 weeks. However, the increase in the high- and moderate-dose curcumin groups was greater than that in the low-dose curcumin and model groups at all time points evaluated (1, 2, 4 and 8 weeks; all P < 0.05). At 8 weeks, both the action potential amplitude and the MNCV (1.65-fold and 1.22-fold greater than those of the low-dose curcumin group, respectively) in the high-dose curcumin group had increased to those observed in control animals. These electrophysiological features were not significantly different between the high-dose and moderate-dose curcumin groups, or between the low-dose curcumin and model groups (all P > 0.05). These results suggest that curcumin administration to mice following sciatic nerve injury promoted the functional recovery of sciatic nerves.
Figure 2.
Action potential amplitude (mV) and motor nerve conductive velocity (m/s) at 1, 2, 4, and 8 weeks following curcumin administration.
(A) Action potential amplitude; (B) motor nerve conductive velocity. Data are expressed as the mean ± SD, n = 5. One-way analysis of variance and Dunnett's test were used to analyze the differences among groups. *P < 0.05, vs. model group; #P < 0.05, vs. low-dose curcumin group. High-, moderate-, low-dose curcumin groups: curcumin 40, 20, and 10 mg/kg/d, respectively.
Effects of curcumin on sciatic nerve myelin sheath examined with Luxol fast blue staining
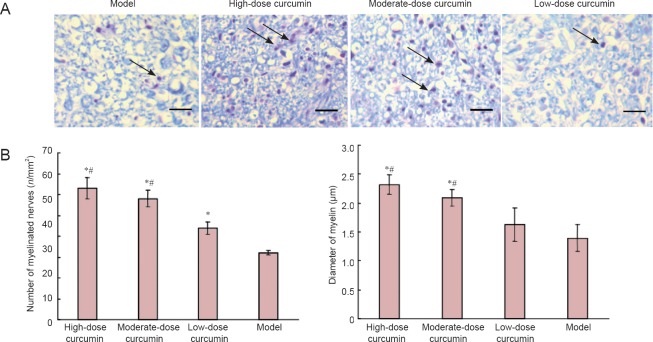
Luxol fast blue staining was performed to detect nerve myelin. The myelin sheath structures examined with Luxol fast blue staining showed no apparent differences among the groups 1, 2, and 4 weeks following curcumin administration. The results at 8 weeks are shown in Figure 3. Myelin is stained blue, while axons remain white with Luxol fast blue. Myelin in the high- and moderate-dose curcumin groups appeared as regular shapes, with a uniform thickness, clear boundary and little hyperplasia surrounding the myelin. By contrast, myelin in the low-dose curcumin group exhibited irregular shapes and thickness, moderately clear borders, and interfascicular hyperplasia of fibrous connective tissues. The myelin in the model group exhibited the worst fiber status. The number and diameter of myelinated fibers 8 weeks following administration were measured using Image-Pro Plus Version 6.0 software (Figure 3). The high- and moderate-dose curcumin groups had myelinated fibers greater in number and larger in diameter than the low-dose curcumin and model groups. The number and diameter of myelinated fibers in the high-dose curcumin group were 1.49-fold and 1.35-fold greater than those in the low-dose curcumin group, indicating marked effects on recovering sciatic nerve myelin.
Figure 3.
Transverse sections of the sciatic nerve myelin sheath showing the number and diameter of myelinated fibers 8 weeks after curcumin administration (Luxol fast blue staining).
(A) Luxol fast blue staining of transverse sections of sciatic nerve myelin. Images were obtained at 40× magnification; scale bar is 200 μm. Arrows indicate the sciatic nerve myelin sheath dyed blue. Myelin in the high- and moderate-dose curcumin groups appears regular and uniform. By contrast, myelin in the low-dose curcumin and model groups appears irregular and exhibits fibrous connective tissues. (B, C) The number (n/mm2) and diameter (μm) of myelinated fibers in the high- and moderate-dose curcumin groups are larger than those in the low-dose curcumin and model groups. Data are expressed as the mean ± SD, n = 5. One-way analysis of variance and Dunnett's test were used to analyze the differences among groups. *P < 0.05, vs. model group; #P < 0.05, vs. low-dose curcumin group. High-, moderate-, low-dose curcumin groups: curcumin 40, 20, 10 mg/kg/d, respectively.
Effect of curcumin on S100 immunoreactivity in L4–6 spinal cord segments detected by immunohistochemical staining
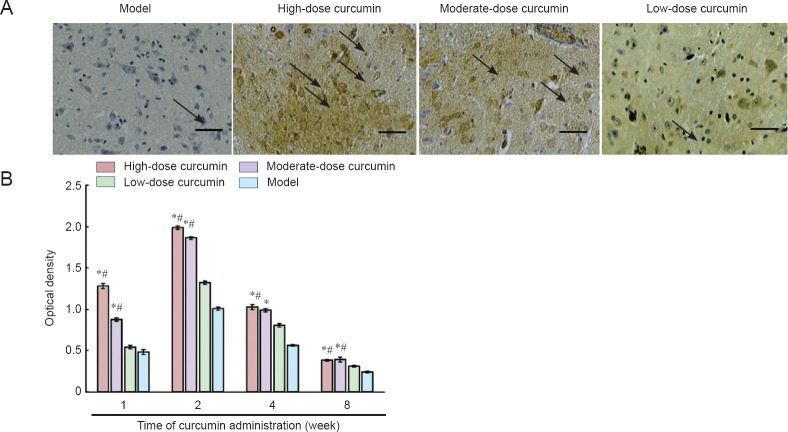
At 1, 2, 4, and 8 weeks following curcumin administration, the density of S-100-positive protein was semiquantitatively described. The integrated optical density values and expression intensity were positively correlated (Figure 4). S100 immunoreactivity was clearly seen under the light microscope as fine brown particles in the cytoplasm. Nuclei were not stained. Starting 1 week after curcumin administration, S100 immunoreactivity in the ipsilateral spinal cord increased. Two weeks after administration, staining density peaked, with the integrated optical density for the high-dose curcumin group 1.79-fold greater than that of low-dose curcumin group. After 4 weeks, the staining intensity decreased. However, for the time period of 1, 2, and 4 weeks examined, the staining intensity in the high- and moderate-dose curcumin groups was significantly stronger than that in the low-dose curcumin and model groups. These results indicated that curcumin at doses of 20 and 40 mg/kg/d increased S100 immunoreactivity.
Figure 4.
S100 immunoreactivity in crosssections of L4–6 spinal cord segments 8 weeks after curcumin administration.
(A) Immunohistochemical staining of S100 protein 2 weeks following curcumin administration at 40× magnification. Scale bar: 200 μm. Arrows indicate positive immunoreactivity for S100, which is seen as fine brown particles in the cytoplasm. (B) Staining intensity. The staining intensity in the high- and moderate-dose curcumin groups is stronger than that in the low-dose curcumin and model groups. Data are expressed as the mean ± SD (n = 5). One-way analysis of variance and Dunnett's tests were used to analyze the difference among groups. *P < 0.05, vs. model group; #P < 0.05, vs. low-dose curcumin group. High-, moderate-, low-dose curcumin groups: curcumin 40, 20, 10 mg/kg/d, respectively.
Effect of curcumin on S100 mRNA levels in L4–6 spinal cord segments detected by quantitative real-time PCR
S100 mRNA was barely detectable in the sciatic nerves of naïve control mice. As shown in Figure 5, after curcumin administration, S100 mRNA levels in L4–6 spinal cord segments increased in each dose group. S100 mRNA levels in the high- and moderate-dose groups peaked at 1 week. The increase in the high- and moderate-dose curcumin groups was significantly greater than those in the low-dose curcumin and model groups (P < 0.05), with S100 mRNA levels for the high-dose curcumin group approximately 2.15-fold greater than those of the low-dose curcumin group. S100 mRNA levels in the low-dose curcumin and model groups increased to peak at 2 weeks. After 8 weeks, S100 mRNA levels were not statistically different among the groups. These results demonstrate that the high- and moderate-dose curcumin groups increased S100 expression.
Figure 5.
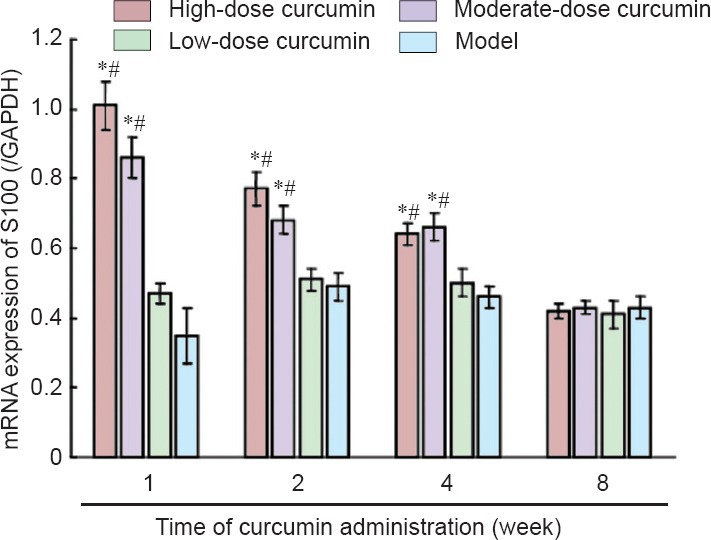
The S100 mRNA expression in L4–6 spinal cord segments 1, 2, 4, and 8 weeks after curcumin administration (quantitative real-time polymerase chain reaction).
Data are expressed as the mean ± SD (n = 5). One-way analysis of variance and Dunnett's test were used to analyze the difference among groups. *P < 0.05, vs. model group; #P < 0.05, vs. low-dose curcumin group. High-, moderate-, low-dose curcumin groups: curcumin 40, 20, 10 mg/kg/d, respectively. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Effect of curcumin on S100 protein levels in L4–6 spinal cord segments detected by western blot assay
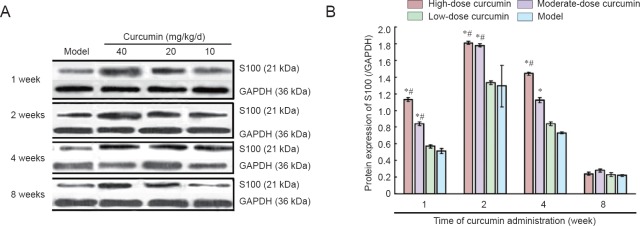
S100 protein levels in each dose group were upregulated and peaked 2 weeks after curcumin administration. The increases in both high- and moderate-dose curcumin groups were significantly greater than those in both low-dose curcumin and model groups (all P < 0.05). At 2 weeks, S100 protein levels in the high-dose curcumin group were 1.4-fold greater than those of the low-dose curcumin group. These results indicated that S100 expression in the high- and moderate-dose curcumin groups was significantly increased compared with that in the low-dose curcumin and model groups. After 8 weeks, S100 protein levels were not statistically different among the groups (all P > 0.05; Figure 6).
Figure 6.
S100 protein expression in L4–6 spinal cord segments 1, 2, 4, and 8 weeks after curcumin administration.
(A) Western blot assay results for the S100 protein; (B) grayscale ratio of S100/GAPDH protein. S100 protein levels peak 2 weeks after curcumin administration in each curcumin group and are dose-dependent for each time period. Data are expressed as the mean ± SD (n = 5). One-way analysis of variance and Dunnett's test were used to analyze the difference among groups. *P < 0.05, vs. model group; #P < 0.05, vs. low-dose curcumin group. High-, moderate-, low-dose curcumin groups: curcumin 40, 20, 10 mg/kg/d, respectively. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Curcumin has been reported to have a wide range of protective effects in the central nervous system (Arora et al., 2011; Khuwaja et al., 2011; Angius et al., 2012; Ozdemir et al., 2012; Kim et al., 2013). However, curcumin effects on peripheral nerve regeneration are not clearly understood. This study examined the effect of curcumin on peripheral nerve regeneration and the mechanism involved. Electrophysiological experiments were performed to examine the functional recovery of the sciatic nerve. Curcumin administration at a high dose of 40 mg/kg/d and a moderate dose of 20 mg/kg/d for 1 week promoted functional recovery of the sciatic nerve following its complete amputation. This outcome was supported by the number and diameter of myelinated nerve fibers observed with Luxol fast blue staining. Both the number and diameter of regenerated nerve myelin in the high- and moderate-dose curcumin groups were larger than those in the low-dose curcumin and model groups. This finding provides direct structural evidence for sciatic nerve recovery at the tissue level following curcumin administration.
The dose of curcumin used to treat neuronal disease and injury varies depending on species, disease categories, or route of administration (Agthong et al., 2015; Da Silva Morrone et al., 2016; Huang et al., 2016; Jangra et al., 2016; Ma et al., 2016; Song et al., 2016; Yu et al., 2016). In a rat model, either intraperitoneal or oral administration of curcumin at doses of 10, 50, or 100 mg/kg/d for approximately 7 weeks have been shown to be effective in attenuating Alzheimer's disease or oxidative stress (Da Silva Morrone et al., 2016; Huang et al., 2016). Studies similar to the current one have used intraperitoneal administration of curcumin at doses of 50, 100, and 300 mg/kg/d for 4 weeks in both diabetic and normal rats, including rats with sciatic nerve crush injury, and found that the treatment improved nerve regeneration in a dose-dependent manner (Ma et al., 2013, 2016).
Using an equivalent dosage conversion, we found that a dose of 10 mg/kg in rats is similar to 20 mg/kg in mice. In the current study, moderate- (20 mg/kg/d) and high-dose (40 mg/kg/d) intragastric administration of curcumin for 1 week promoted nerve regeneration and functional recovery within 8 weeks. These doses were lower than those administered intraperitoneally in rats (50, 100, and 300 mg/kg/d; Ma et al., 2013, 2016) and orally in mice (100, 200, and 400 mg/kg/d; Jangra et al., 2016). These findings indicate that lower curcumin doses of 20 mg/kg/d and 40 mg/kg/d in mice effectively improve peripheral nerve regeneration, whereas a dose of 10 mg/kg in mice might be too low to be effective. Thus, 20 mg/kg approximates the lowest effective dose in mice. Using the equivalent dosage conversion, we found that 20 mg/kg/d in mice is approximately 1.62 mg/kg/d in humans. Although further investigation would be needed, this indicates that a healthy adult weighing 60 kg may benefit from the neuroprotective effect of curcumin by consuming at least 97.2 mg daily. Cox et al. (2015) have reported the beneficial effects of curcumin on cognition and mood in healthy adults aged 60–85 years, and they suggest a daily oral intake of 400 mg.
To explore the molecular mechanism for the curcumin-induced promotion of sciatic nerve regeneration, we determined S100 expression levels. Immunohistochemical staining, quantitative real-time PCR, and western blot assays were performed to detect S100 expression at the tissue, mRNA, and protein levels, respectively. S100 mRNA levels were upregulated, peaking 1 week following curcumin administration, and S100 protein levels peaked at 2 weeks. Thus, S100 mRNA levels peaked 1 week earlier than S100 protein levels. This is likely because mRNA degrades quickly at the same time that protein accumulates. The accumulated S100 protein indicates the proliferation of sciatic nerve Schwann cells. Proliferating Schwann cells promote the sustained regeneration and functional recovery of sciatic nerves (Wang et al., 2015; Wu et al., 2015; Jiang et al., 2016).
S100 expression levels serve as markers for the proliferation of Schwann cells in sciatic nerve regeneration research (Wang et al., 2015; Wu et al., 2015; Jiang et al., 2016). Wu et al. (2015) used S100 protein levels in Schwann cells to indicate the degree and duration of a third-degree hindpaw burn injury affecting the sciatic nerve. Wang et al. (2015) reported that ginsenoside Re greatly increases S100 expression in Schwann cells to promote rat sciatic nerve regeneration, and this is dependent on extracellular signal-regulated kinase 1/2 and c-Jun N-terminal kinase 1/2 signaling pathways. Jiang et al. (2016) detected S100 protein in Schwann cells after frankincense extract administration to rats and demonstrated sciatic nerve regeneration after a crush injury. In the current study, intragastric administration of curcumin daily for 1 week induced S100 immunoreactivity in Schwann cells; the Schwann cells improved myelin structure and functional recovery of the sciatic nerve in 8 weeks.
In conclusion, curcumin administration promoted sciatic nerve regeneration in a dose-dependent manner, in particular at doses of 20 and 40 mg/kg/d, and this regeneration was associated with the upregulation of S100 mRNA, protein, and immunoreactivity. The findings of this study indicate that developing the use of curcumin for improving peripheral nerve injury is warranted.
Footnotes
Funding: This research was supported by the Jilin Provincial Science & Technology Development Project Fund of China, No. 20150311038YY.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Smith T, Pack M, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Agthong S, Kaewsema A, Charoensub T. Curcumin ameliorates functional and structural abnormalities in cisplatin-induced neuropathy. Exp Neurobiol. 2015;24:139–145. doi: 10.5607/en.2015.24.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T, Gilani AH, Hosseinmardi N, Semnanian S, Enam SA, Fathollahi Y. Curcuminoids rescue long-term protentation impaired by amyloid peptide in rat hippocampal slices. Synapse. 2011;65:572–582. doi: 10.1002/syn.20876. [DOI] [PubMed] [Google Scholar]
- Angius D, Wang H, Spinner RJ, Gutierrez-Cotto Y, Yaszemski MJ, Windebank AJ. A systematic review of animal models used to study nerve regeneration in tissue engineered scaffolds. Biomaterials. 2012;33:8034–8039. doi: 10.1016/j.biomaterials.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora V, Kuhad A, Tiwari V, Chopra K. Curcumin ameliorates reserpine-induced pain-depression dyad: behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology. 2011;36:1570–1581. doi: 10.1016/j.psyneuen.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Babu A, Prasanth KG, Balaji B. Effect of curcumin in mice model of vincristine-induced neuropathy. Pharm Biol. 2015;53:838–848. doi: 10.3109/13880209.2014.943247. [DOI] [PubMed] [Google Scholar]
- Cao J, Jiang YW, Sun Y, Zhang XZ, Li LS, Man YH. Immunosuppression of NF-κB by intragastric brazilein in motor neuron of spinal cord connected with injured sciatic nerve in mouse. Biomed Res (India) 2012;23:199–206. [Google Scholar]
- Cox KH, Pipingas A, Scholey AB. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol. 2015;29:642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- Da Silva Morrone M, Schnorr CE, Behr GA, Gasparotto J, Bortolin RC, Moresco KS, Bittencourt L, Zanotto-Filho A, Gelain DP, Moreira JC. Oral administration of curcumin relieves behavioral alterations and oxidative stress in the frontal cortex hippocampus, and striatum of ovariectomized Wistar rats. J Nutr Biochem. 2016;32:181–188. doi: 10.1016/j.jnutbio.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- Du JS, Zhao Q, Zhang YL, Wang Y, Ma M. 7,8-Dihydroxycoumarin may promote sciatic nerve regeneration by suppressing NF-κB expression in mice. Mol Med Rep. 2013;8:1525–1530. doi: 10.3892/mmr.2013.1682. [DOI] [PubMed] [Google Scholar]
- Fang B, Liang M, Yang G, Ye Y, Xu H. Expression of S100A6 in rat hippocampus after traumatic brain injury due to lateral head acceleration. Int J Mol Sci. 2014;15:6378–6390. doi: 10.3390/ijms15046378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Goozee KG, Shah TM, Sohrabi HR, Rainey-Smith SR, Brown B, Verdile G, Martins RN. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer'sdisease. Br J Nutr. 2016;115:449–465. doi: 10.1017/S0007114515004687. [DOI] [PubMed] [Google Scholar]
- He Y, Wang P, Wei P, Feng H, Ren Y, Yang J, Rao Y, Shi J, Tian J. Effects of curcumin on synapses in APPswe/PS1dE9 mice. Int J Immunopathol Pharmacol. 2016;29:217–225. doi: 10.1177/0394632016638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari K, Asadollahi S, Jamshidian M, Abrishamchi SN, Nouroozi M. Prediction of neuropsychological outcome after mild traumatic brain injury using clinical parameters serum S100B protein and findings on computed tomography. Brain Inj. 2015;29:33–40. doi: 10.3109/02699052.2014.948068. [DOI] [PubMed] [Google Scholar]
- Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- Huang HC, Zheng BW, Guo Y, Zhao J, Zhao JY, Ma XW, Jiang ZF. Antioxidative and neuroprotective effects of curcumin in an alzheimer's disease rat model co-treated with intracerebroventricular streptozotocin and subcutaneous D-galactose. J Alzheimers Dis. 2016;52:899–911. doi: 10.3233/JAD-150872. [DOI] [PubMed] [Google Scholar]
- Jaisin Y, Thampithak A, Meesarapee B, Ratanachamnong P, Suksamrarn A. Curcumin I protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis. Neurosci Lett. 2011;489:192–196. doi: 10.1016/j.neulet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Jangra A, Kwatra M, Singh T, Pant R, Kushwah P, Sharma Y, Saroha B, Datusalia AK, Bezbaruah BK. Piperine augments the protective effect of curcumin against lipopolysaccharide-induced neurobehavioral and neurochemical deficits in mice. Inflammation. 2016;39:1025–1038. doi: 10.1007/s10753-016-0332-4. [DOI] [PubMed] [Google Scholar]
- Jeon Y, Kim CE, Jung D, Kwak K, Park S, Lim D, Kim S, Baek W. Curcumin could prevent the development of chronic neuropathic pain in rats with peripheral nerve injury. Curr Ther Res Clin Exp. 2013;74:1–4. doi: 10.1016/j.curtheres.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia YX, Li JR, Mao CY, Yin WT, Jiang RH. Glycyrrhizin improves p75NTR-associated sciatic nerve regeneration in a BALB/c mouse model. Exp Ther Med. 2014;7:1141–1146. doi: 10.3892/etm.2014.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Ma J, Wei Q, Feng X, Qiao L, Liu L, Zhang B, Yu W. Effect of frankincense extract on nerve recovery in the rat sciatic nerve damage model. Evid Based Complement Alternat Med 2016. 2016 doi: 10.1155/2016/3617216. 3617216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuwaja G, Khan MM, Ishrat T, Ahmad A, Raza SS. Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsons’ in rats: behavioral, neurochemical and immunohistochemical studies. Brain Res. 2011;1368:254–263. doi: 10.1016/j.brainres.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Kim JR, Kwon GB, Namgung U, Song KS, Lee JH. Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration. Tissue Eng Part C Methods. 2013;19:233–243. doi: 10.1089/ten.tec.2012.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn M, Dreßler J, Bauer M, Schober K, Franke H, Ondruschka B. Immunohistochemical investigation of S100 and NSE in cases of traumatic brain injury and its application for survival time determination. J Neurotrauma. 2015;32:430–440. doi: 10.1089/neu.2014.3524. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Lin CC. Rescuing apoptotic neurons in Alzheimer's disease using wheat germ agglutinin-conjugated and cardiolipin-conjugated liposomes with encapsulated nerve growth factor and curcumin. Int J Nanomed. 2015;10:2653–2672. doi: 10.2147/IJN.S79528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesko MM, O’Brien SJ, Childs C, Bouamra O, Rainey T, Lecky F. Comparison of several prognostic tools in traumatic brain injury including S100B. Brain Inj. 2014;28:987–994. doi: 10.3109/02699052.2014.890743. [DOI] [PubMed] [Google Scholar]
- Liu H, Li Z, Qiu D, Gu Q, Lei Q, Mao L. The inhibitory effects of different currcuminoids on β-amyloid protein β-amyloid precursor protein and β-site amyloid precursor protein cleaving enzyme 1 in swAPP HEK293 cells. Neurosci Lett. 2010;485:83–88. doi: 10.1016/j.neulet.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Ma J, Liu J, Yu H, Wang Q, Chen Y, Xiang L. Curcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injury. Neurosci Lett. 2013;547:26–31. doi: 10.1016/j.neulet.2013.04.054. [DOI] [PubMed] [Google Scholar]
- Ma J, Yu H, Liu J, Chen Y, Wang Q, Xiang L. Curcumin promotes nerve regeneration and functional recovery after sciatic nerve crush injury in diabetic rats. Neurosci Lett. 2016;610:139–143. doi: 10.1016/j.neulet.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Co. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- Mazzone GL, Nistri A. S100β as an early biomarker of excitotoxic damage in spinal cord organotypic cultures. J Neurochem. 2014;130:598–604. doi: 10.1111/jnc.12748. [DOI] [PubMed] [Google Scholar]
- Menon VP, Sudheer AR. Anti-oxidant and antiinflmmatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- Moini Zanjani T, Ameli H, Labibi F, Sedaghat K, Sabetkasaei M. The attenuation of pain behavior and serum COX-2 concentration by curcumin in a rat model of neuropathic Pain. Korean J Pain. 2014;27:246–252. doi: 10.3344/kjp.2014.27.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir M, Attar A, Kuzu I. Regenerative treatment in spinal cord injury. Curr Stem Cell Res Ther. 2012;7:364–369. doi: 10.2174/157488812802481481. [DOI] [PubMed] [Google Scholar]
- Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- Shakeri M, Mahdkhah A, Panahi F. S100B protein as a post-traumatic biomarker for prediction of brain death in association with patient outcomes. Arch Trauma Res. 2013;2:76–80. doi: 10.5812/atr.8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Nie Q, Li Z, Du G. Curcumin improves neurofunctions of 6-OHDA-induced parkinsonian rats. Pathol Res Pract. 2016;212:247–251. doi: 10.1016/j.prp.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Stankowska DL, Krishnamoorthy VR, Ellis DZ, Krishnamoorthy RR. Neuroprotective effects of curcumin on endothelin-1 mediated cell death in hippocampal neurons. Nutr Neurosci. 2015 doi: 10.1080/1028415X.2015.1119377. doi: 10.1080/1028415X.2015.1119377. [DOI] [PubMed] [Google Scholar]
- Uher T, Bob P. Cerebrospinal fluid S100B levels reflect symptoms of depression in patients with non-inflammatory neurological disorders. Neurosci Lett. 2012;529:139–143. doi: 10.1016/j.neulet.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Wang HM, Zhao YX, Zhang S, Liu GD, Kang WY. PPARgamma agonist curcumin reduces the amyloid-beta-stimulated inflammatory responses in primary astrocytes. J Alzheimers Dis. 2010;20:1189–1199. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- Wang L, Yuan D, Zhang D, Zhang W, Liu C, Cheng H, Song Y, Tan Q. Ginsenoside Re promotes nerve regeneration by facilitating the proliferation, differentiation and migration of Schwann cells via the ERK- and JNK-dependent pathway in rat model of sciatic nerve crush injury. Cell Mol Neurobiol. 2015;35:827–840. doi: 10.1007/s10571-015-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Su C, Li R, Wang H, Ren Y, Sun H, Yang J, Sun J, Shi J, Tian J, Jiang S. Mechanisms and effects of curcumin on spatial learning and memory improvement in APPswe/PS1dE9 mice. J Neurosci Res. 2014;92:218–231. doi: 10.1002/jnr.23322. [DOI] [PubMed] [Google Scholar]
- Wolf H, Frantal S, Pajenda G, Leitgeb J, Sarahrudi K, Hajdu S. Analysis of S100 calcium binding protein B serum levels in different types of traumatic intracranial lesions. J Neurotrauma. 2015;32:23–27. doi: 10.1089/neu.2013.3202. [DOI] [PubMed] [Google Scholar]
- Wu SH, Huang SH, Cheng KI, Chai CY, Yeh JL, Wu TC, Hsu YC, Kwan AL. Third-degree hindpaw burn injury induced apoptosis of lumbar spinal cord ventral horn motor neurons and sciatic nerve and muscle atrophy in rats. Biomed Res Int 2015. 2015 doi: 10.1155/2015/372819. 372819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Elliot J, Seider WD. Brain-derived neurotrophic factor reuses spinal motor neurons from axotomy-induced cell death. Nature. 1992;360:253. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- Yang J, Song S, Li J, Liang T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson's disease rat. Pathol Res Pract. 2014;210:357–362. doi: 10.1016/j.prp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Yu LS, Fan YY, Ye G, Li J, Feng XP, Lin K, Dong M, Wang Z. Curcumin alleviates brain edema by lowering AQP4 expression levels in a rat model of hypoxia-hypercapnia-induced brain damage. Exp Ther Med. 2016;11:709–716. doi: 10.3892/etm.2016.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Browne A, Child D, Tanzi RE. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J Biol Chem. 2010;285:28472–28480. doi: 10.1074/jbc.M110.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]