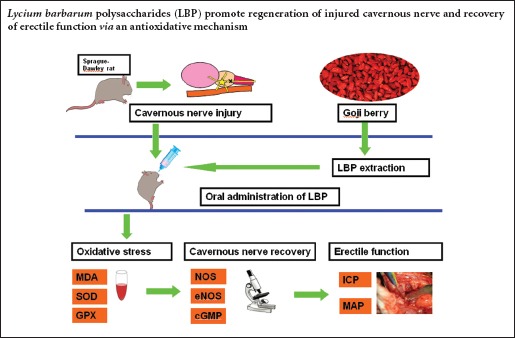
Keywords: nerve regeneration, erectile dysfunction, cavernous nerve, Lycium barbarum polysaccharides, oxidative stress, superoxide dismutase, glutathione peroxidase, malondialdehyde, intracavernous pressure, neural regeneration
Abstract
Polysaccharides extracted from Lycium barbarum exhibit antioxidant properties. We hypothesized that these polysaccharides resist oxidative stress-induced neuronal damage following cavernous nerve injury. In this study, rat models were intragastrically administered Lycium barbarum polysaccharides for 2 weeks at 1, 7, and 14 days after cavernous nerve injury. Serum superoxide dismutase and glutathione peroxidase activities significantly increased at 1 and 2 weeks post-injury. Serum malondialdehyde levels decreased at 2 and 4 weeks. At 12 weeks, peak intracavernous pressure, the number of myelinated axons and nicotinamide adenine dinucleotide phosphate-diaphorase-positive nerve fibers, levels of phospho-endothelial nitric oxide synthase protein and 3-nitrotyrosine were higher in rats administered at 1 day post-injury compared with rats administered at 7 and 14 days post-injury. These findings suggest that application of Lycium barbarum polysaccharides following cavernous nerve crush injury effectively promotes nerve regeneration and erectile functional recovery. This neuroregenerative effect was most effective in rats orally administered Lycium barbarum polysaccharides at 1 day after cavernous nerve crush injury.
Introduction
Technological developments have allowed for the use of nerve-sparing surgery techniques and robotic-assisted laparoscopic techniques in radical prostatectomy. However, in some patients erectile dysfunction persists after radical prostatectomy (Vordermark, 2008; Wang and Eriksson, 2014; Cohen and Glina, 2015; Jacques and Glina, 2015; Kim and Lee, 2015; Putora et al., 2016). In some cases, erectile dysfunction is the result of a cavernous nerve injury during surgery (Vordermark, 2008; Wang and Eriksson, 2014; Cohen and Glina, 2015; Kim and Lee, 2015; Putora et al., 2016). Recovery from erectile dysfunction requires rapid regeneration of the injured cavernous nerve, otherwise injury to the cavernous nerve could lead to fibrosis of the corpus cavernosum (Iacono et al., 2005, 2008). Injury to the cavernous nerve during radical prostatectomy is the main cause of erectile dysfunction, despite modern surgical methods, such as the nerve-sparing technique (Walz et al., 2010; Harris et al., 2013; Khoder et al., 2015; Natali et al., 2015), and return of erectile function is usually a protracted process. A recent study in a radiation-induced cavernous nerve injury model suggests that oxidative stress plays an important role in recovery of the injured nerve (Kimura et al., 2012; Lanza et al., 2012; Wang et al., 2015a). Therefore, reducing oxidative stress may be a potential approach to improve cavernous nerve recovery.
Oxidative stress results from decreased levels of anti-oxidants and increased levels of reactive oxygen species (Zhao et al., 2014). The imbalance between antioxidant capacity and oxidative reactions may directly or indirectly result in cellular damage (Halliwell and Aruoma, 1991). Oxidative stress has been shown to play an inhibitory role in cavernous nerve regeneration (Li et al., 2007), and malondialdehyde (MDA) can be used as a biomarker for oxidative damage. Additionally, superoxide dismutase (SOD), which converts two superoxide anions into a hydrogen peroxide, can be used to measure oxidative stress. Glutathione peroxidase (GPX) is an antioxidant enzyme that converts hydrogen peroxide into water, and it can also directly reduce lipid peroxides (Li et al., 2007; Cheng and Kong, 2011; Shan et al., 2011). Ozkara et al. (2006) demonstrated neurotomy and manipulation of the cavernous nerve can lead to oxidative stress, and erectile function significantly decreases by 3 weeks post-unilateral neurotomy in adult male rats. Lagoda et al. (2007, 2011) reported that both FK506 (a prototypical immunophilin ligand) and sildenafil (the first clinically approved PDE5 inhibitor) increase GPX activity, thereby protecting erectile function. These studies provide evidence that antioxidants reduce the severity of oxidative stress by scavenging reactive oxygen species produced during oxidative stress, thereby protecting erectile function.
Lycium barbarum, which belongs to the solanaceae family, has been widely used in the last decades as a popular functional food with generous biological activities and pharmacological functions. Lycium barbarum is commonly known as the goji berry, and the polysaccharides that are extracted from Lycium barbarum generally consist of six monosaccharides (galactose, glucose, rhamnose, arabinose, mannose, and xylose) that are the active elements involved in Lycium barbarum (Jin et al., 2013). Some studies have used Lycium barbarum polysaccharides (LBP) to treat oxidative stress damage, due to their antioxidant characteristics (Li et al., 2007; Cheng and Kong, 2011; Shan et al., 2011). In these studies, LBP was shown to reduce age-related, exercise-related, and alcohol-induced oxidative stress. Moreover, one study showed that LBP reduced neuronal damage by increasing antioxidant efficacy in a mouse model (Li et al., 2011). However, there are no studies to date that have analyzed the effect of LBP oral administration on cavernous nerve regeneration. We hypothesized that LBP would provide protective effects and promote nerve regeneration against oxidative stress-induced neuronal damage in cavernous nerve injury.
Materials and Methods
Ethics statement and experimental animals
All animal studies were approved by the Medical Animal Care and Welfare Committee of Zhongnan Hospital, Wuhan University, China (approval No. 2012-1806) and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were anesthetized by an intraperitoneal injection of 2% sodium pentobarbital (40 mg/kg). The animals were sacrificed by ether anesthesia.
A total of 200 specific pathogen-free, male, Sprague-Dawley (SD) rats, aged 3 months and weighing 250–300 g, were purchased from the Center for Experimental Animal of Medical College of Wuhan University in China (animal license No. SCXK (E) 2008-0004). The rats were housed in cages in the animal facility, and had free access to standard rat chow and tap water. The rats were equally and randomly divided into the following groups: sham, injured, experimental A, experimental B, and experimental C. In experimental A, B, and C groups, the rats were administered LBP at 1, 7, and 14 days after cavernous nerve injury.
LBP extraction
Lycium barbarum was purchased from a Chinese herb market in Wuhan City, China. LBP was prepared according to previously described methods (Luo et al., 2006) and measured by Professor Qiong Luo from the Department of Nutrition and Food Health, School of Public Health, Wuhan University, China. Briefly, the dried samples were ground, and the powder was dropped into boiling water. The mixture was then allowed to rest for 2 hours at 37°C. Crude polysaccharides were obtained by free-drying the mixture. To remove the lipids, the crude polysaccharides were refluxed in 300 mL solvent (chloroform:methanol = 2:1). The air-dried residue was dropped into 300 mL boiling water and extracted. The desired LBP product was collected and vacuum-dried, after precipitating with 95% alcohol, dehydrated alcohol, and acetone. The polysaccharide content was measured using the phenolsulfuric method (Li et al., 2007; Cheng and Kong, 2011; Shan et al., 2011). Results showed that polysaccharide content in the extract reached 95.74 %.
Establishment of cavernous nerve crush injury models
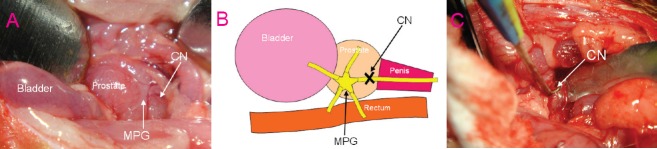
After the rats were anaesthetized, body temperature was maintained isothermically with a heating pad at 37°C. A midline abdominal skin incision provided exposure for the bladder and prostate. The bilateral major pelvic ganglion was dissected from the lateral areas of the prostate with the aid of a surgical microscope (Shanghai Medical Instruments Co., Ltd., Shanghai, China). From the bilateral major pelvic ganglion, cavernous nerves were identified towards the corpus cavernosum (Figure 1A). The bilateral cavernous nerves were dissected and crush injury was performed in the injured group and the three experimental groups (A–C) (Figure 1B). Specifically, the tips of a micro-hemostatic clamp (Cheng-He Microsurgical Instruments Factory, Ningbo, Zhejiang Province, China) were positioned at a 90-degree angle, and the cavernous nerves were crushed for 2 minutes on each side. No additional surgical intervention was performed in the sham group.
Figure 1.
Construction of cavernous nerve crush injury in experimental animal models.
(A) Anatomical location of CN and MPG (× 100). (B) Schematic diagram of CN crush injury. (C) CN stimulation by a bipolar stainless steel electrode (× 100). CN: Cavernous nerve; MPG: major pelvic ganglion.
LBP administration
In experimental A, B, and C groups, the rats were intragastrically administered LBP (10 mg/kg/d) for 2 consecutive weeks at 1, 7, and 14 days post-injury.
Oxidative stress assessment
MDA levels, as well as SOD and GPX activities, were measured in serum at 1, 2, 4, and 12 weeks to assess oxidative stress in vivo. Oxidative stress was measured using MDA, SOD, and GPX assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China) according to the manufacture protocols.
Erectile function measurement
At 12 weeks after model establishment, erectile function was evaluated using intracavernous pressure (ICP) upon unilateral cavernous nerve electrostimulation. The bilateral cavernous nerves were exposed through a repeated lower midline abdominal incision. The overlying skin of the penis was incised, and the right crus of the penis was exposed by ischiocavernous muscle dissection. A 23-gauge scalp-vein needle filled with heparin solution (250 U/mL) was connected to polyethylene-50 tubing that was 20 cm in length. The tubing was carefully inserted into the right crus of the penis to measure ICP. A 22-gauge scalp-vein needle was inserted into the left carotid artery through the side incision of the neck to measure systemic mean arterial pressure (MAP). The unilateral cavernous nerve was directly electrostimulated using bipolar stainless steel. Each probe was 0.2 mm in diameter (Figure 1C), and monophasic rectangular pulses were delivered by a generator. The stimulus parameters were as follows: 1.5 mA amplitude, 20 Hz frequency, 0.2 ms pulse width, and 60-second duration. ICP and MAP were continuously recorded by a data acquisition system (Chengdu TME Technology Co., Ltd., Chengdu, Sichuan Province, China).
Toluidine blue staining
Pathological changes to the cavernous nerve were assessed by toluidine blue staining. After functional measurement, nerve tissue (3–5 mm) was harvested for toluidine blue staining from the bilateral cavernous nerve trunks, distal to the site of the cavernous nerve injury. The samples were fixed for 24 hours with 3% cold glutaraldehyde, dehydrated in 100% alcohol, and fixed for 2 hours in a 1% solution of osmium tetroxide. The samples were infiltrated with a 1:1 mixture of araldite and propylene oxide for 3 hours, and embedded in undiluted epoxy resin. Ultrathin sections (1 μm thick) of the embedded samples were cut on a ultramicrotome (LKB Produkter A.B., Broma, Sweden). The ultrathin sections were then stained for 2 minutes with 1% toluidine blue. Under a light microscope (Olympus BX60, Tokyo, Japan), the stained sections were observed, and recovery of the cavernous nerve was assessed by the number of myelinated axons, which were identified by the most darkly stained area under high-power lens. Three different visual fields were used to quantify the average number of myelinated axons.
Nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase staining
Nitric oxide synthase (NOS)-containing nerve fibers of the dorsal nerves were evaluated by NADPH-diaphorase staining (Wang et al., 2015a). Corpus cavernosum tissues were placed into phosphate-buffered saline (PBS) containing 0.002% picric acid and 2% formaldehyde. After 4 hours of fixation, the tissues were transferred into 30% sucrose at 4°C until further use. Cryosections were cut into 10-μm thick sections, adhered to glass slides, and air dried for 5 minutes. The sections were incubated with a buffer (0.2 mM nitroblue tetrazolium, 0.1 mM NADPH, and 0.2% Triton X-100) for 1 hour at room temperature. The reaction was terminated by rinsing in buffer until a deep blue staining was detected. Coverslips were added to the glass slides with PBS-buffered glycerine (1:9) as the mounting medium. The presence of NADPH-diaphorase-positive nerves was evidenced as a blue stain in the dorsal nerves. The staining pattern was evaluated by quantifying the number of positive nerves with a light microscope (Olympus BX60, Tokyo, Japan).
Western blot assay
Protein expression levels of 3-nitrotyrosine and endothelial nitric oxide synthase (eNOS) were examined by western blot assay. Total protein was extracted from the corpus cavernous tissue using mammalian protein extraction reagent (Pierce, Rockford, IL, USA) and quantified using the bicinchoninic acid protein assay kit (Pierce). The extracted total proteins were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to polyvinyl difluoride membranes. After blocking with 5% fat-free milk for 30 minutes, the membranes were incubated overnight with rabbit anti-rat 3-nitrotyrosine (ab52309; 1:1,000), eNOS (ab5589; 1:1,000), phospho-eNOS (ab51038; 1:500), and β-actin (ab8227; 1:1,000) antibodies (Abcam, Cambridge, UK) at 4°C for 24 hours. The secondary goat anti-rabbit horseradish peroxidase-labeled antibody (1:2,000; Abcam) was added and incubated at 4°C for 1 hour. Finally, the protein bands were visualized using an enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). The gray values were measured using an ultraviolet photometry imaging system (UVP LLC, Upland, CA, USA). The housekeeping gene β-actin was used as an internal control for normalization to determine protein expression levels.
Cyclic guanosine monophosphate (cGMP) level measurement
Corpus cavernosum tissue weighing approximately 50 mg was quickly removed and frozen in liquid nitrogen following detection of MAP and ICP. The frozen tissue was homogenized in cold sodium acetate buffer. cGMP was extracted following the method described below. Each sample was mixed with 2 mL dehydrated alcohol, followed by centrifugation at 3,000 × g for 10 minutes at 4°C. The supernatant was collected and dried at 60°C. Following re-dissolution, all samples were assayed for measurements of cGMP level using the cGMP Immunoassay Kit (K372-100, BioVision, CA, USA) in accordance with manufacture instructions.
Statistical analysis
Data are shown as the mean ± SD. One-way analysis of variance and Tukey post-hoc tests were used for assessing differences between groups. If a P-value was < 0.05, the differences were considered significant. Statistical analyses were performed using SPSS (SPSS 13.0 for Windows; SPSS Inc., Chicago, IL, USA).
Results
LBP effects on oxidative stress in serum in a cavernous nerve crush injury rat model
At 1 week post-injury, there were no differences in serum MDA levels between the five groups (P > 0.05). Serum MDA levels were lowest in the experimental A group at 2 and 4 weeks (P < 0.05). At 2 weeks, serum MDA levels in the experimental B group were less than in the experimental C group (P < 0.05). At 4 weeks, there was no significant difference in serum MDA levels between experimental B and C groups (P > 0.05). At 1 and 2 weeks, SOD and GPX activities in the serum were highest in the experimental A group (P < 0.05). At 2 weeks, SOD and GPX activities in the serum were higher in the experimental B group than in the experimental C group (P < 0.05), but there was no significant difference in SOD and GPX activities in the serum between experimental C, sham, and injured groups (P > 0.05). At 4 weeks, SOD and GPX activities in the serum were higher in the experimental A, B, and C groups than in the sham and injured groups (P < 0.05), but there were no statistically difference between the three experimental groups (P > 0.05). At 12 weeks, the three oxidative stress parameters (MDA, SOD, and GPX) in the serum were not significantly different between the five groups (P > 0.05; Tables 1–3).
Table 1.
Effect of Lycium barbarum polysaccharides on malondialdehyde levels (nmol/mL) in the serum of rats after cavernous nerve crush injury
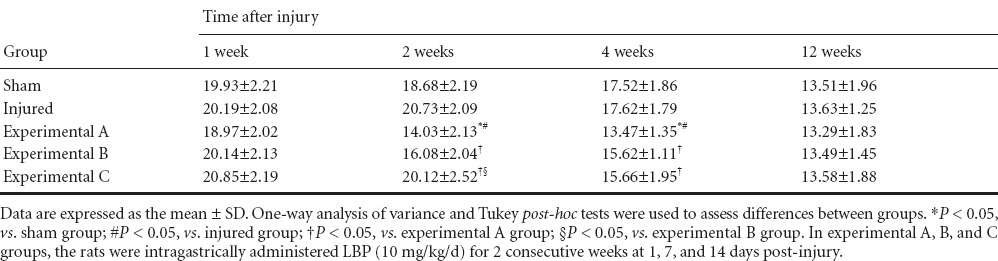
Table 3.
Effect of Lycium barbarum polysaccharides on glutathione peroxidase levels (U/mL) in the serum of rats after cavernous nerve crush injury
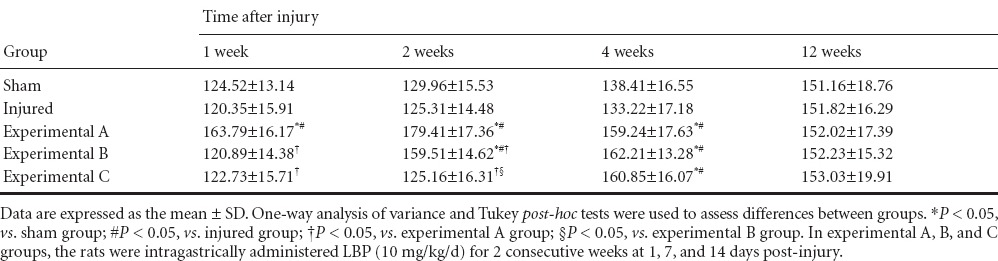
Table 2.
Effect of Lycium barbarum polysaccharides on superoxide dismutase levels (U/mL) in the serum of rats after cavernous nerve crush injury
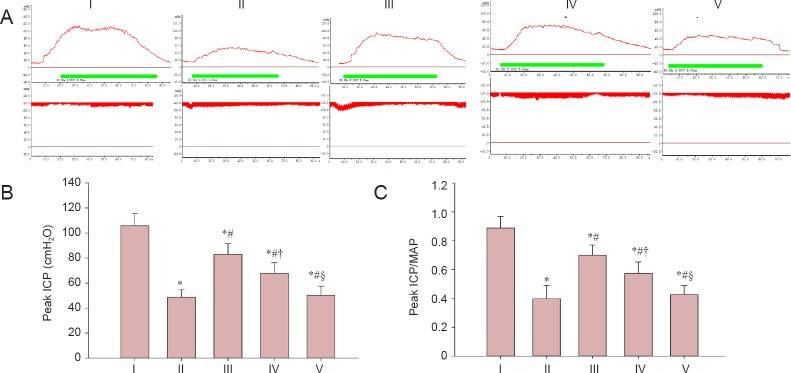
LBP effects on ICP and MAP in a cavernous nerve crush injury rat model
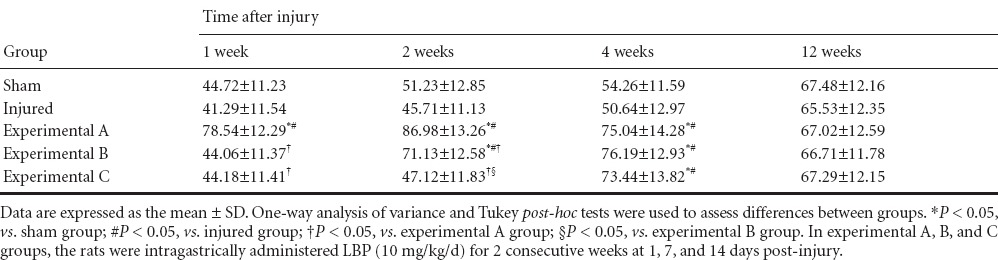
As shown in Figure 2, peak ICP and peak ICP/MAP ratio of rats in the injured group were decreased compared with the sham group (P < 0.05), which represented a state of erectile dysfunction. The peak ICP and peak ICP/MAP ratio were less in the experimental A group than in the sham group (P < 0.05), but higher than in the injured, experimental B, and C groups (P < 0.05) at 12 weeks. Compared with the experimental C group, the peak ICP and peak ICP/MAP ratio were higher in the experimental B group (P < 0.05). There was no significant difference in peak ICP and peak ICP/MAP ratio between the experimental C group and the injured group (P > 0.05).
Figure 2.
Erectile function evaluated by electrical stimulation of cavernous nerve.
(A) ICP and MAP in response to pelvic ganglion stimulation. The red line (above) represents ICP. The green line represents electrostimulation time. The red line (below) represents MAP. (B) Mean peak ICP. (C) Mean peak ICP/MAP ratio. Data are expressed as the mean ± SD. One-way analysis of variance and Tukey post-hoc tests were used to assess differences between groups. *P < 0.05, vs. sham group; #P < 0.05, vs. injured group; †P < 0.05, vs. experimental A group; §P < 0.05, vs. experimental B group. In experimental A, B, and C groups, the rats were intragastrically administered LBP (10 mg/kg/d) for 2 consecutive weeks at 1, 7, and 14 days post-injury. ICP: Intracavernous pressure; MAP: mean arterial pressure. I: Sham; II: injured; III: experimental A; IV: experimental B; V: experimental C.
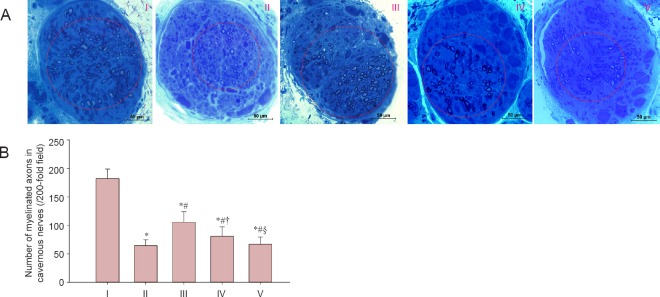
LBP effects on morphology of the cavernous nerve in rats after cavernous nerve crush injury
As shown in Figure 3, the number of myelinated axons in the cavernous nerve was greater in the experimental A group than in the injured, experimental B, and experimental C groups (P < 0.05), but less than in the sham group (P < 0.05). The number of myelinated axons in the cavernous nerve in the experimental B group was greater than in the experimental C group (P < 0.05). However, no significant difference in the number of myelinated axons in the cavernous nerve was detected between the experimental C group and the injured group (P > 0.05).
Figure 3.
LBP effect on cavernous nerve morphology in rats after cavernous nerve crush injury.
(A) Morphology of the cavernous nerve (toluidine blue staining). Circular areas indicate myelinated axons. (B) The number of myelinated axons in cavernous nerves. Data are expressed as the mean ± SD. One-way analysis of variance and Tukey post-hoc tests were used to assess differences between groups. *P < 0.05, vs. sham group; #P < 0.05, vs. injured group; †P < 0.05, vs. experimental A group; §P < 0.05, vs. experimental B group. In experimental A, B, and C groups, the rats were intragastrically administered LBP (10 mg/kg/d) for 2 consecutive weeks at 1, 7, and 14 days post-injury. I: Sham; II: injured; III: experimental A; IV: experimental B; V: experimental C.
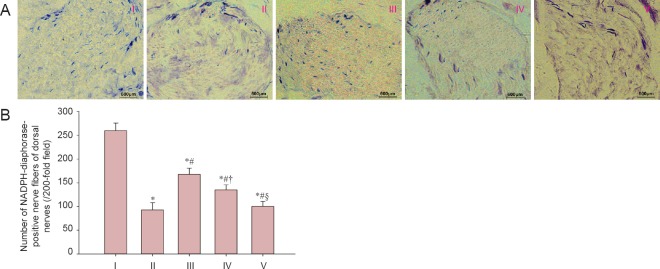
There were more NADPH-diaphorase-positive nerve fibers in dorsal nerves of the experimental A group than in the injured, experimental B, and experimental C groups (P < 0.05). The number of NADPH-diaphorase-positive nerve fibres was significantly higher in the experimental B group compared with the experimental C group (P < 0.05). However, no significant difference in the number of NADPH-diaphorase-positive nerve fibres was determined between the experimental C group and the injured group (P > 0.05; Figure 4).
Figure 4.
LBP effect on NOS-containing nerve fibers in rats after cavernous nerve crush injury.
(A) Images of NADPH-diaphorase staining of dorsal nerves for each group. (B) NADPH-diaphorase-positive nerve fibers. Data are expressed as the mean ± SD. One-way analysis of variance and Tukey post-hoc tests were used to assess differences between groups. *P < 0.05, vs. sham group; #P < 0.05, vs. injured group; †P < 0.05, vs. experimental A group; §P < 0.05, vs. experimental B group. In experimental A, B, and C groups, the rats were intragastrically administered LBP (10 mg/kg/d) for 2 consecutive weeks at 1, 7, and 14 days post-injury. NOS: Nitric oxide synthase; NADPH: nicotinamide adenine dinucleotide phosphate. I: Sham; II: injured; III: experimental A; IV: experimental B; V: experimental C.
LBP effects on 3-nitrotyrosine and eNOS expression in the corpus cavernous tissue of rats after cavernous nerve crush injury
Results from the western blot assay showed significantly decreased 3-nitrotyrosine protein expression in the corpus cavernous tissue of rats in the experimental A and B groups compared with the injured group at 12 weeks (P < 0.05), but expression was greater than in the sham group (P < 0.05). 3-Nitrotyrosine expression in the corpus cavernous tissue of rats in the experimental B group was less than in the experimental C group (P < 0.05), but higher than in the experimental A group (P < 0.05). There was no significant difference in 3-nitrotyrosine expression in the corpus cavernous tissue of rats between the injured group and the experimental C group (P > 0.05; Figure 5).
Figure 5.
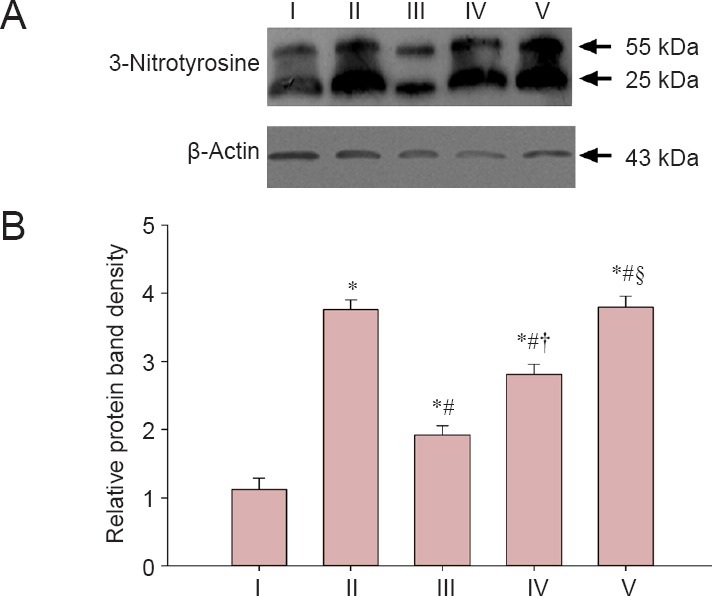
Effect of LBP on the 3-nitrotyrosine expression in the corpus cavernous tissue of rats after cavernous nerve crush injury.
(A) Protein bands of 3-nitrotyrosine and β-actin. (B) The relative gray levels of 3-nitrotyrosine normalized to β-actin. Data are expressed as the mean ± SD. One-way analysis of variance and Tukey post-hoc tests were used to assess differences between groups. *P < 0.05, vs. sham group; #P < 0.05, vs. injured group; †P < 0.05, vs. experimental A group; §P < 0.05, vs. experimental B group. In experimental A, B, and C groups, the rats were intragastrically administered LBP (10 mg/kg/d) for 2 consecutive weeks at 1, 7, and 14 days post-injury. I: Sham; II: injured; III: experimental A; IV: experimental B; V: experimental C.
As shown in Figure 6, there was no significant difference in eNOS expression in the corpus cavernous tissue of rats between the five groups at 12 weeks (P > 0.05). Phospho-eNOS expression in the corpus cavernous tissue of rats significantly increased in the experimental A and B groups compared with the injured group (P < 0.05), although levels were less than in the sham group (P < 0.05). Phospho-eNOS expression in the corpus cavernous tissue of rats was higher in the experimental B group than in the experimental C group (P < 0.05), but less than in the experimental A group (P < 0.05). There was no significant difference in phospho-eNOS expression in the corpus cavernous tissue of rats between the injured group and the experimental C group (P > 0.05).
Figure 6.
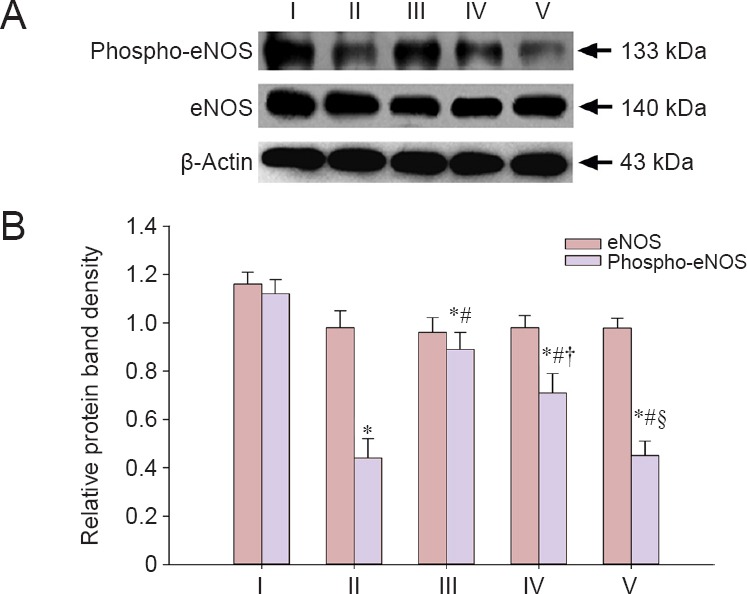
LBP effect on eNOS and phospho-eNOS expression in the corpus cavernous tissue of rats after cavernous nerve crush injury.
(A) Protein bands of eNOS, phospho-eNOS, and β-actin. (B) Relative gray levels of eNOS and phospho-eNOS normalized to β-actin. Data are expressed as the mean ± SD. One-way analysis of variance and Tukey post-hoc tests were used to assess differences between groups. *P < 0.05, vs. sham group; #P < 0.05, vs. injured group; †P < 0.05, vs. experimental A group; §P < 0.05, vs. experimental B group. In experimental A, B, and C groups, the rats were intragastrically administered LBP (10 mg/kg/d) for 2 consecutive weeks at 1, 7, and 14 days post-injury. eNOS: Endothelial nitric oxide synthase. I: Sham; II: injured; III: experimental A; IV: experimental B; V: experimental C.
LBP effects on cGMP levels in the corpus cavernous tissue of rats after cavernous nerve crush injury
Figure 7 depicts cGMP levels in corpus cavernosum tissue of the five groups at 12 weeks. The cGMP levels in the corpus cavernous tissue significantly increased in the experimental A and B groups compared with the injured group (P < 0.05), although levels were less than in the sham group (P < 0.05). The cGMP levels in the corpus cavernous tissue in the experimental B group were higher than in the experimental C group (P < 0.05), but lower than in the experimental A group (P < 0.05). There was no significant different in cGMP levels in the corpus cavernous tissue between the experimental C group and the injured group (P > 0.05).
Figure 7.
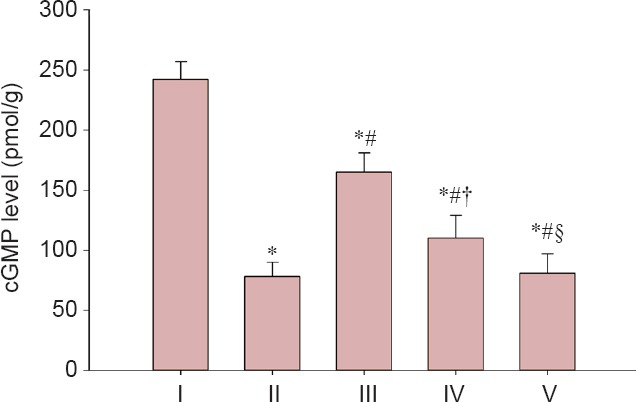
LBP effect on cGMP levels in the corpus cavernous tissue of rats after cavernous nerve crush injury.
Data are expressed as the mean ± SD. One-way analysis of variance and Tukey post-hoc tests were used to assess differences between groups. *P < 0.05, vs. sham group; #P < 0.05, vs. injured group; †P < 0.05, vs. experimental A group; §P < 0.05, vs. experimental B group. In experimental A, B, and C groups, the rats were intragastrically administered LBP (10 mg/kg/d) for 2 consecutive weeks at 1, 7, and 14 days post-injury. cGMP: Cyclic guanosine monophosphate. I: Sham; II: injured; III: experimental A; IV: experimental B; V: experimental C.
Discussion
After radical prostatectomy for clinically localized prostate cancer, erectile dysfunction is a potential surgical complication (Vordermark, 2008; Wang and Eriksson, 2014; Cohen and Glina, 2015; Jacques and Glina, 2015; Kim and Lee, 2015; Putora et al., 2016). Results from the present study suggest that antioxidant administration could prevent oxidative stress-associated damage after cavernous nerve injury. A number of studies have reported that LBP can be used to prevent and treat various diseases, such as hyperlipidemia, diabetes, and cancer (Luo et al., 2004, 2009; Miao et al., 2010; Zhang et al., 2013, 2015; Cai et al., 2015; Tang et al., 2015). LBP also exhibits antioxidant activity in vivo (Amagase et al., 2009; Qiu et al., 2014; Cheng et al., 2015; Ulbricht et al., 2015; Zhu et al., 2015; Zhang et al., 2016). In our study, LBP improved antioxidative functions by promoting antioxidant enzyme activities and the scavenging of excessive free radicals in a rat model of cavernous nerve injury.
Oxidative stress can be evaluated by detecting MDA serum levels, as well as SOD and GPX activity (Li et al., 2007; Cheng and Kong, 2011; Shan et al., 2011; Kimura et al., 2012; Jin et al., 2013; Wang et al., 2015a). To further validate the antioxidative effect of LBP, 3-nitrotyrosine, another oxidative marker, was evaluated in the corpus cavernous tissue. Results showed that cavernous nerve injury led to significantly increased 3-nitrotyrosine formation, which is the end-product of nitric oxide. Expression of 3-nitrotyrosine was decreased in the experimental A and B groups compared with the injured group at 12 weeks. Expression of 3-nitrotyrosine in tissue reflects antioxidant enzyme activity. Therefore, these results demonstrated that LBP exhibited antioxidant efficacy against oxidative stress within the first 2 weeks after cavernous nerve injury. However, the antioxidant efficacy of LBP was not obvious 2 weeks after cavernous nerve injury.
To confirm the neuroprotective effects of LBP, cavernous nerve regeneration was evaluated by toluidine blue staining. The number of myelinated axons of cavernous nerve was more in experimental A group than that in injured group and other two experimental groups. Results provided powerful support for the neuroprotective effect of LBP within the first 2 weeks after cavernous nerve injury. Peak ICP and peak ICP/MAP ratio are well accepted parameters to assess erectile function, and have been used to evaluate erectile dysfunction in many previous animal studies (Ding et al., 2009; Hu et al., 2010; Qiu et al., 2012; Bai and An, 2015; Muniz et al., 2015; Wang et al., 2015c; Gur et al., 2016; Li et al., 2016).
Results for the number of myelinated axons in the cavernous nerve, the number of NADPH-diaphorase-positive nerve fibers, phospho-eNOS protein expression, cGMP levels, peak ICP levels, and peak ICP/MAP ratio are shown in Figure 8. The increased number of myelinated axons in the cavernous nerve histologically reflects nerve regeneration. The nitric oxide/cGMP signal pathway is a key mediator of penile erection, and cGMP is a crucial signal molecule for relaxation of corporal smooth muscle cells within the penis (Komori et al., 2008; Yang et al., 2008; Silva et al., 2015). Immunoassay results showed that the cGMP levels exhibited the same trend as phospho-eNOS expression. Relaxation of corporal smooth muscle can increase blood flow to the penis, which maintains an erection. Therefore, peak ICP and peak ICP/MAP ratio increased.
Figure 8.
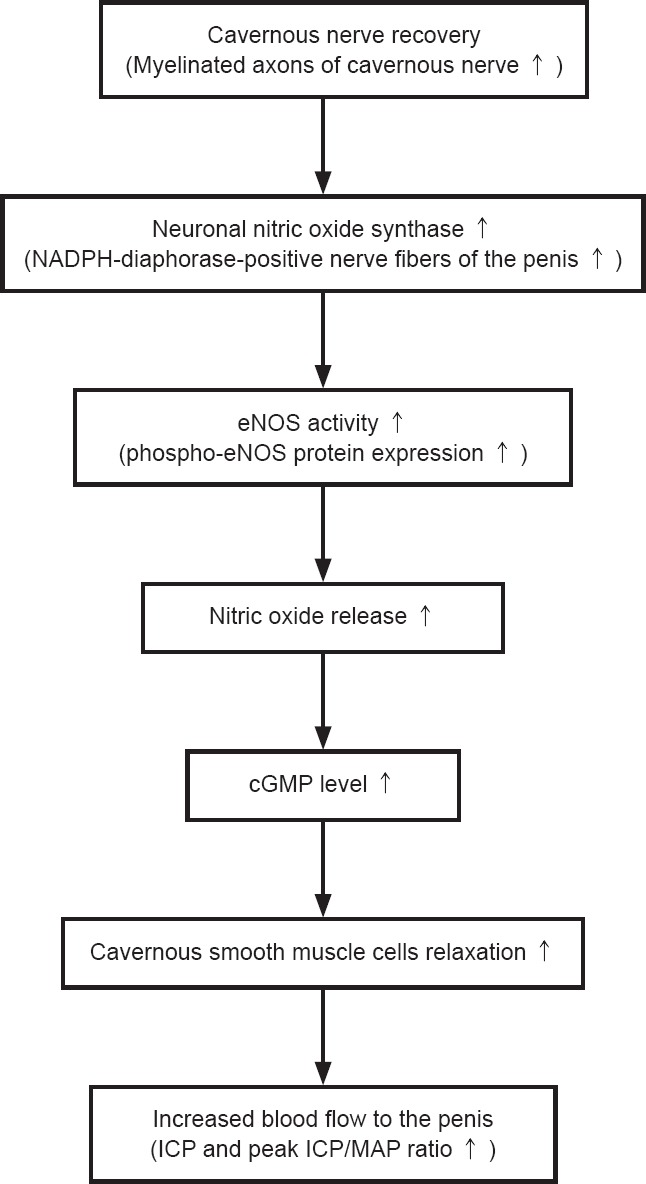
Mechanistic correlation between the number of myelinated axons in the cavernous nerve, the number of NADPH-diaphorase-positive nerve fibers, phospho-eNOS protein expression, cGMP levels, peak ICP levels, and peak ICP/MAP ratio.
NADPH: Nicotinamide adenine dinucleotide phosphate; eNOS: endothelial nitric oxide synthase; cGMP: cyclic guanosine monophosphate; ICP: intracavernous pressure; MAP: mean arterial pressure.
LBP is a complex polysaccharide that consists of acidic heteropolysaccharides and polypeptides (Wang et al., 2015b). Previous studies have demonstrated that polysaccharides exhibit an antioxidant effect by removing reactive oxygen species (Cui et al., 2014; Lei et al., 2014, 2015; Qi et al., 2014; Ren et al., 2014, 2015; Kan et al., 2015; Wang et al., 2016; Wu et al., 2016; Zhuang et al., 2016). A possible mechanism is as follows. (1) Polysaccharides can directly eliminate reactive oxygen species, which are produced via a lipid peroxidation chain reaction. Hydroxyl can combine with hydrogen atoms of the polysaccharide hydrocarbon chain to produce water molecules. (2) Polysaccharides have metal ion complexing actions, and the complexing reaction between metal ions and the hydroxyl of polysaccharide molecules might result in decreased lipid peroxidation. (3) Polysaccharides promote the release of SOD from the cell surface and improve SOD and GPX activity. The highlight of our study is the antioxidative activities of LBP after cavernous nerve crush injury. Some studies also report that LBP has anti-inflammatory action, which might be the result of nuclear factor κB activity down-regulation, which controls transcription of proin-ammatory mediators mediators (Xiao et al., 2012; Teng et al., 2013). Future studies are needed to better understand the molecular mechanisms of anti-inflammatory activities of LBP after cavernous nerve crush injury.
In conclusion, LBP administration improves recovery of the cavernous nerve and restores erectile function within the first 2 weeks after cavernous nerve injury. In the present study, recovery was based on: (a) peak ICP and peak ICP/MAP ratio indicate good functional recovery; (b) an increase in the number of myelinated axons; (c) increased NOS-containing nerve fibers, phospho-eNOS expression, and cGMP level indicate activation of nitric oxide/cGMP signal pathway. Results from the present study suggested that LBP provides a neuroprotective effect by resisting oxidative stress-induced neuronal damage by promoting antioxidant enzyme activity and by scavenging free radicals.
Footnotes
Funding: This work was supported by grants from the National Natural Science Foundation of China, No. 81100492, 81402119 and 81500517; the Natural Science Foundation of Shandong Province of China, No. ZR2014HP055 and ZR2014HL071.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Cooper C, Wysong S, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Amagase H, Sun B, Borek C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr Res. 2009;29:19–25. doi: 10.1016/j.nutres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Bai Y, An R. Resveratrol and sildenafil synergistically improve diabetes-associated erectile dysfunction in streptozotocin-induced diabetic rats. Life Sci. 2015;135:43–48. doi: 10.1016/j.lfs.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Cai H, Liu F, Zuo P, Huang G, Song Z, Wang T, Lu H, Guo F, Han C, Sun G. Practical application of antidiabetic efficacy of lycium barbarum polysaccharide in patients with type 2 diabetes. Med Chem. 2015;11:383–390. doi: 10.2174/1573406410666141110153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Kong H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules. 2011;16:2542–2550. doi: 10.3390/molecules16032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW, He ZX, Sun T, Zhang X, Zhao RJ, Gu L, Cao C, Zhou SF. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther. 2015;9:33–78. doi: 10.2147/DDDT.S72892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DJ, Glina S. Penile rehabilitation after radical prostatectomy. Curr Drug Targets. 2015;16:451–458. doi: 10.2174/1389450116666150202153832. [DOI] [PubMed] [Google Scholar]
- Cui F, Li M, Chen Y, Liu Y, He Y, Jiang D, Tong J, Li J, Shen X. Protective effects of polysaccharides from Sipunculus nudus on Beagle dogs exposed to γ-radiation. PLoS One. 2014;9:e104299. doi: 10.1371/journal.pone.0104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XG, Li SW, Zheng XM, Hu LQ, Hu WL, Luo Y. The effect of platelet-rich plasma on cavernous nerve regeneration in a rat model. Asian J Androl. 2009;11:215–221. doi: 10.1038/aja.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur S, Yurdaarmagan B, Bayatli N, Sikka SC. Effect of short- and long-term sildenafil treatment on erectile dysfunction in rats with partial bladder outlet obstruction. Neurourol Urodyn. 2016;35:108–114. doi: 10.1002/nau.22681. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. Febs Lett. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- Harris CR, Punnen S, Carroll PR. Men with low preoperative sexual function may benefit from nerve sparing radical prostatectomy. J Urol. 2013;190:981–986. doi: 10.1016/j.juro.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Hu W, Cheng B, Liu T, Li S, Tian Y. Erectile function restoration after repair of excised cavernous nerves by autologous vein graft in rats. J Sex Med. 2010;7:3365–3372. doi: 10.1111/j.1743-6109.2010.01730.x. [DOI] [PubMed] [Google Scholar]
- Iacono F, Giannella R, Somma P, Manno G, Fusco F, Mirone V. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005;173:1673–1676. doi: 10.1097/01.ju.0000154356.76027.4f. [DOI] [PubMed] [Google Scholar]
- Iacono F, Prezioso D, Somma P, Chierchia S, Galasso R, Micheli P. Histopathologically proven prevention of post-prostatectomy cavernosal fibrosis with sildenafil. Urol Int. 2008;80:249–252. doi: 10.1159/000127335. [DOI] [PubMed] [Google Scholar]
- Jacques CD, Glina S. Penile rehabilitation after radical prostatectomy. Curr Drug Targets. 2015;16:451–458. doi: 10.2174/1389450116666150202153832. [DOI] [PubMed] [Google Scholar]
- Jin M, Huang Q, Zhao K, Shang P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int J Biol Macromol. 2013;54:16–23. doi: 10.1016/j.ijbiomac.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Kan Y, Chen T, Wu Y, wu J, Wu J. Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int J Biol Macromol. 2015;72:151–157. doi: 10.1016/j.ijbiomac.2014.07.056. [DOI] [PubMed] [Google Scholar]
- Khoder WY, Waidelich R, Seitz M, Becker AJ, Buchner A, Trittschler S, Stief CG. Do we need the nerve sparing radical prostatectomy techniques (intrafascial vs. interfascial) in men with erectile dysfunction? Results of a single-centre study. World J Urol. 2015;33:301–307. doi: 10.1007/s00345-014-1302-9. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SW. Current status of penile rehabilitation after radical prostatectomy. Korean J Urol. 2015;56:99–108. doi: 10.4111/kju.2015.56.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Rabbani ZN, Zodda AR, Yan H, Jackson IL, Polascik TJ, Donatucci CF, Moul JW, Vujaskovic Z, Koontz BF. Role of oxidative stress in a rat model of radiation-induced erectile dysfunction. J Sex Med. 2012;9:1535–1549. doi: 10.1111/j.1743-6109.2012.02716.x. [DOI] [PubMed] [Google Scholar]
- Komori K, Tsujimura A, Takao T, Matsuoka Y, Miyagawa Y, Takada S, Nonomura N, Okuyama A. Nitric oxide synthesis leads to vascular endothelial growth factor synthesis via the NO/cyclic guanosine 3’ 5’-monophosphate (cGMP) pathway in human corpus cavernosal smooth muscle cells. J Sex Med. 2008;5:1623–1635. doi: 10.1111/j.1743-6109.2008.00772.x. [DOI] [PubMed] [Google Scholar]
- Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007;4:908–916. doi: 10.1111/j.1743-6109.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Lagoda G, Xie Y, Sezen SF, Hurt KJ, Liu L, Musicki B, Burnett AL. FK506 neuroprotection after cavernous nerve injury is mediated by thioredoxin and glutathione redox systems. J Sex Med. 2011;8:3325–3334. doi: 10.1111/j.1743-6109.2011.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza C, Raimondo S, Vergani L, Catena N, Sénès F, Tos P, Geuna S. Expression of antioxidant molecules after peripheral nerve injury and regeneration. J Neurosci Res. 2012;90:842–848. doi: 10.1002/jnr.22778. [DOI] [PubMed] [Google Scholar]
- Lei T, Li HF, Fang Z, Lin JB, Wang SS, Xiao LY, Yang F, Liu X, Zhang JJ, Huang ZB, Liao WJ. Polysaccharides from Angelica sinensis alleviate neuronal cell injury caused by oxidative stress. Neural Regen Res. 2014;9:260–267. doi: 10.4103/1673-5374.128218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Yang D, Yeung CM, Yu WY, Chang RC, So KF, Wong D, Lo AC. Lycium barbarum polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal Ischemia/reperfusion injury. PLoS One. 2011;6:e16380. doi: 10.1371/journal.pone.0016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lee YJ, Kim HY, Tan R, Park MC, Kang DG, Lee HS. Beneficial effects of scutellaria baicalensis on penile erection in streptozotocin-induced diabetic rats. Am J Chin Med. 2016;44:305–320. doi: 10.1142/S0192415X1650018X. [DOI] [PubMed] [Google Scholar]
- Li XM, Ma YL, Liu XJ. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J Ethnopharmacol. 2007;111:504–511. doi: 10.1016/j.jep.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Luo Q, Cai Y, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- Luo Q, Li Z, Huang X, Yan J, Zhang S, Cai YZ. Lycium barbarum polysaccharides: Protective effects against heat-induced damage of rat testes and H2O2-induced DNA damage in mouse testicular cells and beneficial effect on sexual behavior and reproductive function of hemicastrated rats. Life Sci. 2006;79:613–621. doi: 10.1016/j.lfs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Luo Q, Li Z, Yan J, Zhu F, Xu RJ, Cai YZ. Lycium barbarum polysaccharides induce apoptosis in human prostate cancer cells and inhibits prostate cancer growth in a xenograft mouse model of human prostate cancer. J Med Food. 2009;12:695–703. doi: 10.1089/jmf.2008.1232. [DOI] [PubMed] [Google Scholar]
- Miao Y, Xiao B, Jiang Z, Guo Y, Mao F, Zhao J, Huang X, Guo J. Growth inhibition and cell-cycle arrest of human gastric cancer cells by Lycium barbarum polysaccharide. Med Oncol. 2010;27:785–790. doi: 10.1007/s12032-009-9286-9. [DOI] [PubMed] [Google Scholar]
- Muniz JJ, Leite LN, De Martinis BS, Carneiro FS, Tirapelli CR. Chronic ethanol consumption induces erectile dysfunction: role of oxidative stress. Life Sci. 2015;141:44–53. doi: 10.1016/j.lfs.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Natali A, Masieri L, Lanciotti M, Giancane S, Vignolini G, Carini M, Serni S. A comparison of different oral therapies versus no treatment for erectile dysfunction in 196 radical nerve-sparing radical prostatectomy patients. Int J Impot Res. 2015;27:1–5. doi: 10.1038/ijir.2014.27. [DOI] [PubMed] [Google Scholar]
- Ozkara H, Alan C, Atukeren P, Uyaner I, Demirci C, Gümüştaş MK, Alici B. Changes of nitric oxide synthase-containing nerve fibers and parameters for oxidative stress after unilateral cavernous nerve resection or manuplation in rat penis. Chin J Physiol. 2006;49:160–166. [PubMed] [Google Scholar]
- Putora PM, Engeler D, Haile SR, Graf N, Buchauer K, Schmid HP, Plasswilm L. Erectile function following brachytherapy external beam radiotherapy, or radical prostatectomy in prostate cancer patients. Strahlenther Onkol. 2016;192:182–189. doi: 10.1007/s00066-015-0928-x. [DOI] [PubMed] [Google Scholar]
- Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G, Wang G, Zhong J. Lycium barbarum polysaccharides protect human lens epithelial cells against oxidative stress-induced apoptosis and senescence. PLoS One. 2014;9:e110275. doi: 10.1371/journal.pone.0110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Chen J, Chen X, Fan Q, Zhang C, Wang D, Li X, Chen X, Chen X, Liu C, Gao Z, Li H, Hu Y. Optimization of selenylation conditions for lycium barbarum polysaccharide based on antioxidant activity. Carbohydr Polym. 2014;103:148–153. doi: 10.1016/j.carbpol.2013.12.032. [DOI] [PubMed] [Google Scholar]
- Qiu X, Villalta J, Ferretti L, Fandel TM, Albersen M, Lin G, Dai Y, Lue TF, Lin CS. Effects of intravenous injection of adipose-derived stem cells in a rat model of radiation therapy-induced erectile dysfunction. J Sex Med. 2012;9:1834–1841. doi: 10.1111/j.1743-6109.2012.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Hu Y, Luo Y, Yang X. Selenium-containing polysaccharides from Ziyang green tea ameliorate high-fructose diet induced insulin resistance and hepatic oxidative stress in mice. Food Funct. 2015;6:3342–3350. doi: 10.1039/c5fo00557d. [DOI] [PubMed] [Google Scholar]
- Ren X, He L, Cheng J, Chang J. Optimization of the solid-state fermentation and properties of a polysaccharide from Paecilomyces cicadae (Miquel) Samson and its antioxidant activities in vitro. PLoS One. 2014;9:e87578. doi: 10.1371/journal.pone.0087578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Zhou J, Ma T, Chai Q. Lycium barbarum polysaccharides reduce exercise-induced oxidative stress. Int J Mol Sci. 2011;12:1081–1088. doi: 10.3390/ijms12021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CN, Nunes KP, Torres FS, Cassoli JS, Santos DM, Almeida Fde M, Matavel A, Cruz JS, Santos-Miranda A, Nunes AD, Castro CH, Machado de Ávila RA, Chávez-Olórtegui C, Láuar SS, Felicori L, Resende JM, Camargos ER, Borges MH, Cordeiro MN, Peigneur S, et al. (2015) PnPP-19, a Synthetic and Nontoxic Peptide Designed from a Phoneutria nigriventer Toxin, Potentiates Erectile Function via NO/cGMP. J Urol. 194:1481–1490. doi: 10.1016/j.juro.2015.06.081. [DOI] [PubMed] [Google Scholar]
- Tang HL, Chen C, Wang SK, Sun GJ. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int J Biol Macromol. 2015;77:235–242. doi: 10.1016/j.ijbiomac.2015.03.026. [DOI] [PubMed] [Google Scholar]
- Teng P, Li Y, Cheng W, Zhou L, Shen Y, Wang Y. Neuroprotective effects of Lycium barbarum polysaccharides in lipopolysaccharide-induced BV2 microglial cells. Mol Med Rep. 2013;7:1977–1981. doi: 10.3892/mmr.2013.1442. [DOI] [PubMed] [Google Scholar]
- Ulbricht C, Bryan JK, Costa D, Culwell S, Giese N, Isaac R, Nummy K, Pham T, Rapp C, Rusie E, Weissner W, Windsor RC, Woods J, Zhou S. An Evidence-Based Systematic Review of Goji (Lycium spp.) by the Natural Standard Research Collaboration. J Diet Suppl. 2015;12:184–240. doi: 10.3109/19390211.2014.904128. [DOI] [PubMed] [Google Scholar]
- Vordermark D. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;359:201. author reply 201-202. [PubMed] [Google Scholar]
- Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, Menon M, Montorsi F, Myers RP, Rocco B, Villers A. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57:179–192. doi: 10.1016/j.eururo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Wang EY, Eriksson HG. Quality of life and functional outcomes 10 years after laparoscopic radical prostatectomy. Ups J Med Sci. 2014;119:32–37. doi: 10.3109/03009734.2013.868560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ding XG, Li SW, Zheng H, Zheng XM, Navin S, Li L, Wang XH. Role of oxidative stress in surgical cavernous nerve injury in a rat model. J Neurosci Res. 2015a;93:922–929. doi: 10.1002/jnr.23545. [DOI] [PubMed] [Google Scholar]
- Wang H, Lau BW, Wang NL, Wang SY, Lu QJ, Chang RC, So KF. Lycium barbarum polysaccharides promotes in vivo proliferation of adult rat retinal progenitor cells. Neural Regen Res. 2015b;10:1976–1981. doi: 10.4103/1673-5374.172315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hu S, Nie S, Yu Q, Xie M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid Med Cell Longev. 2016;2016:5692852. doi: 10.1155/2016/5692852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu C, Li S, Xu Y, Chen P, Liu Y, Ding Q, Wahafu W, Hong B, Yang M. Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. PLoS One. 2015c;10:e0118951. doi: 10.1371/journal.pone.0118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Zhang M, Xie M, Dai Z, Wang X, Hu B, Ye H, Zeng X. Extraction characterization and antioxidant activity of mycelial polysaccharides from Paecilomyces hepiali HN1. Carbohydr Polym. 2016;137:541–548. doi: 10.1016/j.carbpol.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Xiao J, Liong EC, Ching YP, Chang RC, So KF, Fung ML, Tipoe GL. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation. J Ethnopharmacol. 2012;139:462–470. doi: 10.1016/j.jep.2011.11.033. [DOI] [PubMed] [Google Scholar]
- Yang R, Wang J, Chen Y, Sun Z, Wang R, Dai Y. Effect of caffeine on erectile function via up-regulating cavernous cyclic guanosine monophosphate in diabetic rats. J Androl. 2008;29:586–591. doi: 10.2164/jandrol.107.004721. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tang X, Wang F, Zhang Q, Zhang Z. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int J Biol Macromol. 2013;61:270–275. doi: 10.1016/j.ijbiomac.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chen W, Zhao J, Xi W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016;200:230–236. doi: 10.1016/j.foodchem.2016.01.046. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lv X, Wu T, Ma Q, Teng A, Zhang Y, Zhang M. Composition of Lycium barbarum polysaccharides and their apoptosis-inducing effect on human hepatoma SMMC-7721 cells. Food Nutr Res. 2015;59:28696. doi: 10.3402/fnr.v59.28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Fang S, Yuan Y, Guo Z, Zeng J, Guo Y, Tang P, Mei X. Green tea polyphenols protect spinal cord neurons against hydrogen peroxide-induced oxidative stress. Neural Regen Res. 2014;9:1379–1385. doi: 10.4103/1673-5374.137591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Hu S, Zhu L, Ding J, Zhou Y, Li G. Effects of Lycium barbarum polysaccharides on oxidative stress in hyperlipidemic mice following chronic composite psychological stress intervention. Mol Med Rep. 2015;11:3445–3450. doi: 10.3892/mmr.2014.3128. [DOI] [PubMed] [Google Scholar]
- Zhuang C, Xu NW, Gao GM, Ni S, Miao KS, Li CK, Wang LM, Xie HG. Polysaccharide from Angelica sinensis protects chondrocytes from H2O2-induced apoptosis through its antioxidant effects in vitro. Int J Biol Macromol. 2016;87:322–328. doi: 10.1016/j.ijbiomac.2016.02.031. [DOI] [PubMed] [Google Scholar]