Abstract
Objectives
To examine the long-term development of physical and mental health following exposure to a volcanic eruption.
Design
Population-based prospective cohort study.
Setting
In spring 2010, the Icelandic volcano Eyjafjallajökull erupted. Data were collected at 2 time points: in 2010 and 2013.
Participants
Adult residents in areas close to the Eyjafjallajökull volcano (N=1096), divided according to exposure levels, and a non-exposed sample (n=475), with 80% participation rate in 2013.
Main outcome measures
Physical symptoms in the previous year (chronic) and previous month (recent), and psychological distress (General Health Questionnaire-12-item version, GHQ-12), perceived stress (Perceived Stress Scale, PSS-4) and post traumatic stress disorder (PTSD) symptoms (Primary Care PTSD, PC-PTSD).
Results
In the exposed group, certain symptoms were higher in 2013 than in 2010, for example, morning phlegm during winter (OR 2.14; 95% CI 1.49 to 3.06), skin rash/eczema (OR 2.86; 95% CI 1.76 to 4.65), back pain (OR 1.45; 95% CI 1.03 to 2.05) and insomnia (OR 1.53; 95% CI 1.01 to 2.30), in addition to a higher prevalence of regular use of certain medications (eg, for asthma (OR 2.80; 95% CI 1.01 to 7.77)). PTSD symptoms decreased between 2010 and 2013 (OR 0.33; 95% CI 0.17 to 0.61), while the prevalence of psychological distress and perceived stress remained similar. In 2013, the exposed group showed a higher prevalence of various respiratory symptoms than did the non-exposed group, such as wheezing without a cold (high exposure OR 2.35; 95% CI 1.27 to 4.47) and phlegm (high exposure OR 2.81; 95% CI 1.48 to 5.55), some symptoms reflecting the degree of exposure (eg, nocturnal chest tightness (medium exposed OR 3.09; 95% CI 1.21 to 10.46; high exposed OR 3.42; 95% CI 1.30 to 11.79)).
Conclusions
The findings indicate that people exposed to a volcanic eruption, especially those most exposed, exhibit increased risk of certain symptoms 3–4 years after the eruption.
Keywords: Volcanic ash, Respiratory health, Natural disaster, MENTAL HEALTH
Strengths and limitations of this study.
Studies on long-term health effects of volcanic eruptions are rare, let alone follow-up studies on the physical and mental health effects of such a stressful event.
An important strength of this study is that it includes a large population-based cohort exposed to the Eyjafjallajökull eruption, and a matched cohort from a non-exposed population, all contacted at two points in time. Both cohorts yielded a high response rate.
The study is based on multiple self-reported symptoms of physical, especially respiratory, health and various psychometric measurements.
Limitations include reliance on self-reported data and the danger of misclassification that may affect the interpretation of findings.
Introduction
On 14 April 2010, an explosive eruption began in the Icelandic volcano Eyjafjallajökull. It ended 6 weeks later and was classified as a moderate size eruption with index 3 according to the Volcanic Explosive Index (VEI) based on the maximum plume height and magma discharge.1 Ash fall from the eruption is estimated to have been 270 million m3 and the fine grained ash dispersed widely and travelled thousands of kilometres over Europe.1 In addition, resuspension of the ash by wind and human activity in the nearby farmed area raised substantial concerns about the potential long-term effects that inhaling the ash might have on health.2 3
Adverse respiratory symptoms have been reported following exposure to volcanic ash4–11 and clinical examinations have revealed increased cardiovascular disease,6 12 respiratory disease12–15 and mortality.16
Studies on long-term health effects of volcanic eruptions are few, in particular on long-term exposure to volcanic ash and respiratory health.17 It has, however, been reported that long-term exposure to ash fall is associated with increased mortality from respiratory diseases, including chronic obstructive pulmonary disease (COPD) and lung cancer.18
Experiencing a volcanic eruption may affect mental as well as physical health. Psychological symptoms and psychiatric morbidity have been observed in people at different times after natural disasters19 including volcanic eruptions.20 Dose–response patterns after volcanic eruptions have also been reported, with higher rates of psychological distress, such as post traumatic stress disorder (PTSD), among residents who were more exposed to the eruption.21 22
In the months following the Eyjafjallajökull eruption, we conducted a population-based study where residents from exposed areas reported increased prevalence of various physical symptoms.5 In addition, a dose–response pattern was observed, that is, those living closest to the volcano had the highest prevalence of symptoms.5
Using population-based registers, we aimed to examine the association between exposure to the Eyjafjallajökull eruption and the development of self-reported physical and mental health 3–4 years after the eruption ended and compare the results to those obtained 6–9 months after the eruption. On the basis of previous studies, we hypothesised that both physical and mental health symptoms in the exposed population had subsided in the 3–4 years since the 2010 eruption. Furthermore, we hypothesised that highly exposed residents were more likely to report physical and mental symptoms than residents who were less or not at all exposed to the volcanic eruption.
Methods
Study area
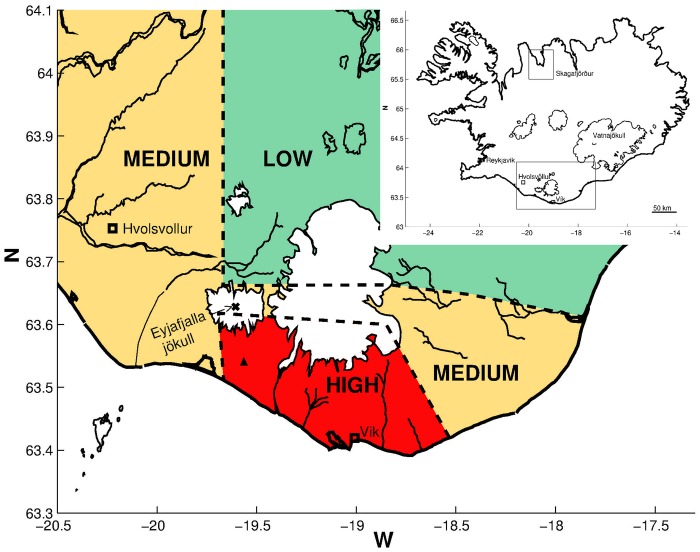
The study area near the Eyjafjallajökull volcano in South Iceland was divided into low, medium and high exposure regions (figure 1), as was done in our previous study.5 To classify different ash exposure levels around the volcano, information based on satellite images of the eruption plume (coarse time resolution) was used as well as information about the emission intensity and observations on the ground.5 In addition, the Environment Agency of Iceland (EAI) provided data on particulate matter less than 10 μm in aerodynamic diameter (PM10) in 2011–2013, from an air monitoring unit at Raufarfell in South Iceland, located in the high exposure region slightly off the road and near a farm, but almost directly 6 km south of the main eruption vent.
Figure 1.
Map of Iceland and study areas (as defined in Carlsen et al5). Inserted map of Iceland in the right corner shows the location of Skagafjörður in Northern Iceland (non-exposed area) and the exposed area in South Iceland. The larger map shows the exposed area with Eyjafjallajökull marked as X, the site of the measuring station with a Δ and the exposed areas divided into low, medium and high exposed areas.
The non-exposed comparison area was in Skagafjörður in North Iceland.
Study population
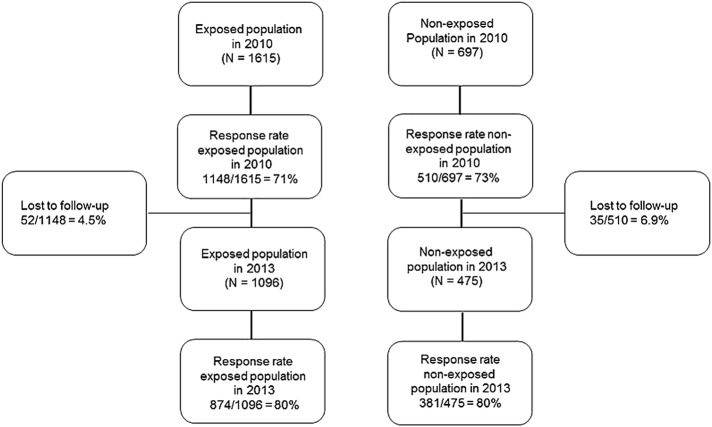
The source population in 2010 included all residents living close to the Eyjafjallajökull volcano (N=2066), a predominantly farming area, where people spend extensive time outdoors. The study population included 1615 residents who were 18–80 years of age, lived in the exposed area during the eruption, could be contacted and spoke Icelandic. The comparison group consisted of a sample of 697 residents of Skagafjörður in Northern Iceland (matched to the exposed population with regard to age, gender and urban/rural habitation). In the first study (6–9 months following the eruption), completed questionnaires were obtained from 71% of the exposed population (1148/1615) and 73% of the non-exposed population (510/697).
Three years later, those who had participated in 2010 were contacted again (December 2013 to February 2014). Fifty-two members of the exposed group and 35 of the non-exposed group could not be found in registers or had moved abroad, leaving the study population with 1096 participants from the exposed area and 475 participants from the non-exposed area.
Data collection
In the 2010 study, participants were given the choice to fill out the questionnaire on paper or online (for details, see Carlsen et al5). Their choice then determined the form of questionnaire they received in 2013. Questionnaires were sent to the exposed population in December 2013 and latest replies to the questionnaires were received in March 2014. The comparison group received questionnaires in February 2014, and the latest replies were received in May 2014. Everyone got a thank you/reminder card a few weeks after the questionnaires had been sent out. Participants who had not replied within a certain time were reminded by email and/or by phone.
All questionnaires had a running number which could be matched with the participant's separately stored ID number to enable the investigation of long-term health effects.
Questionnaires
The questionnaires covered various physical and mental symptoms, as well as demographic information on age, gender, marital status, education level, occupational status, financial situation and household size. Standard questions from the screening part of the European Community Respiratory Health Survey (ECRHS) were used to assess respiratory health and underlying diseases.23 Details on ECRHS questions have been described before.5 Three items from ECRHS (if participant has had COPD, emphysema or chronic bronchitis confirmed by a doctor) were combined into one item (disorders associated with chronic airway obstruction). Psychological distress was measured with the General Health Questionnaire-12-item version (GHQ-12), a well-known and widely used instrument.24 The GHQ-12 is a self-reported screening tool that consists of 12 items, used to assess the severity of mental distress over the past few weeks. A binary cut-off score of >2 was used in the current study. Perceived stress during the last month was evaluated with the Perceived Stress Scale (PSS-4), which is designed to measure the degree to which situations in one’s life are appraised as stressful, unpredictable, uncontrollable and overloading.25 The initial scale includes 14 items, but in our study a validated 4-item version of the PSS-4 list was used, with each of the 4 items scored on a five-point Likert scale (0–4) with a total score ranging from 0 to 16.25 A binary variable was made with a cut-off point at the 90th centile of the PSS-4 scores, identifying individuals in the top 10th centile as having stress symptoms.26 The Primary Care PTSD (PC-PTSD) was used to measure PTSD symptoms and was originally designed to detect the PTSD diagnosis in busy primary care clinics.27 The four-item screening tool reflects four factors that are specific to the PTSD construct: re-experiencing, numbing, avoidance and hyperarousal. A binary cut-off score of >2 was used in our study.
Database and coding
The online survey was built with REDCap (Research Electronic Data Capture).28 Participants answering the questionnaire online were sent a unique link to the online survey by email. Questionnaires on paper were entered into a REDCap database.
Statistical analysis
Demographic characteristics were compared between the exposed population in 2010 and 2013; the exposed and non-exposed populations from 2013 were also compared using the χ2 test. We compared change in the same individuals over time by matching each participant by ID number in the exposed region in 2010 and 2013, resulting in 808 matched pairs who had replied to the same questions on both occasions. To account for the matching variables, conditional logistic regression analysis was used to estimate ORs and 95% CIs for likelihood of experiencing physical and mental symptoms in 2010 and 2013. Conditional logistic regression was used to further analyse those who reported two or more physical symptoms (morning winter phlegm, nocturnal or daytime winter phlegm and/or chronic nocturnal or daytime winter phlegm and skin rash/eczema). Logistic regression analysis was conducted to estimate the relationship between multiple physical symptoms and psychological distress or PTSD symptoms or perceived stress in 2013. Logistic regression was used to calculate ORs and 95% CIs for the association between physical and mental symptoms and residence in the low, medium and high exposure areas and non-exposed area and (2) the low, medium and high exposure areas within the exposed region. These models were adjusted for a priori selected variables; possible confounders were gender, age category, education level and smoking status (never, former, current). Results were considered statistically significant when p values were ≤0.05 or the CIs did not include 1.0. Descriptive statistics for the 24-hour average concentration values of environmental data were performed.
All statistical analyses were performed with RStudio V.0.98.501 (Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2012).
Results
Concentration of ash 2011–2013
PM10 measurements were obtained for 851 of 1095 days (2011–2013); the 244 days missing were mostly in 2013, due to inactive measuring devices. In the high exposure area, the PM10 official health limit of 50 μm/m3 daily average was exceeded 34 times during the whole follow-up period; 6% (18/313) of days measured in 2011, 3% (8/290) in 2012 and 3% (8/248) in 2013. The average 24-hour concentration values were 15.3 μm/m3 in 2011, 15.2 μm3 in 2012 and 15.1 μm3 in 2013. In addition, the maximum 24-hour average PM10 values measured were 307.4 μm/m3 in 2011, 549.9 μm/m3 in 2012 and 152.0 μm/m3 in 2013.
Participants
Valid questionnaires were received from 874 of 1096 in the exposed population (80%) and 381 of 475 (80%) in the non-exposed population (figure 2). Those who did not provide information on gender, age and education were excluded from the analysis (59 from the exposed population and 16 from the non-exposed population). The exposed group differed statistically significantly between 2010 and 2013 regarding age, education, marital status, household size, occupational status and financial status, but was similar regarding gender and smoking status. In 2013, the exposed and non-exposed groups were similar regarding gender, age, education, marital status, financial situation and smoking status (table 1).
Figure 2.
Flow chart of the study population.
Table 1.
Demographic characteristics of the population (South Iceland) exposed to the Eyjafjallajökull volcanic eruption in 2010 and the non-exposed population (North Iceland)
| Exposed 2010 (N=1132) Per cent (n/N) |
Exposed 2013 (N=815) Per cent (n/N) |
Non-exposed 2013 (N=365) Per cent (n/N) |
p Value* Exposed 2010 vs exposed 2013 |
p Value* Exposed 2013 vs non-exposed 2013 |
|
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Gender | 0.17 | 0.81 | |||
| Male | 49.1 (556/1132) | 45.9 (374/815) | 44.9 (164/365) | ||
| Female | 50.9 (576/1132) | 54.1 (441/815) | 55.0 (201/365) | ||
| Age categories | <0.001 | 0.5 | |||
| 18–23 | 11.3 (128/1132) | 4.0 (33/815) | 1.7 (6/363) | ||
| 24–30 | 8.7 (99/1132) | 9.3 (76/815) | 9.4 (34/363) | ||
| 31–40 | 15.4 (174/1132) | 13.1 (107/815) | 14.0 (51/363) | ||
| 41–50 | 20.5 (232/1132) | 19.0 (155/815) | 17.6 (64/363) | ||
| 51–60 | 19.3 (218/1132) | 23.6 (192/815) | 25.6 (93/363) | ||
| 61–70 | 15.7 (178/1132) | 18.4 (150/815) | 19.6 (71/363) | ||
| ≥71 | 9.1 (103/1132) | 12.5 (102/815) | 12.7 (46/363) | ||
| Education | 0.004 | 0.14 | |||
| No formal education | 5.3 (60/1132) | 6.0 (49/815) | 5.5 (20/365) | ||
| Primary education | 36.0 (407/1132) | 29.0 (236/815) | 24.7 (90/365) | ||
| Secondary education | 33.5 (379/1132) | 33.5 (273/815) | 35.1 (128/365) | ||
| Professional or university education | 20.7 (234/1132) | 24.7 (201/815) | 30.1 (110/365) | ||
| Other education* | 4.6 (52/1132) | 6.9 (56/815) | 4.7 (17/365) | ||
| Marital status | 0.006 | 0.85 | |||
| Married or cohabitating | 72.3 (818/1132) | 75.9 (616/812) | 77.5 (282/364) | ||
| Single or divorced | 18.4 (208/1132) | 13.7 (111/812) | 13.7 (50/364) | ||
| Relationship—no cohabitation | 6.9 (78/1132) | 6.2 (50/812) | 5.2 (19/364) | ||
| Widow or widower | 2.5 (28/1132) | 4.3 (35/812) | 3.6 (13/364) | ||
| Household size | 0.009 | <0.001 | |||
| 1 adult | 13.7 (149/1088) | 19.3 (145/751) | 28.7 (96/335) | ||
| 2 adults | 51.3 (558/1088) | 50.1 (376/751) | 51.9 (174/335) | ||
| 3 adults | 21.4 (233/1088) | 18.9 (142/751) | 12.8 (43/335) | ||
| ≥4 adults | 13.6 (148/1088) | 11.7 (88/751) | 6.6 (22/335) | ||
| Occupational status | <0.001 | <0.001 | |||
| Full-time job | 60.8 (679/1117) | 63.1 (487/772) | 57.7 (207/359) | ||
| Part-time job | 9.0 (101/1117) | 9.3 (72/772) | 12.5 (45/359) | ||
| Unemployed | 3.6 (40/1117) | 1.7 (13/772) | 1.4 (5/359) | ||
| Student | 7.0 (78/1117) | 5.3 (41/772) | 5.0 (18/359) | ||
| Homemaker or maternity leave | 8.5 (95/1117) | 3.8 (29/772) | 3.3 (12/359) | ||
| Retired | 6.1 (68/1117) | 12.4 (96/772) | 4.2 (15/359) | ||
| On disability or sick leave | 5.0 (56/1117) | 4.4 (34/772) | 15.9 (57/359) | ||
| Financial situation | 0.038 | 0.38 | |||
| Very good | 4.6 (52/1121) | 5.4 (43/794) | 5.0 (18/360) | ||
| Good | 24.0 (269/1121) | 29.8 (237/794) | 34.7 (125/360) | ||
| Acceptable (making ends meet) | 55.6 (623/1121) | 50.9 (404/794) | 45.8 (165/360) | ||
| Bad | 13.3 (149/1121) | 12.1 (96/794) | 13.3 (48/360) | ||
| Very bad (indebted or bankruptcy) | 2.5 (28/1121) | 1.8 (14/794) | 1.1 (4/360) | ||
| Smoking status 2013 | 0.08 | 0.36 | |||
| Never-smoker | 56.2 (624/1110) | 56.1 (444/791) | 51.7 (186/360) | ||
| Former smoker | 25.9 (288/1110) | 29.3 (232/791) | 32.8 (118/360) | ||
| Current smoker | 17. 8 (198/1110) | 14.5 (115/791) | 15.6 (56/360) | ||
*p Values based on the χ2 test.
Development of health effects in the exposed group between 2010 and 2013
Table 2 presents the development of physical and mental health of the 808 exposed participants answering questionnaires both in 2010 and 2013. In 2013, exposed participants reported a statistically significant increase in respiratory morbidity compared with 2010, such as: morning phlegm during winter (OR 2.14; 95% CI 1.49 to 3.06), winter phlegm during the day or night (OR 2.07; 95% CI 1.32 to 3.26), chronic nocturnal or daytime winter phlegm (OR 2.17; 95% CI 1.33 to 3.56) and regular use of asthma medication (OR 2.80; 95% CI 1.01 to 7.77). The exposed participants in 2013 further reported a statistically significant increase during the last month in skin rash/eczema (OR 2.86; 95% CI 1.76 to 4.65), back pain (OR 1.45; 95% CI 1.03 to 2.05) and myalgia (OR 1.45; 95% CI 1.07 to 2.02). For sleep difficulties, exposed participants in 2013 reported a higher prevalence of insomnia (OR 1.53; 95% CI 1.01 to 2.30), difficulties staying asleep and having trouble falling back asleep (OR 1.58; 95% CI 1.20 to 2.08) and frequently waking up in the middle of the night (OR 1.32; 95% CI 1.01 to 1.73) compared with 2010. In addition, the use of medication for depression (OR 2.20; 95% CI 1.42 to 3.42), any mental morbidity (OR 2.16; 95% CI 1.47 to 3.17) and high blood pressure (OR 2.21; 95% 1.42 to 3.42) was more prevalent among the exposed participants in 2013 than in 2010. Regarding mental symptoms, symptoms of PTSD became less prevalent between the two time points (OR 0.33; 95% CI 0.17 to 0.61), while other mental outcomes remained similar between 2010 and 2013.
Table 2.
Risk of respiratory symptoms, physical and psychological symptoms and drug use in a population exposed to the 2010 Eyjafjallajökull volcanic eruption
| Exposed 2010 (N=808) | Exposed 2013 (N=808) | OR (95% CI)* | |
|---|---|---|---|
| Per cent (n/N) | Per cent (n/N) | ||
| ECRHS (respiratory symptoms) | |||
| Wheezing (past 12 months) | 16.0 (126/788) | 17.7 (137/773) | 1.29 (0.91 to 1.82) |
| If yes, breathlessness at the same time | 8.5 (67/788) | 10.6 (82/773) | 1.56 (1.00 to 2.44) |
| If yes, do you wheeze without a cold? | 10.9 (86/788) | 13.6 (105/773) | 1.48 (0.99 to 2.20) |
| Nocturnal chest tightness (past 12 months) | 12.0 (96/797) | 12.0 (93/777) | 0.98 (0.68 to 1.45) |
| Breathlessness at rest | 7.7 (61/788) | 7.3 (57/773) | 0.79 (0.46 to 1.37) |
| Coughing without a cold | 29.9 (237/793) | 26.4 (207/784) | 0.73 (0.54 to 0.98) |
| Nocturnal cough (past 12 months) | 23.9 (189/791) | 21.7 (169/778) | 0.82 (0.61 to 1.11) |
| Morning winter cough | 12.2 (97/793) | 11.7 (90/769) | 0.95 (0.66 to 1.37) |
| Nocturnal or daytime winter cough | 11.6 (92/790) | 10.1 (77/760) | 0.88 (0.59 to 1.32) |
| If yes, is it chronic?† | 7.6 (60/790) | 7.9 (60/760) | 1.29 (0.81 to 2.06) |
| Morning winter phlegm | 13.9 (109/782) | 19.9 (152/763) | 2.14 (1.49 to 3.06) |
| Nocturnal or daytime winter phlegm | 7.9 (62/783) | 12.3 (92/750) | 2.07 (1.32 to 3.26) |
| If yes, is it chronic?† | 6.6 (52/783) | 10.5 (79/750) | 2.17 (1.33 to 3.56) |
| Dyspnoea | 11.8 (93/791) | 10.3 (79/767) | 0.74 (0.48 to 1.15) |
| Nasal allergy and hay fever | 19.7 (157/797) | 20.0 (152/761) | 1.04 (0.70 to 1.56) |
| Allergic rhinitis | 29.1 (231/793) | 32.7 (248/759) | 1.28 (0.97 to 1.68) |
| Physician diagnosed conditions‡ | |||
| Asthma | 12.1 (96/792) | 12.3 (94/764) | 1.14 (0.64 to 2.05) |
| Asthma diagnosis was confirmed by a doctor | 10.9 (86/792) | 11.4 (87/764) | 1.29 (0.73 to 2.27) |
| Heart disease | 7.2 (57/794) | 8.3 (65/776) | 1.50 (0.76 to 2.95) |
| Disorders associated with chronic airway obstruction§ | 8.9 (72/808) | 9.5 (77/808) | 1.08 (0.77 to 1.51) |
| Other respiratory symptoms¶ | |||
| Shortness of breath | 5.5 (42/769) | 7.5 (55/733) | 1.60 (0.92 to 2.80) |
| Feeling of tightness in chest | 3.3 (25/768) | 4.0 (29/732) | 1.11 (0.58 to 2.15) |
| Cough | 15.2 (118/776) | 12.4 (92/742) | 0.74 (0.51 to 1.06) |
| Phlegm | 11.0 (85/773) | 12.6 (93/741) | 1.20 (0.81 to 1.80) |
| Dry throat | 10.4 (81/778) | 8.8 (65/742) | 0.69 (0.45 to 1.06) |
| Irritation symptoms | |||
| Eye irritation and itch | 11.8 (92/781) | 12.2 (90/739) | 1.04 (0.70 to 1.54) |
| Skin rash/eczema | 6.1 (47/774) | 12.4 (91/734) | 2.86 (1.76 to 4.65) |
| Musculoskeletal symptoms | |||
| Back pain | 18.3 (141/771) | 21.2 (156/735) | 1.45 (1.03 to 2.05) |
| Myalgia | 21.1 (163/773) | 24.5 (185/755) | 1.45 (1.07 to 2.02) |
| Psychological symptoms | |||
| Psychological distress** | 10.2 (66/645) | 8.7 (53/610) | 0.74 (0.43 to 1.27) |
| Perceived stress†† | 6.0 (47/786) | 5.1 (38/743) | 0.82 (0.50 to 1.36) |
| PTSD‡‡ | 6.7 (53/793) | 3.0 (23/766) | 0.33 (0.17 to 0.61) |
| Sleep difficulties | |||
| Insomnia¶ | 13.1 (101/771) | 16.1 (121/750) | 1.53 (1.01 to 2.30) |
| Difficulty falling asleep (yes: sometimes, often and always/every night) | 10.3 (81/789) | 12.7 (99/782) | 1.53 (0.99 to 2.36) |
| Difficulty staying asleep and having trouble falling back asleep (yes: sometimes, often and always/every night) | 35.2 (278/790) | 41.1 (315/767) | 1.58 (1.20 to 2.08) |
| Feeling well rested after a night’s sleep (yes: often and always/every night) | 48.9 (384/786) | 48.6 (375/772) | 1.01 (0.76 to 1.34) |
| Frequently wake up in the middle of the night (yes: sometimes, often and always/every night) | 53.2 (422/793) | 56.7 (442/780) | 1.32 (1.01 to 1.73) |
| Regular drugs use (at least once per week) | |||
| Asthma medication | 3.3 (27/808) | 4.5 (36/808) | 2.80 (1.01 to 7.77) |
| Analgesics | 9.3 (75/808) | 9.8 (79/808) | 1.10 (0.71 to 1.70) |
| Any drug for depression, anxiety, sleeping and other mental symptoms | 28.2 (228/808) | 33.7 (272/808) | 2.16 (1.47 to 3.17) |
| Drugs for other mental symptoms | 0.37 (3/808) | 0.5 (4/808) | 1.50 (0.25 to 8.98) |
| Depression medication | 5.3 (43/808) | 6.7 (54/808) | 2.20 (1.42 to 3.42) |
| Anxiety/sedative medication | 4.5 (36/808) | 5.3 (43/808) | 1.39 (0.76 to 2.54) |
| Hypnotics | 7.3 (59/808) | 6.3 (51/808) | 1.65 (0.90 to 3.00) |
| Blood pressure-lowering medication | 23.3 (188/808) | 27.6 (223/808) | 2.21 (1.42 to 3.42) |
*OR and 95% CI from conditional logistic regression.
†Chronic: more than 3 months/year.
‡Answering ‘yes’ to ‘Has physician ever told you that you had (the disease)?’
§Three items from ECRHS (if participant has had COPD, emphysema or chronic bronchitis confirmed by a doctor) were combined into one item (disorders associated with chronic airway obstruction).
¶Answers ‘yes to a moderate extent’ or ‘yes, to much extent’ to the question ‘Have any of the following symptoms disturbed your daily activities during the last month’.
**Psychological distress was derived from GHQ-12 referring to ‘the previous weeks’, using a binary cut-off score of >2.
††Perceived stress was derived from PSS-4 referring to ‘the recent month’ using a binary cut-off score of 90th centile.
‡‡Primary care PTSD was derived from PC-PTSD referring to ‘the recent month’ using a binary cut-off score of >2.
COPD, chronic obstructive pulmonary disease; ECRHS, European Community Respiratory Health Survey; GHQ-12, General Health Questionnaire-12-item version; PC-PTSD, Primary Care PTSD; PSS-4, Perceived Stress Scale.
Similar analysis for the non-exposed group between 2010 and 2013 indicated no statistically significant changes in symptoms in table 2 (data not shown), except for nocturnal or daytime winter phlegm (OR 2.79; CI 1.16 to 6.94) and skin rash/eczema (OR 3.04; CI 1.19 to 8.54).
Health in 2013 among exposed and non-exposed
Respiratory health
In 2013, a higher prevalence of various respiratory symptoms was observed in the exposed group compared with the non-exposed group, such as wheezing (medium exposure OR 1.88; 95% CI 1.13 to 3.21; high exposure OR 2.20; 95% CI 1.29 to 3.83), wheezing without a cold (high exposure OR 2.35; 95% CI 1.27 to 4.47), coughing without a cold (medium exposure OR 1.64; 95% CI 1.07 to 2.55; high exposure OR 2.01; 95% CI 1.28 to 2.44), morning winter phlegm (medium exposure OR 1.89; 95% CI 1.14 to 3.21; high exposure OR 1.94; 95% CI 1.14 to 3.38), having any disorder associated with chronic airway obstruction (low exposure OR 2.90; 95% CI 1.23 to 6.83), cough (medium exposure OR 2.05; 95% CI 1.13 to 3.86; high exposure OR 2.28; 95% CI 1.21 to 4.42) and phlegm (high exposure OR 2.81; 95% CI 1.48 to 5.55; table 3). Participants in the low exposure region were statistically significantly less prone to experiencing dry throat during the last month (OR 0.18; 95% CI 0.03 to 0.67 (table 3) compared with the non-exposed group.
Table 3.
Risk of respiratory symptoms in 2013 in a population exposed to the 2010 Eyjafjallajökull volcanic eruption, by exposure level
| Non-exposed 2013 (n=365) |
Low exposure 2013* (n=90) |
Medium exposure 2013* (n=428) |
High exposure 2013* (n=267) |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI)† | Per cent (n/N) | OR (95% CI)† | Per cent (n/N) | OR (95% CI)† | Per cent (n/N) | OR (95% CI)† | Per cent (n/N) | |
| ECRHS | ||||||||
| Wheezing (past 12 months) | 1 (ref) | 11.4 (40/351) | 1.42 (0.64 to 2.98) | 14.9 (13/87) | 1.88 (1.13 to 3.21) | 17.1 (71/416) | 2.20 (1.29 to 3.83) | 19.0 (48/253) |
| 1 (ref) | 1.34 (0.70 to 2.76) | 1.59 (0.81 to 3.35) | ||||||
| If yes, breathlessness at the same time | 1 (ref) | 7.1 (25/351) | 1.36 (0.74 to 2.55) | 9.2 (8/87) | 1.05 (0.39 to 2.54) | 10.1 (42/416) | 1.49 (0.79 to 2.87) | 10.7 (27/253) |
| 1 (ref) | 1.33 (0.60 to 3.37) | 1.50 (0.65 to 3.90) | ||||||
| If yes, do you wheeze without a cold? | 1 (ref) | 8.0 (28/351) | 1.78 (0.98 to 3.48) | 12.6 (11/87) | 1.65 (0.69 to 3.79) | 12.3 (51/416) | 2.35 (1.27 to 4.47) | 15.0 (38/253) |
| 1 (ref) | 1.08 (0.53 to 2.39) | 1.46 (0.70 to 3.3) | ||||||
| Nocturnal chest tightness (past 12 months) | 1 (ref) | 10.4 (37/357) | 0.43 (0.12 to 1.16) | 4.6 (4/87) | 1.31 (0.77 to 2.25) | 12.9 (54/420) | 1.40 (0.80 to 2.49) | 13.2 (34/252) |
| 1 (ref) | 3.09 (1.21 to 10.46) | 3.42 (1.30 to 11.79) | ||||||
| Breathlessness at rest | 1 (ref) | 7.9 (28/355) | 0.68 (0.22 to 1.81) | 7.0 (6/86) | 0.90 (0.47 to 1.75) | 7.9 (33/417) | 0.77 (0.38 to 1.56) | 7.1 (18/253) |
| 1 (ref) | 1.39 (0.56 to 4.23) | 1.18 (0.44 to 3.72) | ||||||
| Coughing without a cold | 1 (ref) | 19.0 (67/353) | 1.31 (0.69 to 2.44) | 24.1 (21/87) | 1.64 (1.07 to 2.55) | 25.2 (107/424) | 2.01 (1.28 to 2.44) | 29.1 (74/254) |
| 1 (ref) | 1.26 (0.73 to 2.28) | 1.53 (0.86 to 2.82) | ||||||
| Nocturnal cough (past 12 months) | 1 (ref) | 19.4 (69/355) | 0.85 (0.43 to 1.61) | 17.4 (15/86) | 1.18 (0.77 to 1.82) | 21.6 (91/421) | 1.30 (0.83 to 2.07) | 22.6 (57/252) |
| 1 (ref) | 1.40 (0.78 to 2.68) | 1.55 (0.83 to 3.03) | ||||||
| Morning winter cough | 1 (ref) | 10.8 (38/351) | 1.17 (0.48 to 2.64) | 11.0 (9/82) | 1.44 (0.82 to 2.56) | 12.3 (51/416) | 1.24 (0.67 to 2.30) | 10.7 (27/253) |
| 1 (ref) | 1.25 (0.60 to 2.85) | 1.08 (0.49 to 2.58) | ||||||
| Nocturnal or daytime winter cough | 1 (ref) | 9.3 (32/345) | 1.34 (0.56 to 3.02) | 14.1 (12/85) | 1.18 (0.65 to 2.20) | 10.1 (42/415) | 0.99 (0.51 to 1.96) | 8.6 (21/245) |
| 1 (ref) | 0.90 (0.44 to 2.01) | 0.76 (0.34 to 1.77) | ||||||
| If yes, is it chronic‡ | 1 (ref) | 5.2 (18/345) | 1.73 (0.83 to 3.82) | 8.2 (7/85) | 1.13 (0.34 to 3.32) | 7.7 (32/415) | 1.69 (0.76 to 3.89) | 7.3 (18/245) |
| 1 (ref) | 1.56 (0.62 to 4.77) | 1.51 (0.57 to 4.8) | ||||||
| Morning winter phlegm | 1 (ref) | 12.0 (42/349) | 1.79 (0.87 to 3.59) | 18.6 (16/86) | 1.89 (1.14 to 3.21) | 19.8 (81/410) | 1.94 (1.14 to 3.38) | 20.4 (51/250) |
| 1 (ref) | 1.08 (0.60 to 2.05) | 1.09 (0.59 to 2.14) | ||||||
| Nocturnal or daytime winter phlegm | 1 (ref) | 7.4 (26/349) | 0.85 (0.29 to 2.17) | 7.1 (6/85) | 1.68 (0.92 to 3.19) | 12.7 (52/408) | 1.63 (0.86 to 3.18) | 12.9 (31/241) |
| 1 (ref) | 2.02 (0.88 to 5.46) | 1.97 (0.83 to 5.47) | ||||||
| If yes, is it chronic?‡ | 1 (ref) | 6.3 (22/349) | 1.83 (0.95 to 3.68) | 3.5 (3/85) | 0.51 (0.11 to 1.65) | 11.0 (45/408) | 1.92 (0.97 to 3.95) | 12.0 (29/241) |
| 1 (ref) | 3.64 (1.26 to 15.42) | 3.87 (1.31 to 16.63) | ||||||
| Dyspnoea | 1 (ref) | 10.1(36/355) | 0.47 (0.13 to 1.28) | 5.7 (5/88) | 1.15 (0.65 to 2.08) | 10.6 (44/415) | 1.06 (0.57 to 2.0) | 10.6 (26/246) |
| 1 (ref) | 2.66 (1.02 to 9.11) | 2.45 (0.91 to 8.59) | ||||||
| Nasal allergy and hay fever | 1 (ref) | 19.0 (66/348) | 0.86 (0.42 to 1.66) | 16.7 (14/84) | 1.10 (0.71 to 1.73) | 19.4 (79/408) | 1.19 (0.73 to 1.92) | 19.8 (49/247) |
| 1 (ref) | 1.28 (0.70 to 2.5) | 1.38 (0.72 to 2.76) | ||||||
| Allergic rhinitis | 1 (ref) | 27.6 (96/348) | 0.72 (0.40 to 1.29) | 23.5 (20/65) | 1.11 (0.76 to 1.62) | 30.8 (126/409) | 1.45 (0.97 to 2.18) | 37.6 (92/245) |
| 1 (ref) | 1.57 (0.92 to 2.78) | 2.03 (1.16 to 3.67) | ||||||
| Physician diagnosed conditions§ | ||||||||
| Asthma | 1 (ref) | 10.3 (36/349) | 0.99 (0.43 to 2.14) | 12.8 (11/86) | 0.99 (0.57 to 1.73) | 11.9 (49/412) | 0.97 (0.53 to 1.76) | 11.8 (29/246) |
| 1 (ref) | 1.00 (0.50 to 2.21) | 0.97 (0.46 to 2.21) | ||||||
| Asthma diagnosis was confirmed by a doctor | 1 (ref) | 9.2 (32/349) | 1.01 (0.57 to 1.83) | 12.8 (11/86) | 1.12 (0.48 to 2.47) | 11.2 (46/412) | 0.94 (0.50 to 1.76) | 10.6 (26/246) |
| 1 (ref) | 0.90 (0.44 to 1.98) | 0.83 (0.38 to 1.90) | ||||||
| Heart disease | 1 (ref) | 8.7 (31/356) | 1.49 (0.65 to 3.30) | 13.8 (12/87) | 0.69 (0.35 to 1.34) | 6.5 (27/417) | 0.93 (0.48 to 1.84) | 8.8 (22/251) |
| 1 (ref) | 0.46 (0.22 to 1.00) | 0.63 (0.29 to 1.41) | ||||||
| Disorders associated with chronic airway obstruction¶ | 1 (ref) | 5.5 (20/365) | 2.90 (1.23 to 6.83) | 13.3 (12/90) | 1.86 (0.95 to 3.83) | 8.4 (36/428) | 1.77 (0.86 to 3.78) | 8.2 (22/267) |
| 1 (ref) | 0.64 (0.32 to 1.36) | 0.61 (0.29 to 1.34) | ||||||
| Other respiratory symptoms** | ||||||||
| Shortness of breath | 1 (ref) | 5.7 (19/334) | 0.62 (0.14 to 2.07) | 3.6 (3/84) | 1.52 (0.73 to 3.29) | 6.7 (27/401) | 2.10 (0.99 to 4.64) | 9.4 (22/233) |
| 1 (ref) | 2.40 (0.80 to 10.37) | 3.56 (1.16 to 15.54) | ||||||
| Feeling of tightness in chest | 1 (ref) | 3.3 (11/333) | 0.56 (0.08 to 2.4) | 2.4 (2/84) | 1.01 (0.39 to 2.76) | 3.8 (15/399) | 1.08 (0.39 to 3.03) | 4.3 (10/234) |
| 1 (ref) | 1.80 (0.47 to 11.84) | 2.05 (0.50 to 13.79) | ||||||
| Other respiratory symptoms** | ||||||||
| Cough | 1 (ref) | 9.4 (31/331) | 1.24 (0.48 to 2.98) | 9.2 (8/87) | 2.05 (1.13 to 3.86) | 12.4 (50/404) | 2.28 (1.21 to 4.42) | 13.9 (33/238) |
| 1 (ref) | 1.69 (0.80 to 4.06) | 1.85 (0.84 to 4.54) | ||||||
| Phlegm | 1 (ref) | 7.5 (25/333) | 1.03 (0.35 to 2.69) | 7.1 (6/84) | 1.86 (1.0 to 3.64) | 11.1 (45/404) | 2.81 (1.48 to 5.55) | 16.0 (38/237) |
| 1 (ref) | 1.80 (0.78 to 4.91) | 2.72 (1.16 to 7.50) | ||||||
| Dry throat | 1 (ref) | 7.9 (27/340) | 0.18 (0.03 to 0.67) | 2.4 (2/84) | 0.86 (0.46 to 1.63) | 9.1 (37/408) | 1.04 (0.54 to 2.01) | 11.0 (26/236) |
| 1 (ref) | 4.66 (1.36 to 29.30) | 5.71 (1.62 to 36.26) | ||||||
*Regions are seen in figure 1.
†OR and 95% CI from multivariate logistic regression adjusted for age category, gender, education and smoking status.
‡Answers ‘yes to a moderate extent’ or ‘yes, to much extent’ to the question ‘Have any of the following symptoms disturbed your daily activities during the last month’.
§Answering ‘yes’ to ‘Has physician ever told you that you had (the disease)?’
¶Three items from ECRHS (if participant has had COPD, emphysema or chronic bronchitis confirmed by a doctor) were combined into one item (disorders associated with chronic airway obstruction).
**Chronic: more than 3 months/year.
COPD, chronic obstructive pulmonary disease; ECRHS, European Community Respiratory Health Survey.
We found differences in reported respiratory symptoms by level of exposure. Using the low exposure group as a reference, the following statistically significant differences were observed (see table 3 for details): higher likelihood of nocturnal chest tightness (medium exposed OR 3.09; 95% CI 1.21 to 10.46; high exposed OR 3.42; 95% CI 1.30 to 11.79), chronic nocturnal or daytime winter phlegm (medium exposed OR 3.64; 95% CI 1.26 to 15.42; high exposed OR 3.87; 95% CI 1.31 to 16.63), dyspnoea (medium exposed OR 2.66; 95% CI 1.02 to 9.11), allergic rhinitis (high exposed OR 2.03; 95% CI 1.16 to 3.67), shortness of breath (high exposed OR 3.56; 95% CI 1.16 to 15.54), phlegm (high exposed OR 2.72; 95% CI 1.16 to 7.50) and dry throat (medium exposed OR 4.66; 95% CI 1.36 to 29.30; high exposed OR 5.71; 95% CI 1.62 to 36.26).
Physical and mental health, sleep difficulties and use of medication
In 2013, exposed participants in some exposure regions were less likely to report back pain (low exposed OR 0.50; 95% CI 0.25 to 0.95; medium exposed OR 0.61; 95% CI 0.40 to 0.93), myalgia (low exposed OR 0.49; 95% CI 0.25 to 0.91; medium exposed OR 0.64; 95% CI 0.42 to 0.97), insomnia (low exposed OR 0.43; 95% CI 0.19 to 0.90) and regular use of analgesics (low exposed OR 0.16; 95% CI 0.04 to 0.45), compared with the non-exposed group. No statistically significant differences were reported regarding psychological distress or perceived stress by level of exposure when compared with the non-exposed group (table 4).
Table 4.
Physical and psychological health, sleep difficulties and drug use in a population exposed to the 2010 Eyjafjallajökull volcanic eruption by exposure level
| Non-exposed 2013 (n=365) |
Low exposure 2013* (n=90) |
Medium exposure 2013* (n=428) |
High exposure 2013* (n=267) |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI)† | Per cent (n/N) | OR (95% CI)† | Per cent (n/N) | OR (95% CI)† | Per cent (n/N) | OR (95% CI)† | Per cent (n/N) | |
| Musculoskeletal symptoms‡ | ||||||||
| Back pain | 1 (ref) | 26.5 (91/344) | 0.50 (0.25 to 0.95) | 16.5 (14/85) | 0.61 (0.40 to 0.93) | 19.3 (77/399) | 0.85 (0.54 to 1.33) | 24.5 (58/237) |
| 1 (ref) | 1.20 (0.65 to 2.35) | 1.71 (0.90 to 3.40) | ||||||
| Myalgia | 1 (ref) | 27.5 (95/346) | 0.49 (0.25 to 0.91) | 18.6 (16/86) | 0.64 (0.42 to 0.97) | 24.2 (99/409) | 0.71 (0.45 to 1.10) | 25.4 (62/244) |
| 1 (ref) | 1.30 (0.72 to 2.46) | 1.45 (0.78 to 2.81) | ||||||
| Psychological health | ||||||||
| Psychological distress§ | 1 (ref) | 24.5 (80/327) | 1.08 (0.57 to 2.0) | 22.6 (19/84) | 0.92 (0.59 to 1.42) | 21.7 (84/387) | 1.10 (0.70 to 1.76) | 24.0 (58/242) |
| 1 (ref) | 0.87 (0.49 to 1.59) | 1.07 (0.59 to 1.99) | ||||||
| Perceived stress¶ | 1 (ref) | 11.1 (38/341) | 0.92 (0.36 to 2.14) | 10.7 (9/84) | 0.87 (0.48 to 1.58) | 10.5 (42/401) | 0.93 (0.49 to 1.77) | 10.4 (25/241) |
| 1 (ref) | 0.98 (0.46 to 2.38) | 1.09 (0.48 to 2.73) | ||||||
| PTSD** | 1 (ref) | †† | ||||||
| 1 (ref) | 0 (0/88) | ‡‡ | 3.2 (13/410) | ‡‡ | 4.8 (12/248) | |||
| Sleep difficulties | ||||||||
| Insomnia‡ | 1 (ref) | 20.7 (70/338) | 0.43 (0.19 to 0.90) | 10.6 (9/85) | 0.80 (0.51 to 1.26) | 17.3 (70/405) | 0.63 (0.38 to 1.04) | 14.9 (36/242) |
| 1 (ref) | 1.88 (0.93 to 4.25) | 1.50 (0.71 to 3.50) | ||||||
| Difficulty falling asleep (yes: sometimes, often and always/every night) | 1 (ref) | 40.6 (145/357) | 1.09 (0.64 to 1.85) | 36.4 (32/88) | 1.05 (0.73 to 1.52) | 35.8 (151/422) | 1.11 (0.75 to 1.65) | 38.8 (99/255) |
| 1 (ref) | 0.99 (0.60 to 1.64) | 1.05 (0.62 to 1.79) | ||||||
| Difficulty staying asleep and having trouble falling back asleep (yes: sometimes, often and always/every night) | 1 (ref) | 41.5 (147/354) | 1.02 (0.60 to 1.73) | 41.2 (35/85) | 1.14 (0.80 to 1.64) | 41.3 (171/414) | 1.09 (0.74 to 1.64) | 41.4 (104/251) |
| 1 (ref) | 1.15 (0.70 to 1.88) | 1.12 (0.67 to 1.89) | ||||||
| Feeling well rested after a night’s sleep (yes: often and always/every night) | 1 (ref) | 50.1 (176/351) | 0.89 (0.53 to 1.51) | 43.7 (38/87) | 1.23 (0.86 to 1.77) | 51.9 (217/418) | 0.87 (0.59 to 1.29) | 43.8 (109/249) |
| 1 (ref) | 1.39 (0.86 to 2.27) | 0.99 (0.59 to 1.66) | ||||||
| Frequently wake up in the middle of the night (yes: sometimes, often and always/every night) | 1 (ref) | 64.2 (228/355) | 0.67 (0.40 to 1.15) | 54.7 (47/86) | 0.69 (0.48 to 1.0) | 53.9 (227/421) | 0.95 (0.63 to 1.42) | 62.1 (157/253) |
| 1 (ref) | 1.08 (0.66 to 1.77) | 1.49 (0.88 to 2.52) | ||||||
| Regular drugs use (at least once per week) | ||||||||
| Asthma medication | 1 (ref) | 4.9 (18/365) | 0.68 (0.15 to 2.3) | 4.4 (4/90) | 0.84 (0.36 to 1.97) | 3.7 (16/428) | 0.95 (0.40 to 2.29) | 4.5 (12/267) |
| 1 (ref) | 1.22 (0.38 to 5.46) | 1.50 (0.45 to 6.86) | ||||||
| Analgesics | 1 (ref) | 21.7 (65/365) | 0.16 (0.04 to 0.45) | 3.3 (3/90) | 0.67 (0.41 to 1.1) | 11.7 (50/428) | 0.44 (0.25 to 0.78) | 8.6 (23/267) |
| 1 (ref) | 4.52 (1.56 to 19.20) | 2.92 (0.96 to 12.7) | ||||||
| Any drug for depression, anxiety, sleeping and other mental symptoms | 1 (ref) | 28.8 (105/365) | 1.11 (0.62 to 1.97) | 31.1 (28/90) | 1.46 (0.98 to 2.19) | 33.4 (143/428) | 1.31 (0.86 to 2.02) | 33.7 (90/267) |
| 1 (ref) | 2.98 (0.85 to 18.87) | 3.85 (1.07 to 24.63) | ||||||
| Drugs for other mental symptoms | 1 (ref) | 1.1 (4/365) | 0 (0/90) | 0.5 (2/428) | 0.7 (2/267) | |||
| 1 (ref) | ‡‡ | ‡‡ | ||||||
| Depression medication | 1 (ref) | 6.0 (22/365) | 0.37 (0.06 to 1.41) | 2.2 (2/90) | 1.11 (0.53 to 2.38) | 6.1 (26/428) | 1.48 (0.70 to 3.22) | 7.9 (21/267) |
| 1 (ref) | 2.98 (0.85 to 18.87) | 3.85 (1.07 to 24.63) | ||||||
| Anxiety/sedative medication | 1 (ref) | 5.5 (20/365) | 0.59 (0.13 to 1.97) | 3.3 (3/90) | 1.19 (0.56 to 2.6) | 5.8 (25/428) | 0.83 (0.35 to 1.95) | 4.5 (12/267) |
| 1 (ref) | 2.00 (0.66 to 8.80) | 1.39 (0.41 to 6.37) | ||||||
| Hypnotics | 1 (ref) | 8.8 (32/365) | 1.96 (0.62 to 8.68) | 3.3 (3/90) | 0.97 (0.49 to 1.89) | 5.6 (24/428) | 0.86 (0.43 to 1.73) | 7.5 (20/267) |
| 1 (ref) | 2.08 (0.69 to 9.04) | 2.35 (0.75 to 10.37) | ||||||
| Blood pressure-lowering medication | 1 (ref) | 23.0 (84/365) | 1.21 (0.65 to 2.24) | 27.8 (25/90) | 1.44 (0.94 to 2.22) | 27.1 (116/428) | 1.29 (0.82 to 2.05) | 28.1 (75/267) |
| 1 (ref) | 1.22 (0.69 to 2.21) | 1.08 (0.59 to 2.01) | ||||||
| Irritation symptoms | ||||||||
| Eye irritation and itch | 1 (ref) | 7.1 (24/336) | 0.85 (0.29 to 2.18) | 7.1 (6/84) | 1.70 (0.93 to 3.21) | 11.8 (48/407) | 1.83 (0.97 to 3.55) | 13.3 (31/233) |
| 1 (ref) | 1.92 (0.83 to 5.21) | 2.06 (0.87 to 5.74) | ||||||
| Skin rash/eczema | 1 (ref) | 11.6 (39/337) | 0.77 (0.27 to 1.90) | 7.4 (6/81) | 1.24 (0.71 to 2.22) | 10.8 (44/406) | 1.93 (1.09 to 3.49) | 16.3 (38/233) |
| 1 (ref) | 1.58 (0.69 to 4.28) | 2.41 (1.03 to 6.62) | ||||||
*Regions are seen in figure 1.
†OR and 95% CI from multivariate logistic regression adjusted for age category, gender, education and smoking status.
‡Answers ‘yes to a moderate extent’ or ‘yes, to much extent’ to the question ‘Have any of the following symptoms disturbed your daily activities during the last month’.
§Psychological distress was derived from GHQ-12 referring to ‘the previous weeks’, using a binary cut-off score of >2.
¶Perceived stress was derived from PSS-4 referring to ‘the recent month’ using a binary cut-off score of 90th centile.
**Primary care PTSD was derived from PC-PTSD referring to ‘the recent month’ using a binary cut-off score of >2.
††The non-exposed group did not receive questions regarding PTSD symptoms specifically related to the Eyjafjallajökull eruption.
‡‡Data not applicable.
GHQ-12, General Health Questionnaire-12-item version; PC-PTSD, Primary Care PTSD; PSS-4, Perceived Stress Scale.
Compared with the low exposed group, the medium or high exposed groups showed higher prevalence in the use of analgesics (medium exposure region OR 4.52; 95% CI 1.56 to 19.20), any drug for depression, as well as anxiety, sleeping problems and other mental symptoms (high exposed OR 3.85; 95% CI 1.07 to 24.63), depression medication (high exposed OR 3.85; 95% CI 1.07 to 24.63) and skin rash/eczema (high exposed OR 2.41; 95% CI 1.03 to 6.62; see table 4). Logistic regression was not applicable for the PTSD scores since there were no reports of PTSD symptoms in the low exposure region. No statistically significant difference was detected between reported PTSD symptoms between the medium and high exposed participants in 2013 (p=0.842). Compared with the low exposed group, no statistically significant difference was reported among the medium or high exposed groups regarding psychological distress (medium exposed OR 0.87; 95% CI 0.49 to 1.59; high exposed OR 1.07; 95% CI 0.59 to 1.99) and perceived stress (medium exposed OR 0.98; 95% CI 0.46 to 2.38; high exposed OR 1.09; 95% CI 0.48 to 2.73).
Multiple symptoms
The prevalence of having two or more symptoms (morning winter phlegm, nocturnal or daytime winter phlegm and/or chronic nocturnal or daytime winter phlegm and skin rash/eczema) increased from 6.4% in 2010 to 12.4% in 2013 (OR 2.65; 95% CI 1.58 to 4.43). Furthermore, having multiple symptoms in 2013 was associated with perceived stress (OR 2.86; 95% CI 1.23 to 6.23) and PTSD symptoms (OR 3.21; 95% CI 1.13 to 8.33), but not psychological distress (adjusting for gender, age, smoking and education).
Discussion
The findings from this prospective study in 2010 and 2013 on a population exposed to a volcanic eruption show that some respiratory and physical symptoms increased with time in the exposed group. In 2013, exposed participants reported an increase in various respiratory symptoms, in addition to symptoms like skin rash/eczema, back pain and myalgia. Similarly, the exposed participants were more likely to experience insomnia, sleep difficulties and having two or more physical symptoms, compared with 2010. The use of medication for psychological morbidity and high blood pressure was also more prevalent among the exposed participants in 2013 than in 2010.
A further analysis on health status by exposure level in 2013 showed that participants living in exposed areas reported more respiratory symptoms, such as wheezing, coughing without a cold, morning phlegm during winter and having been diagnosed with any disorder associated with chronic airway obstruction, compared with those living in the non-exposed region. Participants from medium and high exposure regions were at significantly increased odds of upper respiratory symptoms compared with those from the low exposure region. Interestingly, exposed participants were less likely than non-exposed participants to report symptoms related to pain and sleep difficulties, but within the exposed group only, the more exposed showed a higher prevalence of factors like regular use of medications.
Contrary to our hypothesis, these results imply that physical, in particular respiratory, symptoms have not subsided in the 3–4 years since the volcanic eruption ended. However, in accordance with the second hypothesis, we found that a dose–response relationship between ash exposure and physical symptoms still exists.
Respiratory health
Studies on long-term health effects of volcanic ash on respiratory health are few and their results vary. Investigating long-term health effects, such as chronic lung disease or serious respiratory symptoms following volcanic eruptions, may be difficult, as the disease may not become apparent until many years after the initial exposure.9 Previous studies have shown adverse respiratory effects of exposure to volcanic ash, but most of these are based on short-term follow-ups (from a few weeks up to a year following exposure).4 5 8 10 12–14
Our findings differ from the results of Rojas-Ramos et al10 who reported that the increased incidence of respiratory symptoms and a decrease in lung function in the immediate period after the eruption in Popocatepetl in Mexico returned to normal levels 7 months later.
Acute symptoms like chest tightness, coughing and eye irritation were more common among exposed than non-exposed loggers following the Mount St. Helen’s eruption; however, as resuspension of the ash diminished, the symptoms subsided.29–31 A 4-year follow-up indicated no need for further follow-up as those affected seemed to have recovered.11 This is in contrast to our findings, which indicates that symptoms may be more persistent than previously reported.
Our study 6–9 months after the Eyjafjallajökull eruption showed a dose–response tendency between levels of exposure to the volcano and certain symptoms.5 This study indicates that this dose–response pattern still exists 3–4 years later. Other studies comparing health effects by level of exposure to volcanic ash have yielded similar findings.32–34
Our hypothesis that physical symptoms had subsided in the more than 3 years since the eruption is refuted, especially regarding respiratory symptoms. The most likely explanation lies in the continued exposure to respirable particles in the ambient air in the exposed region. The most troublesome volcanic compound regarding chronic lung pathogenesis is crystalline silica, such as cristobalite, quartz or tridymite polymorphs.35 36 Since the Eyjafjallajökull ash contained only 1.4–3.2 wt% crystalline silica (quartz or cristobalite), the potential hazard of developing silicosis due to its persistence in the environment is considered negligible.2 9 However, fresh ash from the Eyjafjallajökull volcano contained up to 25% respirable particles (<10 μm). Exposure to particles of <PM10 aerodynamic diameter, irrespective of origin, is considered a key element in the effects of air pollution on health.37 Following a volcanic eruption, ash deposits can be remobilised by wind or human activities for many years, accompanied by negative health effects.2 Resuspension of the Eyjafjallajökull ash on windy days resulted in unusually high concentrations of particulate matter in the air in Southern and Southwestern Iceland.3 During the summer and fall of 2010, the official Icelandic PM10 health limit (50 μm/m3 daily average)38 was exceeded 25 times.3 5 According to the EAI, the daily average PM10 values did not decrease during the follow-up time of this study, and the maximum values remained elevated in the most exposed area. This indicates that the population continued to be exposed to ash for years after the eruption, which may explain our findings of increased pulmonary and other health symptoms between 2010 and 2013.
Our study is the first to indicate a link between exposure to a volcanic eruption and long-term skin irritation. Fresh ash may have an acid coating and be abrasive to the skin, causing irritation and debilitation which can lead to infections.39 40 Eye and skin irritation commonly subside shortly after exposure to ash and long-term toxic injury to these organs is unusual.36 In this study, however, the prevalence of skin rash/eczema more than doubled between 2010 and 2013 and was most common in the high exposure region, even 3–4 years after the eruption ended.
Mental health
Rates of psychological distress, perceived stress and PTSD symptoms decreased between 2010 and 2013. This is similar to the findings of Ohta et al20 on volcanic eruption evacuees who reported that psychological distress decreased with time (between 6 and 44 months) after evacuation from a volcanic disaster. Dose–response patterns have been found between level of volcanic exposure and psychological distress in the Mount St. Helens and Mount Merapi eruptions.21 22 While the rates of perceived stress and psychological distress were similar in the exposed regions, this study does not warrant decisive conclusions about levels of exposure and mental symptoms. However, PTSD symptoms were only found in the medium and high exposure areas in 2013. In addition, those who reported two or more physical symptoms in 2013 were more likely to show symptoms of PTSD and perceived stress. This may provide guidance for preventive measures and treatment in the future.
Our findings indicate that sleep difficulties among exposed participants in 2013 had increased since 2010, which is consistent with previous studies that report sleep difficulties as late as 16 years after a disaster.41–43 Norris et al44 identified sleep disturbances in 23% of 160 studies on disaster victims that they reviewed.
In our study, musculoskeletal symptoms, back pain and myalgia increased significantly between 2010 and 2013. Somatic symptoms are a common sign of distress in the aftermath of disasters, and of those musculoskeletal symptoms are the most common and long-lasting.45 Other symptoms include headache, fatigue and irritable bowel syndrome.46–48 It has been suggested that physical symptoms merit more attention after disasters47 48 and our findings support that.
Strengths and limitations
The main strengths of the study include the use of a matched design and a large population-based cohort, allowing for prospective assessments of physical and mental health within subpopulations, and high response rates both in 2010 and 2013, which minimises the risk of selection bias. The use of multiple measuring tools for mental outcomes: psychological distress, perceived stress and PTSD symptoms, based on standardised psychological tests, further strengthen the study.
Regarding limitations, non-participation may have influenced the results. It is possible that those who were lost to the follow-up study in 2013 included a disproportionate number of people who had developed serious respiratory symptoms. Their non-response in our follow-up study could thus underestimate the true risk associated with long-term ash exposure. However, it is also possible that individuals who experience symptoms are more likely to participate, which would lead to overestimation of ORs. The high response rate in 2013 lowers this potential selection bias. It is also possible that recall bias may have influenced the self-reported data; however, the specific dose–response relationship found between ash exposure and physical symptoms renders that unlikely. Information bias may have occurred in our study if participants in the higher exposed areas are more concerned or informed about the health effects of volcanic eruptions, making them more aware of symptoms and thus affecting their replies to the questionnaires. The same classification of exposure levels (low, medium and high) was used in 2010 and 2013, based on satellite images, emission intensity and observations on the ground.5 Reclassification of the exposed region might have been valid in this study, as exposure to resuspended ash may differ from the original exposure. The PM10 measurements in this study were only obtained from one location, in Raufarfell, located in the high exposure region. Information is lacking on whether the exposure levels in the medium and low exposure regions had changed during the follow-up period. However, the dose–response patterns of physical symptoms are consistent between the two studies, indicating that the conditions regarding exposure levels had not changed.
Conclusions
Our findings indicated an association between long-term exposure to volcanic ash and increased respiratory and physical symptoms. Similarly, we found a dose–response relationship between exposure and symptoms, with greater risk of exacerbating respiratory and physical symptoms in the more exposed areas.
These findings call for continued health monitoring and adequate treatment of residents in the exposed region. The potential risks of long-term exposure to ash must not be underestimated and finding a way to prevent or minimise the risk to exposed residents is of great importance. This study draws attention to the possibility of long-term physical and mental illnesses following natural hazards and has implications for planning preventive and treatment strategies.
Footnotes
Contributors: All authors planned and designed the study and had full access to the data. HH, AH, HKC and GP gathered the data and HH and AH did the statistical analysis. HH drafted the article and all authors critically revised the manuscript for important intellectual content. GP and AH obtained funding and supervised the study. AH (guarantor) takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The study was funded through a grant in 2010 from the government in Iceland. The preparation of this paper was also supported by the Nordic Centre of Excellence for Resilience and Societal Security—NORDRESS, which is funded by the Nordic Societal Security Program (grant number 68825).
Competing interests: None declared.
Ethics approval: This study was approved by the Icelandic Data Protection Authority (no. S4878/2010) and the Science Bioethics Committee (no. 10-099-V3).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: AH and GP hold all the data used in this study. The data set holds additional information about health of children and mental health, which the authors seek to publish later. The data are anonymised, but could hold identifiable details; thus, we do not wish to share the data.
References
- 1.Gudmundsson MT, Thordarson T, Höskuldsson A et al. . Ash generation and distribution from the April–May 2010 eruption of Eyjafjallajökull, Iceland. Sci Rep 2012;2:572 10.1038/srep00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwell CJ, Baxter PJ, Hillman SE et al. . Physicochemical and toxicological profiling of ash from the 2010 and 2011 eruptions of Eyjafjallajökull and Grímsvötn volcanoes, Iceland using a rapid respiratory hazard assessment protocol. Environ Res 2013;127:63–73. 10.1016/j.envres.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 3.Thorsteinsson T, Jóhannsson T, Stohl A et al. . High levels of particulate matter in Iceland due to direct ash emissions by the Eyjafjallajökull eruption and resuspension of deposited ash. J Geophys Res Solid Earth 2012;117:1–9. 10.1029/2011JB008756 [DOI] [Google Scholar]
- 4.Carlsen HK, Gislason T, Benediktsdottir B et al. . A survey of early health effects of the Eyjafjallajokull 2010 eruption in Iceland: a population-based study. BMJ Open 2012;2:e000343 10.1136/bmjopen-2011-000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsen HK, Hauksdottir A, Valdimarsdottir UA et al. . Health effects following the Eyjafjallajökull volcanic eruption: a cohort study. BMJ Open 2012;2:e001851 10.1136/bmjopen-2012-001851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fano V, Cemigliaro A, Scondotto S et al. . Health effects of environmental contamination due to volcanic ash from Mount Etna (Sicily, Italy) in autumn 2002. Epidemiology 2006;17:S159–S159. 10.1097/00001648-200611001-00400 [DOI] [Google Scholar]
- 7.Gudmundsson G. Respiratory health effects of volcanic ash with special reference to Iceland. A review. Clin Respir J 2011;5:2–9. 10.1111/j.1752-699X.2010.00231.x [DOI] [PubMed] [Google Scholar]
- 8.Horton RJ, McCaldin RO. Observations on air pollution aspects of Iraz'u volcano, Costa Rica. Public Health Rep 1964;79:925–9. 10.2307/4592281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwell CJ, Baxter PJ. The respiratory health hazards of volcanic ash: a review for volcanic risk mitigation. Bull Volcanol 2006;69:1–24. 10.1007/s00445-006-0052-y [DOI] [Google Scholar]
- 10.Rojas-Ramos M, Catalan-Vazquez M, Pozzo ALM-D et al. . A seven months prospective study of the respiratory effects of exposure to ash from Popocatepetl volcano, Mexico. Environ Geochem Health 2001;23:379–92. 10.1023/A:1012244311557 [DOI] [Google Scholar]
- 11.Buist AS, Vollmer WM, Johnson LR et al. . A four-year prospective study of the respiratory effects of volcanic ash from Mt. St. Helens. Am Rev Respir Dis 1986;133:526–34. 10.1164/arrd.1986.133.4.526 [DOI] [PubMed] [Google Scholar]
- 12.Lombardo D, Ciancio N, Campisi R et al. . A retrospective study on acute health effects due to volcanic ash exposure during the eruption of Mount Etna (Sicily) in 2002. Multidiscip Respir Med 2013;8:51 10.1186/2049-6958-8-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxter PJ, Ing R, Falk H et al. . Mount St Helens eruptions, May 18 to June 12, 1980. An overview of the acute health impact. JAMA 1981;246:2585–9. [PubMed] [Google Scholar]
- 14.Cadelis G, Tourres R, Molinie J et al. . Exacerbations of asthma in Guadeloupe (French West Indies) and volcanic eruption in Montserrat (70 km from Guadeloupe). Rev Mal Respir 2013;30:203–14. 10.1016/j.rmr.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 15.Gordian ME, Ozkaynak H, Xue JP et al. . Particulate air pollution and respiratory disease in Anchorage, Alaska. Environ Health Perspect 1996;104:290–7. 10.1289/ehp.96104290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newnham RM, Dirks KN, Samaranayake D. An investigation into long-distance health impacts of the 1996 eruption of Mt Ruapehu, New Zealand. Atmos Environ 2010;44:1568–78. 10.1016/j.atmosenv.2009.12.040 [DOI] [Google Scholar]
- 17.Hansell AL, Horwell CJ, Oppenheimer C. The health hazards of volcanoes and geothermal areas. Occup Environ Med 2006;63:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higuchi K, Koriyama C, Akiba S. Increased mortality of respiratory diseases, including lung cancer, in the area with large amount of ashfall from Mount Sakurajima volcano. J Environ Public Health 2012;2012:257831 10.1155/2012/257831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leon GR. Overview of the psychosocial impact of disasters. Prehosp Disaster Med 2004;19:4–9. 10.1017/S1049023X00001424 [DOI] [PubMed] [Google Scholar]
- 20.Ohta Y, Araki K, Kawasaki N et al. . Psychological distress among evacuees of a volcanic eruption in Japan: a follow-up study. Psychiatry Clin Neurosci 2003;57:105–11. 10.1046/j.1440-1819.2003.01086.x [DOI] [PubMed] [Google Scholar]
- 21.Shore JH, Tatum EL, Vollmer WM. Evaluation of mental effects of disaster, Mount St. Helens eruption. Am J Public Health 1986;76:76–83. 10.2105/AJPH.76.Suppl.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warsini S, Buettner P, Mills J et al. . Post-traumatic stress disorder among survivors two years after the 2010 Mount Merapi volcano eruption: a survey study. Nurs Health Sci 2015;17:173–80. 10.1111/nhs.12152 [DOI] [PubMed] [Google Scholar]
- 23.Burney PG, Luczynska C, Chinn S et al. . The European Community Respiratory Health Survey. Eur Respir J 1994;7:954–60. 10.1183/09031936.94.07050954 [DOI] [PubMed] [Google Scholar]
- 24.Hankins M. The reliability of the twelve-item general health questionnaire (GHQ-12) under realistic assumptions. BMC Public Health 2008;8:355 10.1186/1471-2458-8-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 26.Hauksdóttir A, McClure C, Jonsson SH et al. . Increased stress among women following an economic collapse—a prospective cohort study. Am J Epidemiol 2013;177:979–88. 10.1093/aje/kws347 [DOI] [PubMed] [Google Scholar]
- 27.Prins A, Ouimette P, Kimerling R et al. . The primary care PTSD screen (PC-PTSD): development and operating characteristics. Prim Care Psychiatry 2003;9:9–14. 10.1185/135525703125002360 [DOI] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R et al. . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein R, McCawley M, Attfield M et al. . Evaluation of the potential pulmonary hazards of breathing-zone exposures to Mount St. Helens volcanic ash in loggers: the results of short-term toxicological, environmental and epidemiological studies. Interim Report No. GHE-80-112, DHHS (NIOSH), Atlanta, GA, 1981. [Google Scholar]
- 30.Merchant JA, Baxter P, Bernstein R et al. . Health implications of the Mount St. Helen's eruption: epidemiological considerations. Ann Occup Hyg 1982;26:911–19. 10.1093/annhyg/26.8.911 [DOI] [PubMed] [Google Scholar]
- 31.Bernstein R, McCawley M, Attfield M et al. . Epidemiological assessment of the risk for adverse pulmonary effects from persistent occupational exposures to Mount St. Helens volcanic ash (tephra). Mount St Helens: one year later. Cheney, WA: Eastern Washington University Press, 1982. [Google Scholar]
- 32.Forbes L, Jarvis D, Potts J et al. . Volcanic ash and respiratory symptoms in children on the island of Montserrat, British West Indies. Occup Environ Med 2003;60:207–11. 10.1136/oem.60.3.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yano E, Yokoyama Y, Higashi H et al. . Health effects of volcanic ash: a repeat study. Arch Environ Health 1990;45:367–73. 10.1080/00039896.1990.10118757 [DOI] [PubMed] [Google Scholar]
- 34.Yano E, Yokoyama Y, Nishii S. Chronic pulmonary effects of volcanic ash: an epidemiologic study. Arch Environ Health 1986;41:94–9. 10.1080/00039896.1986.9937416 [DOI] [PubMed] [Google Scholar]
- 35.Demichela M, Maschiob G, Milazzoc M et al. . Vulnerability assessment for human targets due to ash fallout from Mt. Etna. Chem Eng Trans 2013;32:445–50. [Google Scholar]
- 36.Weinstein P, Horwell CJ, Cook A. Volcanic emissions and health. Volcanic Emissions and Health. In: Selinus O, ed.. Essentials of medical geology. Springer, 2013:217–38. [Google Scholar]
- 37.Lähde A, Gudmundsdottir SS, Joutsensaari J et al. . In vitro evaluation of pulmonary deposition of airborne volcanic ash. Atmos Environ 2013;70:18–27. 10.1016/j.atmosenv.2012.12.048 [DOI] [Google Scholar]
- 38.Regulation for ambient hydrogen sulfide ndano, bensene, carbon monoxide, particulate matter, and lead and information to the public [in Icelandic], Ministry for the Environment and Natural Resources. 251/2002 (2002).
- 39.Amaral A, Rodrigues A. Volcanogenic contaminants: chronic exposure. Encyclopedia Environ Health 2011;5:645–53. [Google Scholar]
- 40.Heggie TW. Geotourism and volcanoes: health hazards facing tourists at volcanic and geothermal destinations. Travel Med Infect Dis 2009;7:257–61. 10.1016/j.tmaid.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 41.Tempesta D, Curcio G, De Gennaro L et al. . Long-term impact of earthquakes on sleep quality. PLoS ONE 2013;8:e55936 10.1371/journal.pone.0055936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thordardottir EB, Valdimarsdottir UA, Hansdottir I et al. . Posttraumatic stress and other health consequences of catastrophic avalanches: a 16-year follow-up of survivors. J Anxiety Disord 2015;32:103–11. 10.1016/j.janxdis.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 43.van der Velden PG, Wong A, Boshuizen HC et al. . Persistent mental health disturbances during the 10 years after a disaster: four-wave longitudinal comparative study. Psychiatry Clin Neurosci 2013;67:110–18. 10.1111/pcn.12022 [DOI] [PubMed] [Google Scholar]
- 44.Norris FH, Friedman MJ, Watson PJ et al. . 60,000 disaster victims speak: part I. An empirical review of the empirical literature, 1981–2001. Psychiatry 2002;65:207–39. [DOI] [PubMed] [Google Scholar]
- 45.Wahlström L, Michélsen H, Schulman A et al. . Longitudinal course of physical and psychological symptoms after a natural disaster. Eur J Psychotraumatol 2013;4:1–7. 10.3402/ejpt.v4i0.21892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afari N, Ahumada SM, Wright LJ et al. . Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med 2014;76:2–11. 10.1097/PSY.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidson JR, McFarlane AC. The extent and impact of mental health problems after disaster. J Clin Psychiatry 2006;67:9–14. [PubMed] [Google Scholar]
- 48.Keskinen-Rosenqvist R, Michélsen H, Schulman A et al. . Physical symptoms 14 months after a natural disaster in individuals with or without injury are associated with different types of exposure. J Psychosom Res 2011;71:180–7. 10.1016/j.jpsychores.2011.01.015 [DOI] [PubMed] [Google Scholar]