Abstract
Objectives
Early identification of developmental vulnerability is vital. This study aimed to estimate the prevalence of moderate or high developmental risk on the Parents' Evaluation of Developmental Status (PEDS) at 6-month, 12-month and 18-month well-child checks; identify associated risk factors; and examine documentation of the PEDS at well-child checks.
Design, participants
A prospective birth cohort of 2025 children with 50% of those approached agreeing to participate. Demographic data were obtained via questionnaires and linked electronic medical records. Telephone interviews were conducted with parents to collect PEDS data.
Primary and secondary outcomes
Multiple logistic regression analyses identified risk factors for moderate or high developmental risk on the PEDS. A Cumulative Risk Index examined the impact of multiple risk factors on developmental risk and documentation of the PEDS at the well-child checks.
Results
Of the original cohort, 792 (39%) had 6-month, 649 (32%) had 12-month and 565 (28%) had 18-month PEDS data. Parental concerns indicating moderate or high developmental risk on the PEDS were 27% (95% CI 24 to 30) at 6 months, 27% (95% CI 24 to 30) at 12 months and 33% (95% CI 29 to 37) at 18 months. Factors associated with moderate or high developmental risk were perinatal risk (OR 12 months: 1.7 (95% CI 1.1 to 2.7)); maternal Middle Eastern or Asian nationality (OR 6 months: 1.6 (95% CI 1.1 to 2.4)), (OR 12 months: 1.7 (95% CI 1.1 to 2.7)); and household disadvantage (OR 6 months: 1.5 (95% CI 1.0 to 2.2). As the number of risk factors increased the odds increased for high or moderate developmental risk and no documentation of the PEDS at well-child checks.
Conclusions
Children with multiple risk factors are more likely to have parental concerns indicating developmental vulnerability using the PEDS and for these concerns to not be documented.
Strengths and limitations of this study.
This is the first study to examine the impact of cumulative risk on developmental vulnerability in children under 2 years in an area of socioeconomic disadvantage and cultural diversity.
This is the first study to examine the impact of cumulative risk on documentation of the PEDS at 6-month,12-month and 18-month well-child checks.
Strength of this study was the use of data linkage with the mother and child's electronic medical record.
Significant loss of follow-up of mothers and infants from the time of recruitment at birth to the 18-month follow-up.
Maternal mental health was unable to be examined due to missing data in the electronic medical record (EMR).
Note that the Child Health Nurses in this paper that are referred to are called Child and Family Health Nurses in the State in Australia where this research was undertaken.
Background
Although ∼4% of Australian children start school with an identified developmental disorder, a further 18% exhibit subtler developmental difficulties (developmental vulnerability)—lacking the necessary skills to flourish in formal education.1 Children experiencing these vulnerabilities typically remain unidentified prior to starting school, and hence, are unlikely to have received support to address their needs. These inequalities in early childhood development (ECD) can worsen with increased risks of school failure, unemployment and adult mortality and morbidity.2–4 Timely access to early intervention through effective early identification of children who are developmentally vulnerable, presents an invaluable opportunity to address and close the ‘ECD gap’.4 5
Developmental surveillance is one mechanism that has been proposed to facilitate earlier identification of developmental vulnerability and improved access to services. It is defined by the National Health and Medical Research Council of Australia (NH&MRC) and the American Academy of Paediatrics (AAP) and involves:
Ongoing contact of families and children within a universal system;
Health professionals trained in child development and health promotion;
Monitoring and responding to developmental concerns over time from infancy to the preschool period.5 6
In Australia, state departments in New South Wales (NSW), Victoria and Western Australian have implemented universal developmental surveillance programmes. These programmes use the Parents' Evaluation of Developmental Status (PEDS) as a standardised tool in the child's personal health record (PHR) to elicit parental concerns and identify developmental vulnerability at the 6-month, 12-month, 18-month, 24-month, 36-month and 48-month well-child checks.7–9 The PHR is given to all parents in NSW after the birth of a baby. It records the child's health, illnesses, injuries, and growth and development as well as immunisations. The PHR is a tool that facilitates communication between parents and healthcare professionals and it is used by parents and health professionals to document and track a child's health, growth and development over time. The PEDS covers expressive and receptive language, fine and gross motor skills, behaviour, socialisation, self-care and learning. It is recommended that the PEDS questionnaire be completed by parents and then discussed at the well-child check by the primary healthcare provider (a child health nurse or general practitioner (GP)). An estimate of developmental vulnerability (‘high’, ‘moderate’, ‘low-risk but concerned/elevated risk for mental health problems category’ or ‘no developmental risk’) is derived from the parental concerns and a clinical pathway of counselling and monitoring of the areas of concern, further screening, assessment and/or early intervention is recommended—commensurate with the level of risk. In the USA, the PEDS has a reported sensitivity of 91–97% and specificity of 73–86% for detecting children at moderate or high developmental risk.10
A recent systematic review demonstrated that approximately one-third of parents globally have concerns on the PEDS that indicate moderate or high developmental risk.9 Male gender, low birthweight, poor child health, poor maternal mental health, low family socioeconomic status (SES) and minority ethnicity were associated with a higher prevalence of parental concerns indicating high developmental risk on the PEDS.9 There was emerging evidence to suggest a dose response relationship between the number of risk factors and developmental risk on the PEDS.9 In addition, in the USA, the greater the number of risk factors experienced by the child and their family, the more likely the child was to not have access to comprehensive health services.11 However, only three of the studies in this systematic review were Australian studies, and of these, none examined developmental risk in the first 18 months of developmental surveillance. In addition, none of the studies in the review have examined how the collection of this PEDS data is documented in the PHR.9
The aim of this study was to estimate the prevalence of parental concerns indicating moderate or high developmental risk on the PEDS at 6-month, 12-month and 18-month well-child checks in a culturally diverse and socioeconomically disadvantaged area of NSW, and to identify associated child, parent, family and neighbourhood factors. The focus on the first and second years of life is particularly important to promote earlier identification and intervention of developmentally vulnerable children. In addition, to provide results that are relevant to service development, the documentation of the PEDS in the PHR at the 6-month, 12-month and 18-month well-child checks was also examined.
Methods
Participants and setting
The ‘Watch Me Grow’ (WMG) study was designed to evaluate the feasibility, accuracy, barriers and enablers of the current developmental surveillance systems in South Western Sydney—an area of significant social disadvantage located about 35 km from the Sydney central business district.12 A key component of the WMG study was the establishment of a longitudinal birth cohort. The WMG study protocol, recruitment methods, representativeness and baseline risk factors have previously been reported.12 13 In summary, the WMG cohort consisted of 2025 parent–infant dyads recruited at birth, that was broadly representative of the culturally diverse and socially disadvantaged local population it sampled.13 Of these 2025 participants enrolled in the WMG study at birth, 1078 (53%) were able to be contacted at 6 months by phone, of which 1052 consented to continue (52%), 26 withdrew (1%) and 792 (39%) had PEDS data available. At 12 months, 875 (43%) were able to be contacted by phone, of which 857 consented to continue (42%), 18 withdrew (1%) and 649 (32%) had PEDS data available. At 18 months, 671 were able to be contacted by phone (33%), of which 632 consented to continue (31%), 39 withdrew (2%) and 565 (28%) had PEDS data available.
Measurement and procedures—measurement of child, parent, family and neighbourhood factors
The impact of risk factors on developmental vulnerability was examined using the bioecological framework14 by measuring child, parent, family and neighbourhood factors available in the WMG cohort and demonstrated in the recent systematic review to be associated with parental concerns, indicating high developmental risk on the PEDS.9 Risk factor measures and the method and timing of their collection in the WMG cohort are listed in table 1. Data were self-reported by parents using baseline and 18-month follow-up questionnaires specifically designed for this study. These questionnaires were derived from the extant literature and examination of questionnaires from other Australian cohort studies, such as the Longitudinal Study of Australian Children.15 16 Additional information routinely collected as part of the mothers' and babies' antenatal and postnatal care was obtained through data linked with their electronic medical record (EMR). Socio-Economic Indexes for Areas (SEIFA) data for the families were calculated using the suburb of residence obtained from data linkages to give information on neighbourhood factors. SEIFA is a composite of indicators that rank geographic areas according to their socioeconomic characteristics based on five-yearly census data of people, families and dwellings across Australia. The lowest SEIFA decile indicates the highest neighbourhood disadvantage.17
Table 1.
Baseline and follow-up measures
| Measure | Instrument/source | Birth | 6 months | 12 months | 18 months |
|---|---|---|---|---|---|
| Child | |||||
| Gender, gestational age, birthweight | EMR (birth) | X | |||
| Admission special care nursery (SCN) or neonatal intensive care unit (NICU) | EMR (postnatal) | X | |||
| Parent | |||||
| Partner status (mother) | Survey | X | |||
| Maternal education | Survey (LSAC adapted15) | X | |||
| Father employed | Survey | X | X | ||
| Nationality | EMR (demographic) | X | |||
| Household | |||||
| Primary language | Survey | X | |||
| Annual income | Survey (LSAC adapted15) | X | X | ||
| Neighbourhood | |||||
| SEIFA decile 1 (most disadvantaged) by suburb17 | EMR (demographic) | X | |||
| Service Use | |||||
| Had well-child check | Parent report | X | X | X | |
| Parental concerns indicating developmental risk using the PEDS10 | PHR | X | X | X | |
EMR, electronic medical record; LSAC, Longitudinal Survey of Australian Children; PEDS, Parents' Evaluation of Developmental Status; PHR, personal health record; SEIFA, Socio-Economic Indexes for Areas.
Measurement of parental concerns indicating moderate and high developmental risk on the PEDS
At each 6-month, 12-month and 18-month follow-up, parents recruited at baseline into the study were contacted by phone and asked (through a standard questionnaire developed by the researchers) whether they had taken their child for the recommended well-child checks as outlined in their child's personal health record (PHR), which health service(s) they used and whether a standardised screening tool (the PEDS) had been completed. The procedure for this was as follows: parents were asked by researchers whether they had taken their child for the well-child checks with the question: “Since your baby was 6 (12 or 18) months of age, have any checks in the ‘Blue Book’ [Personal Health Record] been done at a GP or baby clinic (e.g. Early Childhood Health Centre)?” Parents were then asked to turn to the appropriate page in the PHR to ascertain whether the PEDS had been documented either by the parent or primary healthcare provider, and what the results were (table 1). PEDS data were recorded verbatim by the researchers when it was available. Alternatively, if it was not completed, researchers administered the missing PEDS over the phone for the well-child check that was due.
As demonstrated in a previous paper, PEDS data were more likely to be collected in the 6-month, 12-month and 18-month groups of families at greater household and/or neighbourhood advantage.13 Imputation of this missing outcome data was not possible, because the PEDS results at 6 months may be quite different to those at 18 months, due to the changing developmental skills needed as children age and loss to follow-up was not random.18 As complete PEDS data over three time points were available for only 314 children, we elected to treat each follow-up period as a discrete group and thus examine the results for the 6-month, 12-month and 18-month groups separately, rather than across time.
Statistical analysis
Based on the PEDS scoring, parental concerns were categorised as indicating—high, moderate or low/no developmental risk. Estimates of the prevalence of parental concerns on the PEDS indicating moderate or high risk were calculated with corresponding 95% CIs. Univariate logistic regression was used to test for associations between moderate or high developmental risk at each check and child, parent, family and neighbourhood factors.
Multivariate regression was used to model the association between parental concerns indicating moderate or high developmental risk and factors noted in the univariate logistic regression to be significant (p<0.05). In addition, risk factors that were significant in the recent systematic review on the topic9 were also included in the model. At the child level, these were: male gender; perinatal risk, which was defined as a child who was low birthweight (<2500 g) and/or preterm (<37 weeks' gestation); and/or had an admission to special care nursery or neonatal intensive care. At the parent level, these were: mothers of Middle Eastern or Asian nationality, as per Australian Bureau of Statistics (ABS) coding, as they represented the two key minority groups in the local population.19 At the family level, these were: English not being the primary household language; and household disadvantage. Household disadvantage was defined as an annual household income <$A25 001; and/or the mother not completing high school and/or father being unemployed. At the neighbourhood level, it was a SEIFA score in the lowest decile.17 Individual variables that made up the perinatal and household disadvantage composite variables were selected to reflect the different components of perinatal risk and wealth on developmental vulnerability and to avoid potential collinearity in the regression models.20 Maternal mental health data at antenatal booking in the EMR were not included as these were only available for half of the follow-up participants.
The individual or composite risk factors used in the multivariate regression for the 6-month, 12-month and 18-month for all groups (ie, male gender, perinatal risk, maternal Middle Eastern or Asian nationality, English not being the primary language, household disadvantage and neighbourhood disadvantage) were summed to create a Cumulative Risk Index for each child that ranged from 0 to 6. The odds of being at moderate or high developmental risk on the PEDS were compared with the number of risk factors (five and six risk factors were combined because there were <10 participants with six risk factors). The odds of PEDS documentation in the PHR at well-child check was compared with the number of risk factors (four, five and six risk factors combined because there were <10 participants with five or six risk factors). All analyses were conducted in STATA V.13 (StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP, 2013).
Results
Participants and their characteristics
The mean age of children when PEDS data were either documented in the PHR or collected by researchers for the 6-month group was 8.1 (SD 3.2) months, for 12-month group was 13.9 (SD 3.0) months and for 18-month group was 19.9 (SD 2.1) months. Table 2 outlines the characteristics of the 6-month, 12-month and 18-month groups that had PEDS data. Just fewer than half (45–47%) of the infants were male; 6–7% of infants were born with low birthweight, 7–9% were preterm and 13–16% were admitted to the special care nursery/neonatal intensive care after birth. Overall, around 20% of the infants were defined as being at perinatal risk; while, 6–8% of infants had mothers who did not have a partner at the time of birth, and 14% of mothers had not completed high school. Around 41% of all mothers in the group were of Middle Eastern or Asian nationality. Approximately a third of families did not have English as their primary household language. Around a quarter of households were defined as being at household disadvantage and around a third lived in a neighbourhood at the lowest SEIFA decile in NSW.
Table 2.
Participants and their characteristics
| Characteristic | Baseline—birth N=2013 n (%) |
6-month PEDS N=792 n (%) |
12-month PEDS N=649 n (%) |
18-month PEDS N=565 n (%) |
|---|---|---|---|---|
| Child | ||||
| Male gender | 964 (48.0) | 344 (46.5) | 281 (46.1) | 244 (44.9) |
| Low birthweight (<2500 g) | 151 (7.5) | 49 (6.6) | 45 (7.4) | 38 (7.0) |
| Preterm (<37 weeks) | 180 (9) | 57 (7.7) | 57 (9.3) | 51 (9.4) |
| Admitted SCN/NICU | 301 (15) | 102 (13.8) | 94 (15.4) | 86 (15.8) |
| Parents | ||||
| Single parent at time of birth | 157 (9.0) | 62 (8.0) | 42 (6.6) | 34 (6.0) |
| Mother did not complete high school | 316 (18.5) | 110 (14.5) | 90 (14.4) | 81 (14.6) |
| Father unemployed at birth | 109 (6.7) | 37 (5.0) | 39 (6.3) | 33 (6.1) |
| Mother nationality | ||||
| Australia, UK, North America, New Zealand | 933 (46.4) | 355 (48.0) | 300 (49.2) | 255 (47.0) |
| Middle East | 273 (13.6) | 97 (13.1) | 92 (15.0) | 104 (19.1) |
| Asia (includes India, Bangladesh) | 575 (28.6) | 210 (28.4) | 157 (25.8) | 122 (22.5) |
| Other | 231 (11.5) | 78 (10.5) | 61 (10.0) | 62 (11.4) |
| Household | ||||
| Primary language not English at birth | 589 (33.7) | 240 (31.0) | 209 (32.7) | 180 (32.0) |
| Household annual income at birth $A<25 001 | 277 (17.6) | 94 (13.2) | 75 (13.0) | 60 (11.8) |
| Neighbourhood | ||||
| SEIFA decile 1 (most disadvantaged) at baseline | 872 (44.2) | 274 (37.3) | 221 (36.5) | 200 (37.0) |
| Composite measures | ||||
| Perinatal risk | 403 (20.0) | 135 (18.2) | 123 (20.2) | 109 (20.1) |
| Maternal Middle Eastern or Asian Nationality | 848 (42.2) | 307 (41.5) | 249 (40.8) | 226 (41.6) |
| Household disadvantage | 540 (34.1) | 187 (26.3) | 157 (26.8) | 134 (25.8) |
NICU, neonatal intensive care unit; PEDS, Parents' Evaluation of Developmental Status; SCN, special care nursery; SEIFA, Socio-Economic Indexes for Areas.
Prevalence of parental concerns on the PEDS indicating moderate or high developmental risk
Between a quarter and a third of children had parental concerns indicating moderate or high developmental risk (tables 3–5) with 215 (27.2%; 95% CI 24.2 to 30.4) in the 6-month group (table 3); 174 (26.8%; 95% CI 23.6 to 30.4) in the 12-month group (table 4); and 184 (32.6%; 95% CI 28.8 to 36.6) in the 18-month group (table 5). Of these moderate or high developmental risk children, there were 21 (2.7%; 95% CI 1.7 to 4.1) in the 6-month group, 34 (5.2%; 95% CI 3.8 to 7.3) in the 12-month group and 29 (5.1%; 95% CI 3.6 to 7.3) in the 18-month group whose parents indicated high developmental risk on the PEDS.
Table 3.
Risk factors for moderate or high developmental risk in the 6-month group
| Risk factor N (n)% Whole sample |
Total 792 (100) | High 21 (2.7%; 95% CI 1.7 to 4.1) |
High/moderate 215 (27.2%; 95% CI 24.2 to 30.4) |
Low/no 577 (72.9%; 95% CI 69.6 to 75.8) |
unadjusted OR (high/moderate vs low/no)− |
p Value |
|---|---|---|---|---|---|---|
| Child level | ||||||
| Male gender | 344 | 12 (3.5) | 97 (28.2) | 247 (71.8) | 1.1 (0.8 to 1.4) | 0.8 |
| Low birthweight (<2500g) | 49 | 3 (6.1) | 16 (32.7) | 33 (67.4) | 1.3 (0.7 to 2.4) | 0.8 |
| Preterm (<37 weeks) | 57 | 2 (3.5) | 17 (29.8) | 40 (70.2) | 1.1 (0.6 to 2.0) | 0.7 |
| SCN/NICU | 102 | 3 (2.9) | 31 (30.4) | 71 (69.6) | 1.2 (0.7 to 1.8) | 0.7 |
| Parent level | ||||||
| Mother did not complete high school | 110 | 3 (2.7) | 37 (33.6) | 73 (66.4) | 1.4 (0.9 to 2.2) | 0.1 |
| Father unemployed at birth | 37 | 1 (2.7) | 15 (40.5) | 22 (59.5) | 1.9 (1.0 to 3.8) | 0.06 |
| Mother nationality | ||||||
| Australia, UK, North America, New Zealand | 355 | 7 (2.0) | 82 (23.1) | 273 (76.9) | 1.0 (ref) | |
| Middle East | 97 | 6 (6.1) | 38 (39.2) | 59 (60.8) | 2.1 (1.3 to 3.5) | 0.002 |
| Asian (includes India, Bangladesh) | 210 | 6 (2.8) | 67 (31.9) | 143 (68.1) | 1.6 (1.1 to 2.3) | 0.02 |
| Other | 78 | 1 (1.3) | 18 (23.1) | 60 (76.9) | 0.9 (0.6 to 1.8) | 0.9 |
| Family level | ||||||
| English not primary language | 240 | 7 (2.9) | 76 (31.7) | 164 (68.3) | 1.4 (1.0 to 1.9) | 0.06 |
| Household annual income at birth<$A25 001 | 94 | 5 (5.3) | 35 (37.2) | 59 (62.7) | 1.7 (1.1 to 2.7) | 0.02 |
| Neighbourhood level | ||||||
| SEIFA lowest decile | 274 | 10 (3.7) | 90 (32.9) | 184 (67.2) | 1.5 (1.1 to 2.1) | 0.02 |
| Composite measures | ||||||
| Perinatal risk | 135 | 4 (3.0) | 39 (28.5) | 96 (71.5) | 1.1 (0.7 to 1.6) | 0.7 |
| Maternal Middle Eastern or Asian nationality | 307 | 12 (3.9) | 105 (34.2) | 202 (65.8) | 1.7 (1.3 to 2.4) | 0.001 |
| Household disadvantage | 187 | 8 (4.3) | 66 (35.3) | 121 (64.7) | 1.7 (1.2 to 2.4) | 0.005 |
NICU, neonatal intensive care unit; SCN, special care nursery; SEIFA, Socio-Economic Indexes for Areas.
Table 4.
Risk factors for moderate or high developmental risk in the 12-month group
| Total | High | High/moderate | Low/no | unadjusted OR (high/moderate vs low/no) | p Value | |
|---|---|---|---|---|---|---|
| Risk factor N (n)% Whole sample |
649 (100%) | 34 (5.2%; 95% CI 3.8 to 7.3) | 174 (26.8%; 95% CI 23.6 to 30.4) | 475 (73.2%; 95% CI 69.6 to 76.5) | ||
| Child level | ||||||
| Male gender | 281 | 16 (5.7) | 80 (28.5) | 201 (71.5) | 1.3 (0.9 to 1.8) | 0.2 |
| Low birthweight (<2500g) | 45 | 7 (15.5) | 20 (44.4) | 25 (55.6) | 2.4 (1.3 to 4.6) | 0.004 |
| Preterm (<37 weeks) | 57 | 7 (12.3) | 19 (33.3) | 38 (66.7) | 1.5 (0.8 to 2.6) | 0.2 |
| SCN/NICU | 94 | 8 (8.5) | 34 (36.2) | 60 (63.8) | 1.8 (1.1 to 2.8) | 0.01 |
| Parent level | ||||||
| Mother did not complete high school | 90 | 7 (7.8) | 31 (34.4) | 59 (65.6) | 1.5 (0.9 to 2.5) | 0.08 |
| Father unemployed at birth | 39 | 4 (10.0) | 15 (38.5) | 24 (61.5) | 1.8 (0.9 to 3.5) | 0.09 |
| Mother nationality | ||||||
| Australia, UK, North America, New Zealand | 300 | 14 (4.7) | 70 (23.3) | 230 (76.7) | 1.0 (ref) | |
| Middle Eastern | 92 | 6 (6.5) | 35 (38.0) | 57 (62.0) | 2.0 (1.2 to 3.3) | 0.006 |
| Asian (includes India, Bangladesh) | 157 | 10 (6.4) | 44 (28.0) | 113 (72.0) | 1.3 (0.8 to 2.0) | 0.3 |
| Other | 61 | 1 (1.6) | 10 (16.4) | 51 (83.6) | 0.6 (0.3 to 1.3) | 0.2 |
| Family level | ||||||
| English not primary language | 209 | 13 (6.2) | 60 (28.7) | 149 (71.3) | 1.1 (0.8 to 1.7) | 0.4 |
| Household annual income at birth <$A25 001 | 75 | 5 (6.7) | 21 (28.4) | 54 (71.6) | 1.2 (0.7 to 2.0) | 0.6 |
| Neighbourhood level | ||||||
| SEIFA lowest decile | 221 | 15 (6.8) | 67 (30.3) | 154 (69.7) | 1.4 (0.9 to 2.0) | 0.09 |
| Composite measures | ||||||
| Perinatal risk | 123 | 10 (8.1) | 41 (33.3) | 82 (66.7) | 1.6 (1.0 to 2.4) | 0.04 |
| Maternal Middle Eastern or Asian nationality | 249 | 16 (6.4) | 79 (31.7) | 170 (68.3) | 1.6 (1.1 to 2.4) | 0.008 |
| Household disadvantage | 157 | 10 (6.4) | 48 (30.6) | 109 (69.4) | 1.3 (0.9 to 2.0) | 0.2 |
NICU, neonatal intensive care unit; SCN, special care nursery; SEIFA, Socio-Economic Indexes for Areas.
Table 5.
Risk factors for moderate or high developmental risk in the 18-month group
| Risk factor N (n)% | Total | High | High/moderate | Low/no | Unadjusted OR (high/moderate vs low/no) | p Value |
|---|---|---|---|---|---|---|
| Whole sample | 565 (100) | 29 (5.1% 95% CI; 3.6 to 7.3) | 184 (32.6%; 95% CI 28.8 to 36.6) | 381 (67.4%; 95% CI 63.5 to 71.2) | ||
| Child level | ||||||
| Male gender | 244 | 12 (4.9) | 87 (35.7) | 157 (64.3) | 1.3 (0.9 to 1.9) | 0.1 |
| Low birthweight (<2500 g) | 38 | 4 (10.5) | 20 (52.6) | 18 (47.4) | 2.5 (1.3 to 4.8) | 0.007 |
| Preterm (<37 weeks) | 51 | 7 (13.7) | 25 (49.0) | 26 (51.0) | 2.2 (1.2 to 3.9) | 0.009 |
| SCN/NICU N | 86 | 9 (10.4) | 36 (41.8) | 50 (58.2) | 1.6 (1.0 to 2.6) | 0.04 |
| Parent level | ||||||
| Mother did not complete high school | 81 | 4 (4.9) | 25 (30.9) | 56 (69.1) | 0.9 (0.5 to 1.5) | 0.6 |
| Father unemployed at birth | 33 | 2 (6.0) | 13 (39.4) | 20 (60.6) | 1.3 (0.7 to 2.8) | 0.4 |
| Mother nationality | ||||||
| Australia, UK, North America, New Zealand | 255 | 9 (3.5) | 77 (30.2) | 178 (69.8) | 1.0 (ref) | |
| Middle Eastern | 104 | 6 (5.8) | 37 (35.6) | 67 (64.4) | 1.3 (0.8 to 2.1) | 0.3 |
| Asian (includes India, Bangladesh) | 122 | 7 (5.7) | 46 (37.7) | 77 (62.3) | 1.4 (0.9 to 2.2) | 0.1 |
| Other | 62 | 5 (8.1) | 16 (25.8) | 46 (74.2) | 0.8 (0.4 to 1.5) | 0.5 |
| Family level | ||||||
| English not primary language | 180 | 10 (5.5) | 61 (33.9) | 119 (66.1) | 1.1 (0.7 to 1.6) | 0.7 |
| Household annual income at birth <$A25 001 | 60 | 6 (10.0) | 23 (38.3) | 37 (61.7) | 1.4 (0.8 to 2.4) | 0.3 |
| Neighbourhood level | ||||||
| SEIFA lowest decile | 200 | 14 (7.0) | 70 (35.0) | 130 (65.0) | 1.2 (0.8 to 1.7) | 0.3 |
| Composite measures | ||||||
| Perinatal risk | 109 | 10 (9.1) | 44 (40.4) | 65 (59.6) | 1.6 (1.0 to 2.4) | 0.04 |
| Maternal Middle Eastern or Asian nationality | 226 | 13 (5.7) | 83 (36.7) | 143 (63.3) | 1.4 (1.0 to 2.0) | 0.07 |
| Household disadvantage | 134 | 9 (6.7) | 45 (33.6) | 89 (66.4) | 1.0 (0.7 to 1.6) | 0.9 |
NICU, neonatal intensive care unit; SCN, special care nursery; SEIFA, Socio-Economic Indexes for Areas.
Factors associated with parental concerns indicating moderate or high developmental risk on univariate logistic regression
In the 6-month group (table 3), increased prevalence of parental concerns indicating moderate or high developmental risk was associated with maternal Middle Eastern (OR: 2.1 (95% CI 1.3 to 3.5)) or Asian (OR: 1.6 (95% CI 1.1 to 2.3)) nationality; household annual income <$A25 001 (OR: 1.7 (95% CI 1.1 to 2.7))); and neighbourhood disadvantage (OR: 1.5 (95% CI 1.1 to 2.1)). Using composite measures, maternal Middle Eastern or Asian nationality (OR: 1.7 (95% CI 1.3 to 2.4)) and household disadvantage (OR: 1.7 (95% CI 1.2 to 2.4)) were significant (p<0.05).
In the 12-month group (table 4), increased prevalence of parental concerns indicating moderate or high developmental risk was associated with low birthweight (OR: 2.4 (95% CI 1.3 to 4.6)), admission of the infant to special care nursery/neonatal intensive care unit (SCN/NICU) (OR: 1.8 (95% CI 1.1 to 2.8)), and maternal Middle Eastern nationality (OR: 2.0 (95% CI 1.2 to 3.3)). Using composite measures perinatal risk (OR: 1.6 (95% CI 1.0 to 2.4)) and maternal Middle Eastern or Asian nationality (OR: 1.6 (95% CI 1.1 to 2.4)) were significant (p<0.05).
In the 18-month group (table 5), increased prevalence of parental concerns indicating moderate or high developmental risk was associated with low birthweight (OR: 2.5 (95% CI 1.3 to 4.8)); being preterm (OR: 2.2 (95% CI 1.2 to 3.9)); and/or admission to SCN/NICU after birth (OR: 1.6 (95% CI 1.0 to 2.6)). Using composite measures perinatal risk (OR: 1.6 (95% CI 1.0 to 2.4)) was significant (p<0.05).
Factors associated with parental concerns indicating moderate or high developmental risk on multivariate logistic regression
Multivariate logistic regression identified perinatal risk as an independent risk factor for moderate or high developmental risk in the 12-month group (OR: 1.7 (95% CI 1.1 to 2.6)). Maternal Middle Eastern or Asian nationality was a risk factor in the 6-month (OR: 1.6 (95% CI 1.1 to 2.4)) and 12-month group (OR: 1.7 (95% CI 1.1 to 2.7)). Household disadvantage was a risk factor in the 6-month group (OR: 1.5 (95% CI 1.0 to 2.2)) (table 6). In the models, there were 661 observations at 6 months, 546 observations at 12 months and 495 observations at 18 months due to missing observations.
Table 6.
Multivariate analysis of risk factors for moderate or high developmental risk
| Risk factors | 6-month group N=792 OR (p value) |
12-month group N=649 OR (p value) |
18-month group N=565 OR (p value) |
|||
|---|---|---|---|---|---|---|
| Child level | ||||||
| Male gender | 1.0 (0.7 to 1.4) | 0.9 | 1.1 (0.7 to 1.6) | 0.6 | 1.3 (0.9 to 1.9) | 0.2 |
| Perinatal risk | 1.0 (0.6 to 1.6) | 0.9 | 1.7 (1.1 to 2.6) | 0.03 | 1.6 (1.0 to 2.7) | 0.06 |
| Parent level | ||||||
| Maternal middle Eastern or Asian nationality | 1.6 (1.1 to 2.4) | 0.02 | 1.7 (1.1 to 2.7) | 0.03 | 1.5 (1.0 to 2.3) | 0.06 |
| Family level | ||||||
| English not primary language | 1.0 (0.6 to 1.5) | 0.9 | 0.8 (0.5 to 1.3) | 0.3 | 0.9 (0.6 to 1.4) | 0.7 |
| Household disadvantage | 1.5 (1.0 to 2.2) | 0.03 | 1.2 (0.8 to 1.9) | 0.3 | 0.9 (0.6 to 1.4) | 0.6 |
| Neighbourhood level | ||||||
| SEIFA lowest decile | 1.4 (1.0 to 2.0) | 0.1 | 1.3 (0.9 to 2.0) | 0.2 | 1.2 (0.8 to 1.8) | 0.5 |
SEIFA, Socio-Economic Indexes for Areas.
Documentation of the PEDS at the 6-month, 12-month and 18-month well-child check
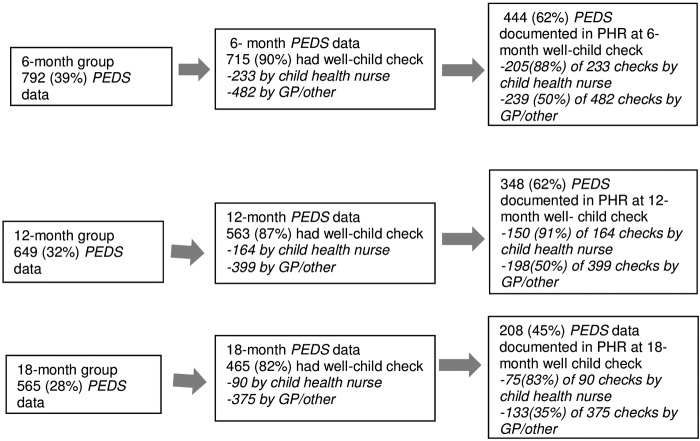
Figure 1 depicts details of the well-child check attendance, including the primary healthcare provider the child consulted for the well-child check and whether the PEDS was documented in the PHR. Of the 792 children in the 6-month group who had PEDS data available (either already documented in the PHR or conducted by researchers over the phone), 715 (90.0%) children had attended a well-child check and 444 (62%) had the PEDS results documented in their PHR.
Figure 1.
Data collection of PEDS outcomes. GP, general practitioner; PEDS, Parents' Evaluation of Developmental Status; PHR, personal health record.
Of the 649 children in the 12-month group who had PEDS data available (either already documented in the PHR or conducted by researchers over the phone), 563 (87%) children had attended a well-child check and 348 (62%) had the PEDS results documented.
Of the 565 children in the 18-month group who had PEDS data available (either already documented in the PHR or conducted by researchers over the phone), 465 (82%) children had attended a well-child check and 208 (45%) had the PEDS results documented. Depending on the time of measurement, 35–50% of children had a PEDS documented in the PHR if they had seen a GP or a health professional other than a child health nurse (eg, paediatrician) for the well-child check, compared with 83–91% of children who had seen a child health nurse.
Cumulative Risk Index and moderate or high developmental risk and documentation of the PEDS in the PHR
Table 7 shows increased odds of being at a moderate or high developmental risk with increasing number of risk factors in the 6-month group (p<0.001), in the 12-month group (p=0.004) and in the 18-month group (p=0.02). There were also increased odds that the PEDS was not documented in the PHR at well-child checks as the number of risk factors increased in the 6-month group (p<0.001), 12-month group (p<0.001) and 18-month group (p<0.001).
Table 7.
Cumulative risk for moderate or high developmental risk/documentation of PEDS in the PHR at well-child check* †
| Number of risk factors (RF) | Moderate or high developmental risk | PEDS not documented in PHR | Moderate or high developmental risk | PEDS not documented in PHR | Moderate or high developmental risk | PEDS not documented in PHR |
|---|---|---|---|---|---|---|
| 6-month group (p<0.001) |
6-month group (p<0.001) | 12-month group (p=0.004) |
12-month group (p<0.001) | 18-month group (p=0.02) | 18-month group (p<0.001) | |
| 0 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 1 | 1.3 (0.7 to 2.5) | 1.3 (0.7 to 2.3) | 1.4 (0.7 to 2.7) | 0.8 (0.4 to 1.5) | 2.2 (1.1 to 4.3) | 2.4 (1.2 to 4.7) |
| 2 | 1.4 (0.7 to 2.6) | 1.6 (0.9 to 2.9) | 1.9 (1.0 to 3.8) | 1.1 (0.6 to 2.1) | 2.2 (1.1 to 4.4) | 2.8 (1.4 to 5.5) |
| 3 | 1.7 (0.9 to 3.3) | 2.6 (1.4 to 4.8) | 1.8 (0.9 to 3.7) | 2.1 (1.0 to 4.0) | 2.7 (1.3 to 5.5) | 4.4 (2.1 to 9.2) |
| 4 | 2.3 (1.1 to 4.7) | 4.3 (2.2 to 8.3) | 2.3 (1.1 to 5.0) | 6.2 (3.0 to 12.5) | 2.2 (1.0 to 4.8) | 8.6 (3.9 to 19.0) |
| 5 | 4.8 (1.8 to, 12.6) | − | 3.2 (1.3 to 8.0) | − | 3.2 (1.2 to 8.9) | − |
*6 months (661 observations), 12 months (546 observations), 18 months (495 observations)—for moderate or high developmental risk. †6 months (596 observations), 12 months (477 observations), 18 months (407 observations) for not documented in PHR if had well-child check.
PEDS, Parents' Evaluation of Developmental Status; PHR, personal health record.
Discussion
In this Australian sample from a culturally diverse and socioeconomically disadvantaged area, the prevalence of developmental vulnerability defined as parental concerns on the PEDS indicating moderate or high developmental risk is substantial with between a quarter and a third of children at this risk level. This finding is consistent with the recent systematic review on the topic.9 However, the proportion of children at high developmental risk (2.7–5.1%) in our study is lower than the estimates from the systematic review of 13.8%—probably due to the fact that our sample was restricted to children aged under 2 years.9 Another possibility is that, due to the attrition in our study sample, those parents who were harder to contact and/or refused to continue to participate had more risk factors which would, as the results indicate, be related to higher proportions of parental concern. Perinatal risk, maternal minority nationality and household disadvantage were independent risk factors in this current study for developmental vulnerability, using this measure, in the 6-month and 12-month groups, which is consistent with the current literature.9 21–25
We found that the greater the number of child, parent, family and neighbourhood risk factors, the higher the odds of moderate or high developmental risk on the PEDS in the 6-month, 12-month and 18-month groups. This finding is in keeping with the bioecological model of ECD with a cumulative burden of multiple risk factors on developmental outcomes.9 11 21 24 26–30 What was somewhat surprising was that this cumulative effect was evident as early as infancy, a time of enormous neurobiological and neuroanatomical adaptation and vulnerability. Studies examining the dose response relationship between the number of risk factors and developmental vulnerability as indicated by the PEDS have included young children in their sample, but none have solely focused on children under 2 years of age.11
The high level of parental concerns, overall in each group demonstrated in this study, supports a comprehensive universal service response to support parents and promote ECD. Services need to be enhanced, in particular, for those children at perinatal risk and for families from culturally and linguistically diverse (CALD) background and/or socioeconomically disadvantaged populations using an approach of proportionate universalism.31 32 This is in keeping with recent state/federal policy initiatives in NSW/Australia that aim to promote parental health literacy around ECD to ensure a deeper understanding of what to expect at different ages, when to be concerned and where to seek help.33 34
In our study, the vast majority of parents reported seeing a primary healthcare provider for a well-child check at 6, 12 and 18 months—as outlined in their PHR, although this dropped to 82% for the 18-month check. However, at best, only 50% of children who saw a GP/other non-child health nurse for their well-child check had PEDS documentation in the PHR. This is concerning given that the PHR is an important tool for parents and healthcare providers to document and discuss a child's health over their multiple health service contacts in the early years. The odds of lack of PEDS documentation increased with multiple risk factors. Thus, children and families with multiple risk factors are more likely to experience developmental vulnerability, and their developmental vulnerability is less likely to be documented and perhaps less likely to identified at well-child checks. However, we cannot rule out its identification through history, observation and another developmental surveillance tool that hasn't been documented in the PHR. Recent quantitative and qualitative research has identified a number of factors that were barriers to early identification of developmental vulnerability, including lack of parental, GP and community knowledge around ECD and developmental surveillance.31 35 Thus, there is work to do with GPs and other non-child health nurse services to enhance the consistency of their early identification of developmental vulnerability through developmental surveillance practice using evidence-based tools.
Limitations
Although the WMG birth cohort is broadly representative of the CALD population from which it is sourced,13 a significant limitation to the study is the lack of follow-up of some mothers and infants from the time of recruitment at birth to the 18-month follow-up, thus impacting on the applicability and power of the study. There were difficulties contacting participants by telephone in a timely manner, which was compounded by changes to contact details of the families. Multiple methods of contact were used by research staff, including mobile phones, emails and landlines. This significant loss to follow-up limited any longitudinal analysis of the prognostic impact of parental concerns indicating developmental vulnerability over time. To address the significant challenge in retaining a cohort at each follow-up point, there needs to be a more effective method of longitudinal follow-up than phone calls which, in the WMG, necessitated significant manpower resources with a disappointing result. Adequate resources for a study director, ongoing participant engagement strategies, such as collaborative research planning, home visiting, study information days and community presentations, are all strategies that have been used with success in other Australian cohort studies.15 16 36
In this study, composite measures of perinatal risk and household disadvantage were used to reflect the different components of perinatal risk and wealth on developmental vulnerability and to avoid potential collinearity in the regression models.20 There is some debate around the use of composite indicators. While a number of longitudinal studies have used this approach, such as the Longitudinal Study of Australia's Children,37 the recent AAP policy regarding measurement of SES does not recommend their use.38
Another limitation in this study is that the measure of race/ethnicity used in the WMG study only reflects maternal nationality as reported by mothers at the time of antenatal check in. This may not be an accurate reflection of the ethnic and cultural milieu at home, which is influenced by multiple factors, including paternal and maternal backgrounds and country of origin. The WMG study did not specifically measure acculturation, but the AAP suggests that language spoken at home is a valid proxy measure for acculturation.38 Maternal mental health was unable to be examined—due to missing data in the EMR, which requires future examination by service providers around whether the Edinburgh Depression Scale is being uniformly administered and its data recorded.
In addition, the PEDS is not a comprehensive developmental assessment that acts as a ‘gold standard’, it is a developmental surveillance tool only. Some children identified as developmentally vulnerable by parental concern on the PEDS will be false positives.10 39 The parental concerns may reflect, for example parental stress due to poverty, the anxiety of having a child who was preterm or underlying mental health issues. This still warrants a system response in terms of support, advice and monitoring. In addition, the PEDS outcome data were collected in two different ways in this study: the first from PEDS results documented in the PHR, and the second from the PEDS being administered over the phone by a researcher if there was no documentation. Although the PEDS has been administered extensively by both methods in the research,9 the fact that the PEDS was collected over the phone for a significant proportion of participants, rather than during a well-child check in the primary healthcare setting, limits the applicability of these findings to clinical practice.
The impact of multiple risk factors was examined in this study using a Cumulative Risk Index of individual and composite child, parent and family risk factors. This is a robust way of examining the impact risk factors in a bioecological model of child development in longitudinal and cross-sectional studies, and suits smaller sample sizes. Its advantage is its simplicity and the fact that the ‘dose’ of a number of risk factors can be investigated. However, it does not allow for investigation of risk factors that are mediators and moderators. In addition, all risk factors are given equal weight which may or may not reflect ‘real life’.26
Conclusion
The prevalence of parental concerns on the PEDS indicating moderate or high developmental risk increases with multiple risk factors. This study has illustrated the impact of cumulative risk on the developing child as early as infancy. We need to take a life course approach in service planning from conception onwards to address the inequity in ECD to positively impact the developing brain when it is most likely to be beneficial. Each stage of integrated service delivery needs to build on to the next—a form of ‘cumulative buffering’ to address the cumulative risks identified here.
Acknowledgments
The authors thank Professor Margot Prior for her contribution to the development of the research proposal, the Child and Family Health Nurses (CFHN) in the Liverpool/Fairfield/Bankstown areas and CFHN Managers Trish Clarke, Victoria Blight and Wendy Geddes, the staff of the postnatal wards at Liverpool and Bankstown hospitals, the staff at South Western Sydney Local Health District (SWSLHD) IM&TD., as well as research staff, including Amelia Walter, Trinh Ha, Susan Harvey, Nicole Lees, Olivia Wong, Laura Nicholls, Banosha Yacob, Cherie Butler, Feroza Khan, Janelle Cleary, Mary Ha, Snehal Akre and Van Nguyen.
Footnotes
Collaborators: ‘Watch Me Grow’ Study Group: Butler C, Cleary JA, Deering A, Descallar J Einfield SL, Garg P, Ha MT, Ha M, Harvey S, Matthey S, McKenzie A, Nguyen V, Nicholls L, Shine T, Short K, Silove N, Walter A, Wong O, Yakob B.
Contributors: SW, VE, KW, BJ, CD, EM, JE, RC and DB developed the study design and participated in the preparation of the manuscript. EA, AH and BO provided assistance in developing the study protocols and databases, and participated in manuscript preparation. LK, AH and AMK gave critical input into the data analysis and manuscript preparation. All authors have read and approved the content of the manuscript.
Funding: This study (APP 1013690) was funded by the National Health and Medical Research Council (NH&MRC) of Australia, through a partnership grant with the New South Wales Department of Health, Kids and Families and in-kind support from University of New South Wales, La Trobe University, SWSLHD and Sydney Children's Hospital Network.
Competing interests: None declared.
Ethics approval: The study was approved by the Human Research Ethics Committees of SWSLHD and the University of New South Wales (HREC/11/LPOOL/281).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The unpublished data are available to all members of the Watch Me Grow group and coresearchers if a research application is put into the WMG governance group. The data are de-identified and kept in a password secured server system at the Academic Unit of Child Psychiatry South West Sydney Local Health District (AUCS), Australia School of Psychiatry and Ingham Institute, University of New South Wales, Australia.
Contributor Information
Collaborators: C Butler, JA Cleary, A Deering, J Descallar, SL Einfield, P Garg, MT Ha, M Ha, S Harvey, S Matthey, V Nguyen, L Nicholls, T Shine, K Short, N Silove, A Walter, O Wong, and B Yakob
References
- 1.Goldfeld S, O'Connor M, Sayers M et al. . Prevalence and correlates of special health care needs in a population cohort of Australian children at school entry. J Dev Behav Pediatr 2012;33:319–27. 10.1097/DBP.0b013e31824a7b8e [DOI] [PubMed] [Google Scholar]
- 2.Fiscella K, Kitzman H. Disparities in academic achievement and health: the intersection of child education and health policy. Pediatrics 2009;123:1073–80. 10.1542/peds.2008-0533 [DOI] [PubMed] [Google Scholar]
- 3.Starfield B. Equity, social determinants, and children's rights: coming to grips with the challenges. Ambul Pediatr 2005;5:134–7. [DOI] [PubMed] [Google Scholar]
- 4.Lynch JW, Law C, Brinkman S et al. . Inequalities in child healthy development: some challenges for effective implementation. Soc Sci Med 2010;71:1244–8;discussion 1254–8 10.1016/j.socscimed.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 5.NH&MRC. Centre for Community Child Health. Child Health screening and Surveillance: A critical review of the literature. Canberra: National Health and Medical Research Council, 2002. [Google Scholar]
- 6.AAP. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics 2006;118:405–20. 10.1542/peds.2006-1231 [DOI] [PubMed] [Google Scholar]
- 7.Glascoe FP. Using parents’ concerns to detect and address developmental and behavioral problems. J Soc Pediatr Nurs 1999;4:24–35. 10.1111/j.1744-6155.1999.tb00077.x [DOI] [PubMed] [Google Scholar]
- 8.Woolfenden S, Goldfeld S, Raman S et al. . Inequity in child health: the importance of early childhood development. J Paediatr Child Health 2013;49:E365–9. 10.1111/jpc.12171 [DOI] [PubMed] [Google Scholar]
- 9.Woolfenden S, Eapen V, Williams K et al. . A systematic review of the prevalence of parental concerns measured by the Parents’ Evaluation of Developmental Status (PEDS) indicating developmental risk. BMC Pediatrics 2014;13:231 10.1186/1471-2431-14-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glascoe F. Collaborating with parents: using Parents’ Evaluation of Developmental Status (PEDS) to detect and address developmental and behavioral problems. 2nd edn Nolensville, TN: PEDSTest.com, LLC 2013. (http://www.pedstest.com). 2013. [Google Scholar]
- 11.Stevens GD. Gradients in the health status and developmental risks of young children: the combined influences of multiple social risk factors. Matern Child Health J 2006;10:187–99. 10.1007/s10995-005-0062-y [DOI] [PubMed] [Google Scholar]
- 12.Eapen V, Woolfenden S, Williams K et al. . Are you available for the next 18 months? Methods and aims of a longitudinal birth cohort study investigating a universal developmental surveillance program: The ‘Watch Me Grow’ study. BMC Pediatrics 2014;14:234 10.1186/1471-2431-14-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolfenden S, Eapen V, Axelsson E et al. . Who is our cohort: recruitment, representativeness, baseline risk and retention in the “Watch Me Grow” study? BMC Pediatr 2016;16:1–11. 10.1186/s12887-016-0582-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bronfenbrenner U. The ecology of human development: experiments by nature and design. Cambridge, MA: Harvard University Press, 1979. [Google Scholar]
- 15.LSAC. Growing up in Australia. The Longitudinal Study of Australian Children. http://www.growingupinaustralia.gov.au/studyqns/index.html (accessed May 2012).
- 16.Nicholson J, Sanson A. A new longitudinal study of the health and wellbeing of Australian children: How will it help? Med J Aust 2003;178:282–4. [DOI] [PubMed] [Google Scholar]
- 17.ABS. Census of population and housing: Socio-Economic Indexes for Areas (SEIFA). Australia: Australian Bureau of Statistics, 2011. http://www.abs.gov.au/ausstats/abs@.nsf/mf/2033.0.55.001 (accessed Sep 2014). [Google Scholar]
- 18.Sterne JA, White IR, Carlin JB et al. . Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ABS. 1249.0—Australian Standard Classification of Cultural and Ethnic Groups (ASCCEG), 2011 2011. http://www.abs.gov.au/ausstats/abs@.nsf/lookup/1249.0main+features22011 (accessed Sep 2014).
- 20.Moore K, Vandivere S, Redd Z. A Sociodemographic risk index. Soc Indicators Res 2006;75:45–81. 10.1007/s11205-004-6398-7 [DOI] [Google Scholar]
- 21.Walker SP, Wachs TD, Grantham-McGregor S et al. . Inequality in early childhood: risk and protective factors for early child development. Lancet 2011;378:1325–38. 10.1016/S0140-6736(11)60555-2 [DOI] [PubMed] [Google Scholar]
- 22.Guralnick MJ. Effectiveness of early intervention for vulnerable children: a developmental perspective. Am J Ment Retard 1997;102:319–45. [DOI] [PubMed] [Google Scholar]
- 23.King EH, Logsdon DA, Schroeder SR. Risk factors for developmental delay among infants and toddlers. Child Health Care 1992;21:39–52. 10.1207/s15326888chc2101_6 [DOI] [PubMed] [Google Scholar]
- 24.Maggi S, Irwin LJ, Siddiqi A et al. . The social determinants of early child development: an overview. J Paediatr Child Health 2010;46:627–35. 10.1111/j.1440-1754.2010.01817.x [DOI] [PubMed] [Google Scholar]
- 25.Patrianakos-Hoobler AI, Msall ME, Marks JD et al. . Risk factors affecting school readiness in premature infants with respiratory distress syndrome. Pediatrics 2009;124 (258–67. 10.1542/peds.2008-1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woolfenden S, Williams K, Eapen V et al. . Developmental vulnerability—Don't investigate without a model in mind. Child Care Health Dev 2015;41:337–45. 10.1111/cch.12181 [DOI] [PubMed] [Google Scholar]
- 27.Msall ME, Bier JA, LaGasse L et al. . The vulnerable preschool child: The impact of biomedical and social risks on neurodevelopmental function. Semin Pediatr Neurol 1998;5:52–61. 10.1016/S1071-9091(98)80019-3 [DOI] [PubMed] [Google Scholar]
- 28.Nicholson J, Carroll J, Brodie A et al. . Child and youth health inequalities in Australia; the status of Australian research 2003. Paper prepared for the Health Inequalities Research Collaboration. Children, Youth and Families Network, 2004. [Google Scholar]
- 29.NRC&IM. Committee on evaluation of children's health;board on children, youth, and families; children's health, the nation's wealth: assessing and improving child health. In: AcademiesPress N , ed. Washington, DC: Division of Behavioral and Social Sciences and Education; National Research Council and Institute of Medicine, 2004. [Google Scholar]
- 30.Hertzman C. Framework for the Social Determinants of Early Child Development. In: Development CoEfEC, ed. Encyclopedia on early childhood development. Canada: University of British Columbia, 2010. [Google Scholar]
- 31.Woolfenden S, Short K, Blackmore R et al. . How do primary health-care practitioners identify and manage communication impairments in preschool children? Aust J Prim Health 2015;21:176–81. 10.1071/PY12152 [DOI] [PubMed] [Google Scholar]
- 32. Marmot M. Fair Society, Healthy Lives (The Marmot Review). Strategic Review of Health Inequalities in England post-2010: The Marmot Review. 2010. http://www.instituteofhealthequity.org/projects/fair-society-healthy-lives-the-marmot-review (accessed May 2012).
- 33.COAG. Investing in the Early Years—A National Early Childhood Development Strategy. An initiative of the Council of Australian Governments 2009. https://www.coag.gov.au/sites/default/files/national_ECD_strategy.pdf (accessed Nov 2015).
- 34.NSW Health. Child Personal Health Record. NSW Kids and Families 2015. http://www.kidsfamilies.health.nsw.gov.au/publications/child-personal-health-record-(blue-book) (accessed Sep 2015).
- 35.Woolfenden S, Posada N, Krchnakova R et al. . Equitable access to developmental surveillance and early intervention—understanding the barriers for children from culturally and linguistically diverse (CALD) backgrounds. Health Expect 2015;18:3286–301. 10.1111/hex.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald J, Comino E, Knight J et al. . Developmental progress in urban aboriginal infants: a cohort study. J Paediatr Child Health 2012;48:114–21. 10.1111/j.1440-1754.2011.02067.x [DOI] [PubMed] [Google Scholar]
- 37.Nicholson JM, Lucas N, Berthelsen D et al. . Socioeconomic inequality profiles in physical and developmental health from 0–7 years: Australian National Study. J Epidemiol Community Health 2012;66:81–7. 10.1136/jech.2009.103291 [DOI] [PubMed] [Google Scholar]
- 38.Cheng TL, Goodman E, Committee on Pediatric Research. Race, ethnicity, and socioeconomic status in research on child health. Pediatrics 2015;135:e225–37. 10.1542/peds.2014-3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon AE, Pastor PN, Avila RM et al. . Socioeconomic disadvantage and developmental delay among US children aged 18 months to 5 years. J Epidemiol Community Health 2013;67:689–95. 10.1136/jech-2013-202610 [DOI] [PMC free article] [PubMed] [Google Scholar]