Abstract
Ketamine has been shown to produce rapid and robust antidepressant effects in depressed individuals, however its abuse potential and adverse psychotomimetic effects limit its widespread use. Dextromethorphan may serve as a safer alternative based on pharmacodynamic similarities to ketamine. In this proof of concept study, behavioral and biochemical analyses were undertaken to evaluate the potential involvement of brain derived neurotrophic factor (BDNF) in the antidepressant-like effects of dextromethorphan in mice, with comparisons to ketamine and imipramine. Male Swiss, Webster mice were injected with dextromethorphan, ketamine or imipramine and their behaviors evaluated in the forced swim test (FST) and open field test. Western blots were used to measure brain derived neurotrophic factor (BDNF) and its precursor, pro-BDNF, protein expression in the hippocampus and frontal cortex of these mice. Our results show dextromethorphan and imipramine each reduced immobility time in the FST without affecting locomotor activity, whereas ketamine reduced immobility time and increased locomotor activity. Ketamine also rapidly (within 40 min) increased pro-BDNF expression in an AMPA receptor-dependent manner in the hippocampus, while DM and imipramine did not alter pro-BDNF or BDNF levels in either the hippocampus or frontal cortex within this timeframe. These data demonstrate that dextromethorphan shares some features with both ketamine and imipramine. Additional studies looking at dextromethorphan may aid in the development of more rapid, safe, and efficacious antidepressant treatment.
Keywords: antidepressant, brain derived neurotrophic factor, dextromethorphan, ketamine, AMPA
INTRODUCTION
Most available pharmaceutical agents for treating depression have similar mechanisms of action targeting monoaminergic systems, remain ineffective in a third of patients, and have a delayed clinical efficacy of several weeks to months (Berton and Nestler, 2006). Notably, accumulating evidence indicates that sub-anesthetic doses of the glutamate N-methyl-D-aspartate (NMDA) antagonist ketamine can produce robust and rapid antidepressant effects even in treatment-resistant individuals (Murrough et al., 2013). However, the widespread use of ketamine is limited by abuse liability and adverse effects (Aan Het Rot et al., 2012).
In the search for alternatives to ketamine, dextromethorphan was postulated to have fast acting antidepressant activity based on pharmacodynamic similarities to ketamine (Lauterbach, 2012). In contrast to ketamine, dextromethorphan has been available over-the-counter for over 50 years as an antitussive, with a wide margin of safety, and thus may serve as a safer alternative to ketamine to be utilized for depressed patients. Recently, dextromethorphan has also been approved for use in the treatment of pseudobulbar affect in combination with a low dose of the cytochrome P450 (CYP) 2D6 inhibitor quinidine (to slow down the first-pass metabolism of dextromethorphan) in the United States and Europe. Importantly, in depressed patients with treatment resistant disease, some subjects reported improvements in mood within 1–2 days of initiating treatment with dextromethorphan/quinidine or having the dose increased to twice a day (Kelly and Lieberman, 2014).
In preclinical studies, we found that dextromethorphan exerts antidepressant-like effects in mice using the forced swim test (FST) and tail suspension test, two well-established and commonly used animal models for predicting antidepressant efficacy (Nguyen et al., 2014, Nguyen and Matsumoto, 2015). Moreover, treatment with the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) antagonist NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt) attenuated the antidepressant-like effects of dextromethorphan in these behavioral tests (Nguyen et al., 2014, Nguyen and Matsumoto, 2015). Earlier studies suggest that AMPA receptors are associated with producing a faster onset of antidepressant efficacy (Adell et al., 2005, Duman, 2014), and are critical to the fast-acting antidepressant effects of ketamine (Maeng et al., 2008, Koike et al., 2011, Lindholm et al., 2012, Zhou et al., 2014). They may therefore contribute to the faster onset of antidepressant actions produced by dextromethorphan (Kelly and Lieberman, 2014).
A growing body of evidence suggests that neural plasticity may be a final common pathway of different antidepressant therapies and may explain the delay in efficacy with conventional antidepressants (Duman, 2014). An important contributor driving neural adaptation and antidepressant-like effects is brain derived neurotrophic factor (BDNF) (Castren and Rantamaki, 2010). BDNF protects neurons from injury, at least in part, by inhibiting apoptosis and by stimulating neurite sprouting and neuronal reorganization (Balaratnasingam and Janca, 2012). BDNF is formed from its precursor, pro-BDNF, and both pro-BDNF and mature BDNF levels are altered in animal models of depression and in humans with this mood disorder (Castren and Rantamaki, 2010, Polyakova et al., 2015). For instance, post-mortem studies have reported reductions in BDNF levels in the prefrontal cortex and hippocampus of suicide victims who were depressed relative to matched controls or patients taking an antidepressant at the time of death (Dwivedi et al., 2001). In contrast, antidepressant treatment has led to upregulation of BDNF in the hippocampus of patients with major depression at the time of death compared to untreated subjects (Chen et al., 2001), and intracerebral infusion of BDNF results in an antidepressant-like effect in animal models of depression (Hoshaw et al., 2005). Interestingly, chronic, but not acute, treatment with classic antidepressant drugs increases BDNF expression (Castren and Rantamaki, 2010), whereas acute and/or chronic treatment with ketamine can promote increases in BDNF, and pro-BDNF, expression (Garcia et al., 2008, Autry et al., 2011, Reus et al., 2011, Yang et al., 2013, Reus et al., 2014, Zhou et al., 2014). Thus, pharmaceutical agents capable of quickly enhancing BDNF, and possibly pro-BDNF, levels may aid in the development of novel, effective, and fast-acting antidepressant drugs.
In the present study, the behavioral effects of dextromethorphan were examined with additional neurobiochemical analyses being made. Specifically, the effects of dextromethorphan on antidepressant-like effects in the FST, the most predictive rodent model for screening potential antidepressant drugs (Cryan and Holmes, 2005), and locomotor activity in the open field test (OFT) were studied in mice. Fast acting and conventional antidepressants represented by ketamine and imipramine, respectively, were used as reference drugs in these studies. In addition, we evaluated the effects of these compounds on pro-BDNF and BDNF protein expression in the hippocampus and frontal cortex, two key areas involved in depression (Palazidou, 2012). Because the precursor and mature forms of BDNF may be independently regulated and have been separately shown to rapidly increase following ketamine administration (Garcia et al., 2008, Autry et al., 2011, Reus et al., 2011, Yang et al., 2013, Reus et al., 2014, Zhou et al., 2014), we evaluated them individually rather than as a ratio or in combination. Also, because the antidepressant-like behaviors of dextromethorphan have only been established recently and at a single time point (Nguyen et al., 2014, Nguyen and Matsumoto, 2015), we used this known antidepressant effective time point to evaluate potential biochemical changes. Finally, as AMPA receptors have been previously shown to be critical for the antidepressant-like effects of dextromethorphan and ketamine (Nguyen and Matsumoto, 2015), we also examined their role in modulating the potential behavioral and biochemical effects discussed herein.
MATERIALS AND METHODS
Animals
Male, Swiss Webster mice (24–28 g; Harlan, Frederick, MD) were housed with food and water ad libitum, with a 12:12 h light–dark cycle. Animals were housed in groups of five for at least one week prior to initiation of experiments. All procedures were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Institutional Animal Care and Use Committee at West Virginia University (Morgantown, WV) approved the protocol and all efforts were made to minimize suffering.
Drugs and chemicals
Dextromethorphan hydrobromide, ketamine hydrochloride and imipramine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). NBQX was obtained from Tocris (Ellisville, MO). All compounds were dissolved in sterile saline (Teknova, Hollister, CA).
Experimental groups
Mice received intraperitoneal (ip) injections of saline or drug solutions (imipramine, ketamine, or dextromethorphan) at a volume of 10 ml/kg of body weight after acclimating to the OFT, 30 min prior to the FST. Pretreatment with NQBX was administered 15 min prior to ketamine. Otherwise, pretreatment with saline was administered prior to a second drug (imipramine, ketamine, or dextromethorphan). The behavioral experiments were carried out between 10:00 a.m. and 2:00 p.m. Immediately after the behavioral tests (N=10 per group, for a total of 50 mice), the animals were killed by decapitation and the hippocampus and frontal cortex were extracted for Western blot analysis (N=8–10 per group).
Open field test (OFT)
Locomotor activity was measured in the OFT utilizing the Photobeam Activity System-Open Field (San Diego Instruments, San Diego, CA), an automated activity monitoring system. Prior to locomotor activity measurements, animals were acclimated to the testing facility for at least 30 min and habituated to the testing chambers for an additional 30 min. Each testing chamber consisted of a Plexiglas housing and a 16 × 16 photobeam array to detect lateral (ambulatory and fine) movements, with a separate 16 photobeam array to detect rearing activity. Subsequent to the acclimation period, animals were treated and placed back in their respective chambers. Ambulatory, fine and rearing movements were quantified and summated as a measure of total locomotor activity for the next 30 min.
Forced swim test (FST)
Immediately after the locomotor measurements, mice were subjected to the FST, which was adapted with some modifications from the behavioral despair test described by Porsolt (Porsolt et al., 2001). Mice were placed in individual cylinders of water (10 cm deep, 12.7 cm diameter) for a total of 6 min. The initial 2 min was an acclimation period and not scored. During the remaining 4 min, immobility time was quantified using ANY-Maze version 4.63 video tracking software (Stoelting Co., Wood Dale, IL). Immobility was defined as no activity other than that required for maintaining the animal's head above the surface of the water. ANY-Maze software settings were as follows: accustomization period = 120 s, test duration = 240 s, minimum immobility time = 2000 ms (2 s), and immobility sensitivity = 65%.
Western blot
Immediately after completion of the behavioral measurements in the FST, animals were sacrificed by decapitation. Brains were then removed and bilateral hippocampus and frontal cortex samples dissected, flash frozen and stored at −80° C. To dissect the frontal cortex, we first cut off the olfactory bulb. We then divided the cortex into two hemispheres and sectioned off the tissue in front of the white matter (corpus callosum). Tissues were homogenized and lysed in RIPA (radioimmunoprecipitation assay) buffer (Teknova, Hollister, CA) containing protease inhibitors (cOmplete ULTRA Protease Inhibitor Cocktail Tablets; Roche, Indianapolis, IN) and phosphatase inhibitors (phosSTOP Phosphatase Inhibitor Cocktail Tablets; Roche), and centrifuged at 13,000×g for 30 min at 4° C. The supernatants were assayed for total protein concentrations using BCA (bicinchoninic acid assay) protein assay kits (Pierce, Rockford, IL). Samples were run at 20 or 30 µg of protein/well using Bolt Bis-Tris Plus 10% pre-cast 15 well gels (Life Technologies, Grand Island, NY) in combination with 2 × Laemmli sample buffer (Bio-Rad, Hercules, CA). After electrophoresis and transfer onto polyvinylidene fluoride (PVDF) membranes, blots were incubated with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature and then with the appropriate primary antibodies overnight at 4° C (rabbit anti-pro-BDNF catalog no. ANT006, 1:600, Alomone Labs, Jerusalem, Israel; rabbit anti-BDNF catalog no. ANT010, 1:200, Alomone Labs; or mouse anti-α-tubulin catalog no. ab7291, 1:100,000, Abcam, Cambridge, MA). After washing with Tris-buffered saline containing 0.1% Tween 20, the blots were incubated with the secondary antibodies (IRDye 800CW goat anti-mouse or 700CW goat anti-rabbit, 1:10,000, LI-COR Biosciences) containing 0.01% SDS for 45 min at room temperature. Visualization was carried out with an Odyssey Classic Infrared Imaging System (LI-COR Biosciences). Detected bands were quantified using Image Studio Lite Software (LI-COR Biosciences), normalized to relevant α-tubulin values, and expressed as a percent of control density within individual blots.
Data analysis
Data from all experiments were analyzed using GraphPad Prism 5.0 (San Diego, CA) by Student’s unpaired t-test or by one-way analysis of variance (ANOVA), followed by Tukey's multiple comparisons tests. Data are represented as mean ± SEM. P<0.05 was considered statistically significant for all data analyzed.
RESULTS
Using previously established antidepressant effective doses of the compounds of interest (Nguyen et al., 2014, Nguyen and Matsumoto, 2015), we confirmed that the fast acting antidepressant drug ketamine and conventional antidepressant imipramine both reduced immobility time in the FST (Fig. 1A and 1B; t=4.31, p<0.001; and t=6.22, p<0.0001, respectively). Similar to the reference drugs, dextromethorphan significantly reduced immobility time (Fig. 1C; t=5.00, p<0.0001).
Figure 1. Antidepressant-like effects of ketamine, imipramine, and dextromethorphan in the forced swim test.
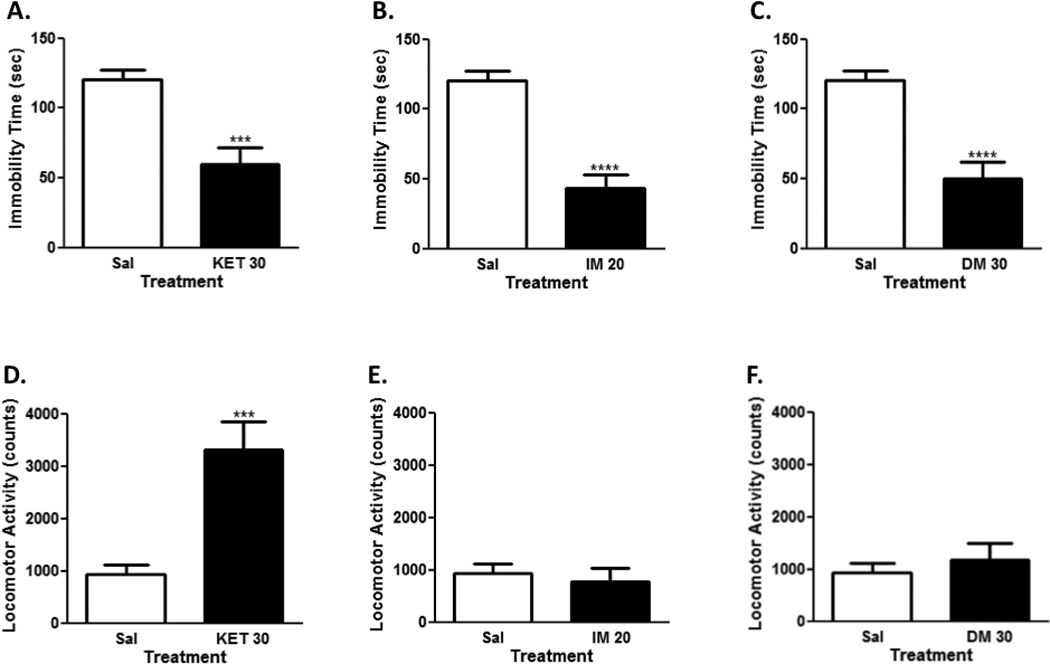
Ketamine (30 mg/kg, ip; N=10), significantly decreased immobility time (A) and increased locomotor activity (D). Imipramine (20 mg/kg, ip; N=10) also significantly decreased immobility time (B), but had no significant effects on locomotor activity (E). Likewise, dextromethorphan (30 mg/kg, ip; N=10) significantly decreased immobility time (C), but had no effects on locomotor activity (F). Data shown are expressed as mean ± SEM. ***p< 0.001, ****p<0.0001 vs. saline-treated group (N=10); Student’s unpaired t-test. Sal, saline; KET, ketamine; IM, imipramine; DM, dextromethorphan.
In the OFT, ketamine produced significant increases in locomotor activity (Fig. 1D; t=4.14, p<0.001). Imipramine and dextromethorphan, on the other hand, did not significantly alter locomotor activity (Fig. 1E and 1F; t=0.48 and t=0.67, n.s., respectively).
As shown in Fig. 2A and 2B, ketamine significantly increased pro-BDNF (~32–34 kDa) levels in the hippocampus (t=2.53, p<0.05), but not the frontal cortex (1.64, n.s.) 40 min post-injection. Imipramine did not alter pro-BDNF protein expression in the hippocampus (t=0.26, n.s.) or frontal cortex (t=1.37, n.s.) at 40 min post-injection. Similar to imipramine, dextromethorphan also did not increase pro-BDNF levels in the hippocampus (t=0.71, n.s.) or frontal cortex (t=0.83, n.s.) 40 min after injection.
Figure 2. Ketamine-induced alternations in pro-BDNF protein expression in the hippocampus, but not frontal cortex.
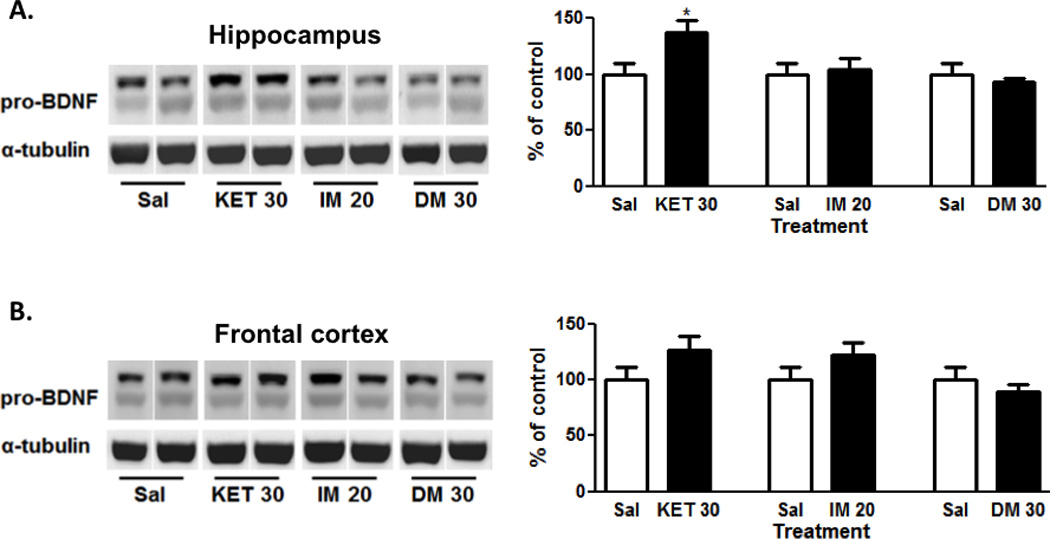
Ketamine (30 mg/kg, ip; N=9), but not imipramine (20 mg/kg, ip; N=10) or dextromethorphan (30 mg/kg, ip; N=10) significantly increased pro-BDNF levels in the hippocampus (A). In contrast, ketamine (30 mg/kg, ip; N=9), imipramine (20 mg/kg, ip; N=10) or dextromethorphan (30 mg/kg, ip; N=9) had no significant effects on pro-BDNF levels in the frontal cortex (B). Representative images of the protein are shown on the left and the cumulative graphed data on the right. Data shown are expressed as mean ± SEM. *p< 0.05 vs. saline-treated group (N=10); Student’s unpaired t-test. Sal, saline; KET, ketamine; IM, imipramine; DM, dextromethorphan.
With regards to the mature form of BDNF (~18 kDa), no alterations in BDNF protein expression were observed in the hippocampus (Fig. 3A) or frontal cortex (Fig. 3B) for any of the drugs tested (ketamine: t=0.13 or 0.03; imipramine: t=0.78 or 0.95; dextromethorphan: t=1.55 or 0.37, respectively).
Figure 3. No alternations in BDNF protein expression in the hippocampus or frontal cortex.
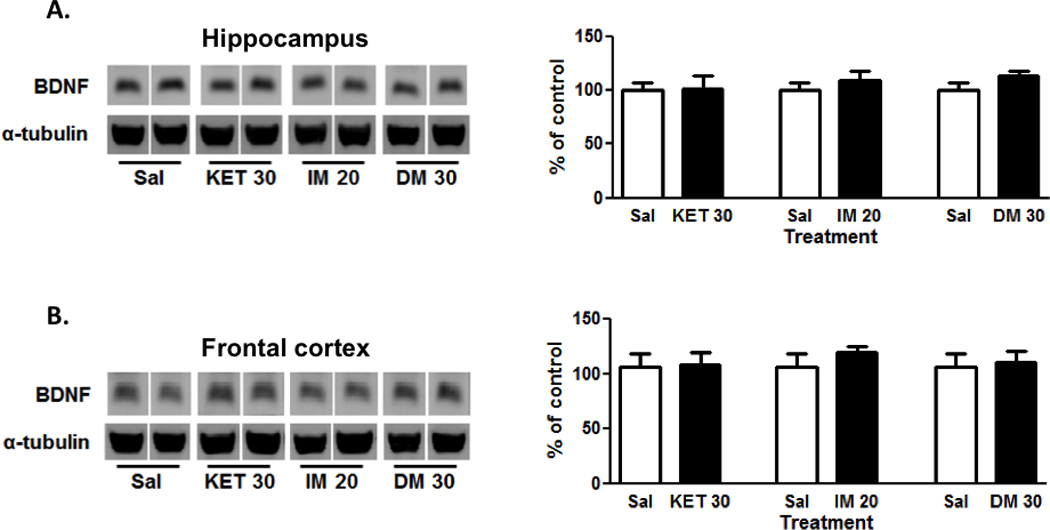
Ketamine (30 mg/kg, ip; N=9), imipramine (20 mg/kg, ip; N=10) or dextromethorphan (30 mg/kg, ip; N=10 for hippocampus, N=9 for frontal cortex) administration had no significant effects on BDNF levels in hippocampus (A) and frontal cortex (B). Representative images of the protein are shown on the left and the cumulative graphed data on the right. Data shown are expressed as mean ± SEM. Sal, saline; KET, ketamine; IM, imipramine; DM, dextromethorphan.
Similar to earlier reports that AMPA receptors mediate many of the antidepressant-relevant behavioral and biochemical effects of ketamine (Maeng et al., 2008, Autry et al., 2011, Koike et al., 2011), we observed here that pretreatment with an AMPA receptor antagonist (NBQX) attenuated the antidepressant-like effects of ketamine in the FST (Fig. 4A), stimulatory effects in the OFT (Fig. 4B), and increases in pro-BDNF protein expression in the hippocampus (Fig. 4C). For the FST antagonism study, the overall ANOVA was significant (F[2,27]=6.85; p<0.01). Post-hoc Tukey’s multiple comparisons tests confirmed that the ketamine treatment group differed significantly from saline (q=5.20, p<0.01), and the NBQX + ketamine group did not differ significantly from saline (q=2.09, n.s.). However, the differences in the antidepressant-like effects of ketamine in the absence and presence of NBQX were not significant (q=3.11, n.s.), suggesting partial attenuation of the effects. In the OFT, the overall ANOVA was significant (F[2,27]=17.73; p<0.0001). Post-hoc Tukey’s test confirmed the ability of NBQX to significantly block the stimulatory effects of ketamine (q=7.55, p<0.001). For hippocampal pro-BDNF levels, the overall ANOVA was also significant (F[2,23]=4.78, p<0.05). Post-hoc Tukey’s multiple comparisons tests revealed that the ketamine treatment group differed significantly from saline (q=3.96, p<0.05), as well as the NBQX + ketamine group (q=3.68, p<0.05). Although not shown, the NBQX treatment group had no significant effects in the FST (t=0.19, n.s.), OFT (t=1.02, n.s.) or on hippocampal pro-BDNF protein expression (t=1.86, n.s.) compared to saline control group.
Figure 4. Attenuation of the behavioral and biochemical effects of ketamine by AMPA receptor antagonism.
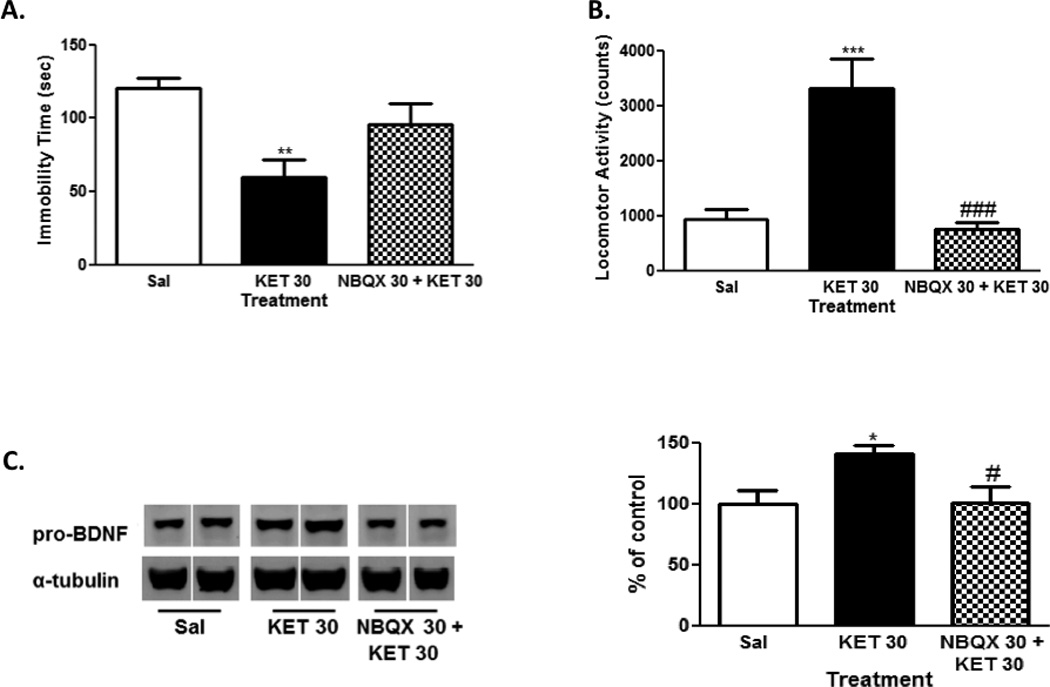
Pretreatment with an AMPA receptor antagonist (NBQX; 30 mg/kg, ip) attenuated ketamine (30 mg/kg, ip)-induced behaviors in forced swim test (A) and open field test (B), and increase in hippocampal pro-BDNF levels (C). Representative images of the protein are shown on the left and the cumulative graphed data on the right (C). In the behavioral data, 10 mice were used for each group. In the biochemical data, 10 mice were used for the saline group and 8 were used for the ketamine and NBQX + ketamine groups. Data shown are expressed as mean ± SEM. *p<0.05, **p<0.01, vs. saline-treated group; #p<0.05, ###p<0.001, vs. ketamine-treated group; Tukey’s post-hoc tests. Sal, saline; KET, ketamine.
DISCUSSION
This pilot study indicates that dextromethorphan may be effective for treating depression and warrants further investigation as a novel fast-acting treatment. In our model, dextromethorphan reduced immobility time in the FST in mice with comparable efficacy to the fast-acting antidepressant ketamine and conventional tricyclic antidepressant imipramine. This is consistent with previous findings from our lab, which showed dose-dependent decreases in immobility time in mice following administration of dextromethorphan, ketamine, and imipramine (Robson et al., 2012, Nguyen et al., 2014, Nguyen and Matsumoto, 2015).
Previous locomotor findings for ketamine and dextromethorphan have varied, with some studies showing no effects and others showing increases in spontaneous locomotor activity (Danysz et al., 1994, Robson et al., 2012, Nguyen et al., 2014, Nguyen and Matsumoto, 2015). We demonstrated here that dextromethorphan did not significantly affect spontaneous locomotor activity in the OFT. In contrast, ketamine elicited a significant increase in locomotor activity which is suggestive of stimulatory effects. Under the same testing conditions, a slighter higher dose of ketamine (40 mg/kg, ip) than used here had exhibited antidepressant-like effects in the FST without affecting locomotor activity (Robson et al., 2012). The coincidence of stimulatory effects with antidepressant-like actions for ketamine in this study suggests that the two effects can be related, though not necessarily dependent on one another. Notably, the fact that ketamine, but not dextromethorphan, had significant stimulatory effects in this study further suggests that dextromethorphan can elicit antidepressant-like efficacy with less abuse liability and reduced side effects compared to ketamine.
Both pro-BDNF and mature BDNF levels are altered in animal models of depression and in depressed human populations (Castren and Rantamaki, 2010, Polyakova et al., 2015). Extending the findings from previous studies, which suggested pro-BDNF associations with ketamine antidepressant efficacy (Autry et al., 2011, Reus et al., 2014), we found that a single administration of ketamine produced a rapid increase in pro-BDNF. We observed the increase in the mouse hippocampus pro-BDNF levels within 40 min post-injection, which is a similar time frame to the detection of antidepressant effects reported for intravenous or intranasal ketamine administration in some depressed human subjects (Diazgranados et al., 2010, Lapidus et al., 2014). With regards to conventional antidepressants, Musazzi and colleagues found that the conventional antidepressants fluoxetine and reboxetine up-regulated pro-BDNF protein expression in the hippocampus of male Sprague-Dawley rats around week 2 to 3 of treatment, but not at week 1 (Musazzi et al., 2009). Unsurprisingly, we did not find a significant change in pro-BDNF expression within 40 minutes of imipramine administration. The concurrence of the decrease in immobility time and the increase in pro-BDNF after ketamine, but not imipramine treatment may in part explain ketamine’s rapid, compared to imipramine’s delayed, onset of antidepressant efficacy. It is important to keep in mind that while ketamine has been shown to increase pro-BDNF expressions without a concomitant increase in locomotor activity in the previous two studies (Autry et al., 2011, Reus et al., 2014), we cannot rule out a possible pro-BDNF association with mobility. Although evidence for this association is lacking at present, Macias et al. had shown that locomotor exercise can alter the expression of pro-BDNF (and BDNF) in the spinal cord of adult rats (Macias et al., 2007).
Regarding mature BDNF, many studies have implicated its crucial role in depression (Castren and Rantamaki, 2010) and have examined the effects of ketamine on BDNF levels (Garcia et al., 2008, Autry et al., 2011, Reus et al., 2011, Yang et al., 2013, Zhou et al., 2014). Indeed, acute administration of ketamine has been shown to increase BDNF levels in rodents in key brain regions involved in depression, including the hippocampus and prefrontal cortex (Garcia et al., 2008, Autry et al., 2011, Reus et al., 2011, Yang et al., 2013, Zhou et al., 2014). Following a single ketamine administration, increases in BDNF levels have occurred as early as 30 min post-injection in the mouse (Autry et al., 2011) and rat (Yang et al., 2013) hippocampus. We, however, observed no changes in BDNF levels at 40 min post-injection, which, compared to the earlier mouse study, may be due to differences in mouse strains (Swiss Webster vs. C57BL/6), ketamine doses used (30 mg/kg, ip vs. 3 mg/kg, ip), and experimental laboratory conditions. Importantly however, some other studies have also reported dissociations between antidepressant-like effects and mature BDNF protein changes following ketamine administration (Garcia et al., 2008, Lindholm et al., 2012). The possibility that other (non-BDNF) signaling pathways also mediate ketamine-induced antidepressant effects is supported by studies in BDNF knockout mice. In particular, treatment with ketamine (50 mg/kg, ip) retained antidepressant-like effects in the FST in heterozygous BDNF knockout (bdnf+/−) C57BL/6 mice and failed to alter BDNF levels in the hippocampus when assessed at 45 min or 7 days after drug administration (Lindholm et al., 2012). These results suggest ketamine can produce antidepressant-like effects independent of the mature BDNF signaling pathway. Moreover, the effects of ketamine on hippocampal BDNF appear to be time-dependent. Autry et al. found an increase in mouse hippocampal BDNF expression within 30 minutes of administration, but not at 24 hours (Autry et al., 2011). Fraga et al., in contrast, found a decrease in BDNF levels in rat hippocampus at one and six hours after the last injection though the animals had reduced immobility time at the six hour time point (Fraga et al., 2013).
The incongruity between an increase in pro-BDNF levels and no corresponding change in BDNF levels in our study remains unclear. This is different from the findings by Autry et al, which showed ketamine increased hippocampal pro-BDNF as well as BDNF within 30 minutes of administration (Autry et al., 2011). It was initially thought that only secreted mature BDNF was biologically active, and that pro-BDNF, which localizes within the cell, served as an inactive precursor. However, emerging evidence indicate that pro-BDNF and mature BDNF elicit different and seemingly opposing biological effects via the p75 neurotrophin receptor (p75NTR) and tropomyosin receptor kinase B (TrkB), respectively (Lu et al., 2005, Castren and Rantamaki, 2010). Activation of p75NTR by pro-BDNF has been shown to induce neuronal atrophy and apoptosis, whereas activation of the TrkB has been associated with growth and survival, suggesting a “yin and yang” model of neurotrophin action (Lu et al., 2005). Which of these activities predominate at given times is not yet clear, and whether the rise in pro-BDNF levels following acute ketamine administration in this study is detrimental at the cellular level requires further investigation. It may be that the dynamic changes in pro-BDNF and BDNF levels are important for the optimal tuning of neuronal plasticity, whereby structural increases are balanced by programmed neuronal death, neurite retraction and synaptic pruning (Castren and Rantamaki, 2010).
Lastly, we also report herein that the increase in pro-BDNF levels in the hippocampus and antidepressant-like effects are mediated in part through AMPA receptors. In a study by Reus and colleagues, ketamine increased pro-BDNF protein expression in the rat hippocampus and nucleus accumbens, with a mitogen-activated protein kinase kinase (MEK) inhibitor blocking the effects of ketamine in the nucleus accumbens (Reus et al., 2014). They also reported that the MEK inhibitor attenuated the antidepressant-like effects of ketamine in the FST. Our findings thus provide an additional, or upstream, mechanism by which ketamine may promote pro-BDNF levels in specific brain regions. These findings are consistent with previous reports implicating the critical role of AMPA receptors in mediating the antidepressant-like effects of ketamine (Maeng et al., 2008, Autry et al., 2011, Koike et al., 2011). In addition, although the antidepressant-like effects of dextromethorphan also appear to be AMPA receptor-mediated (Nguyen and Matsumoto, 2015), its lack of effects on pro-BDNF suggests additional mechanisms are at play downstream of AMPA receptor activation.
Importantly, our data did not evaluate the effects of ketamine, or dextromethorphan, on pro-BDNF or BNDF levels at other time points. In a double blind study, subsequent treatment with dextromethorphan (60 mg/day) plus VPA produced a small increase in plasma BDNF levels from baseline at 12 weeks, and the increase was significantly higher than placebo plus VPA group (Chen et al., 2014). Additionally, in a retrospective study, daily administration of dextromethorphan/quinidine has been shown to produce antidepressant effects in bipolar depressed patients within 1–2 days of treatment (Kelly and Lieberman, 2014), rather than hours as seen with ketamine (Berman et al., 2000, Diazgranados et al., 2010, Murrough et al., 2013). Further studies to determine whether dextromethorphan can promote increases in BDNF or pro-BDNF at later time points or after sub-chronic dosing are worthy of future investigation.
Overall, our results suggest that dextromethorphan may produce antidepressant actions, while reducing problematic stimulatory effects observed with ketamine. Moreover, in contrast to the AMPA-dependent increase in hippocampal pro-BDNF levels of ketamine, dextromethorphan does not rapidly alter pro-BDNF levels, while still producing antidepressant-like effects. Along with earlier studies, these findings indicate that dextromethorphan shares some, but not all, antidepressant-related mechanisms with ketamine. In light of these findings, we encourage additional studies to identify specific molecular mechanisms through which dextromethorphan conveys its antidepressant effects. Furthermore, once these preclinical studies have been validated, it will be necessary to determine the efficacy of dextromethorphan for treating depression in patients.
Acknowledgments
The authors would like to thank Dr. Steven M. Frisch for use of western blot resources. This study was supported by funding from the West Virginia University Research Development Fund. The funding source had no role in the study design; the collection, analysis and interpretation of the data; the writing of the manuscript; or the decision to submit the article for publication. An American Medical Association Foundation Seed-Grant, Neurosurgery Research and Education Foundation Medical Student Research Fellowship, Sigma Xi Grants in Aid of Research, American Association of Pharmaceutical Scientist Pre-Doctoral Fellowship, and American Foundation of Pharmaceutical Education Pre-Doctoral Fellowship supported Brandon Lucke-Wold. An American Foundation of Pharmaceutical Education Pre-Doctoral Fellowship also supported Aric Logsdon. Research reported in this publication was supported by the NIGMS of the National Institutes of Health under award number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
REFERENCES
- Aan Het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biological psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell A, Castro E, Celada P, Bortolozzi A, Pazos A, Artigas F. Strategies for producing faster acting antidepressants. Drug discovery today. 2005;10:578–585. doi: 10.1016/S1359-6446(05)03398-2. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaratnasingam S, Janca A. Brain Derived Neurotrophic Factor: a novel neurotrophin involved in psychiatric and neurological disorders. Pharmacology & therapeutics. 2012;134:116–124. doi: 10.1016/j.pharmthera.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biological psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nature reviews Neuroscience. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Castren E, Rantamaki T. Role of brain-derived neurotrophic factor in the aetiology of depression: implications for pharmacological treatment. CNS drugs. 2010;24:1–7. doi: 10.2165/11530010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biological psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chen SL, Lee SY, Chang YH, Chen PS, Lee IH, Wang TY, Chen KC, Yang YK, Hong JS, Lu RB. Therapeutic effects of add-on low-dose dextromethorphan plus valproic acid in bipolar disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2014;24:1753–1759. doi: 10.1016/j.euroneuro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nature reviews Drug discovery. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Danysz W, Essmann U, Bresink I, Wilk R. Glutamate antagonists have different effects on spontaneous locomotor activity in rats. Pharmacology Biochemistry and Behavior. 1994;48:111–118. doi: 10.1016/0091-3057(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archives of general psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues in clinical neuroscience. 2014;16:11–27. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. Journal of neurochemistry. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Fraga DB, Réus GZ, Abelaira HM, De Luca RD, Canever L, Pfaffenseller B, Colpo GD, Kapczinski F, Quevedo J, Zugno AI. Ketamine alters behavior and decreases BDNF levels in the rat brain as a function of time after drug administration. Revista Brasileira de Psiquiatria. 2013;35:262–266. doi: 10.1590/1516-4446-2012-0858. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain research. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Kelly TF, Lieberman DZ. The utility of the combination of dextromethorphan and quinidine in the treatment of bipolar II and bipolar NOS. Journal of affective disorders. 2014;167:333–335. doi: 10.1016/j.jad.2014.05.050. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behavioural brain research. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biological psychiatry. 2014;76:970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach EC. An extension of hypotheses regarding rapid-acting, treatment-refractory, and conventional antidepressant activity of dextromethorphan and dextrorphan. Medical hypotheses. 2012;78:693–702. doi: 10.1016/j.mehy.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Lindholm JS, Autio H, Vesa L, Antila H, Lindemann L, Hoener MC, Skolnick P, Rantamaki T, Castren E. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf(+)/(−) heterozygous null mice. Neuropharmacology. 2012;62:391–397. doi: 10.1016/j.neuropharm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu Y, Sousa N, Almeida OF. SMAD pathway mediation of BDNF and TGF beta 2 regulation of proliferation and differentiation of hippocampal granule neurons. Development (Cambridge, England) 2005;132:3231–3242. doi: 10.1242/dev.01893. [DOI] [PubMed] [Google Scholar]
- Macias M, Dwornik A, Ziemlinska E, Fehr S, Schachner M, Czarkowska-Bauch J, Skup M. Locomotor exercise alters expression of pro-brain-derived neurotrophic factor, brain-derived neurotrophic factor and its receptor TrkB in the spinal cord of adult rats. European Journal of Neuroscience. 2007;25:2425–2444. doi: 10.1111/j.1460-9568.2007.05498.x. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biological psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. The American journal of psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Cattaneo A, Tardito D, Barbon A, Gennarelli M, Barlati S, Racagni G, Popoli M. Early raise of BDNF in hippocampus suggests induction of posttranscriptional mechanisms by antidepressants. BMC Neuroscience. 2009;10:1–7. doi: 10.1186/1471-2202-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Matsumoto RR. Involvement of AMPA receptors in the antidepressant-like effects of dextromethorphan in mice. Behavioural brain research. 2015;294:26–34. doi: 10.1016/j.bbr.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Robson MJ, Healy JR, Scandinaro AL, Matsumoto RR. Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PloS one. 2014;9:e89985. doi: 10.1371/journal.pone.0089985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazidou E. The neurobiology of depression. British medical bulletin. 2012;101:127–145. doi: 10.1093/bmb/lds004. [DOI] [PubMed] [Google Scholar]
- Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. Journal of affective disorders. 2015;174:432–440. doi: 10.1016/j.jad.2014.11.044. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Chapter 8:Unit 8 10A. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] 2001 doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- Reus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P, Souza CT, Quevedo J. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behavioural brain research. 2011;221:166–171. doi: 10.1016/j.bbr.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Reus GZ, Vieira FG, Abelaira HM, Michels M, Tomaz DB, dos Santos MA, Carlessi AS, Neotti MV, Matias BI, Luz JR, Dal-Pizzol F, Quevedo J. MAPK signaling correlates with the antidepressant effects of ketamine. Journal of psychiatric research. 2014;55:15–21. doi: 10.1016/j.jpsychires.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Elliott M, Seminerio MJ, Matsumoto RR. Evaluation of sigma (sigma) receptors in the antidepressant-like effects of ketamine in vitro and in vivo. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2012;22:308–317. doi: 10.1016/j.euroneuro.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Yang C, Hu YM, Zhou ZQ, Zhang GF, Yang JJ. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Upsala journal of medical sciences. 2013;118:3–8. doi: 10.3109/03009734.2012.724118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. European psychiatry : the journal of the Association of European Psychiatrists. 2014;29:419–423. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]


