Abstract
Background
Major depression is one of the most prevalent psychiatry comorbidities of alcohol use disorders (AUD). Since negative emotions can trigger craving and increase the risk of relapse, treatments that target both conditions simultaneously may augment treatment success. Previous studies showed a potential synergist effect of FDA approved medication for AUD acamprosate and the antidepressant escitalopram. In this study, we investigated the effects of combining acamprosate and escitalopram on ethanol consumption in stress-induced depressed mice.
Methods
Forty singly-housed C57BL/6J male mice were subjected to chronic unpredictable stress. In parallel, 40 group-housed male mice were subjected to normal husbandry. After 3 weeks, depressive- and anxiety-like behaviors and ethanol consumption were assessed. For the next 7 days, mice were injected with saline, acamprosate (200 mg/kg; twice/day), escitalopram (5 mg/kg; twice/day), or their combination (n = 9–11/drug group/stress group). Two-bottle choice limited access drinking of 15% ethanol and tap water was performed 3 hours into dark phase for 2 hours immediately after the dark phase daily injection. Ethanol drinking was monitored for another 7 days without drug administration.
Results
Mice subjected to the chronic unpredictable stress paradigm for 3 weeks showed apparent depression- and anxiety-like behaviors compared to their non-stressed counterparts including longer immobility time in the forced swim test and lower sucrose preference. Stressed mice also displayed higher ethanol consumption and preference in a 2-bottle choice drinking test. During the drug administration period, the escitalopram-only and combined drug groups showed significant reduction in ethanol consumption in non-stressed mice, while only the combined drug group showed significantly reduced consumption in stressed mice. However, such reduction did not persist into the post-drug administration period.
Conclusions
The combination of acamprosate and escitalopram suppressed ethanol intake in both non-stressed and stressed mice, hence this combination is potentially helpful for AUD individuals with or without comorbid depression to reduce alcohol use.
Keywords: Chronic Unpredictable Stress, Acamprosate, Escitalopram, Comorbid Depression
INTRODUCTION
Major depression is one of the most prevalent psychiatric comorbidities of alcohol use disorders (AUD). It has been reported to occur in up to 70% of patients in some studies (Conner et al., 2009; Grant et al., 2004). National epidemiological surveys reported 24.3% of men and 48.5% of women who fulfilled criteria for alcohol dependence also fulfilled those for depression (Kendler et al., 1997). Manifestation of major depression prior to that of alcohol problems, i.e., primary depression, has been associated with later development of alcohol dependence (Boschloo et al., 2012; Kessler et al., 1997). Major depressive episode is associated with an increase in days spent drinking at home alone as well as drinking to enhance depressed mood (Cranford et al., 2011). Comorbid depression also impacts treatment outcomes, including shorter time to relapse, higher dropout rates, and greater consumption of alcohol during treatment (Adamson et al., 2009; Burns et al., 2005). The close relationship between negative moods and alcohol lapse calls for effective and integrated treatment of AUD and comorbid depression to maximize positive treatment outcomes.
Acamprosate was proposed to act as a partial coagonist of N-methyl-D-aspartate (NMDA) receptors (al Qatari et al., 1998) and has been shown to modulate NMDA receptor function and subunit synthesis (Naassila et al., 1998; Rammes et al., 2001). Acamprosate also acts on the type I metabotropic glutamate receptor, in particular mGluR5, to reduce ethanol consumption and ethanol-induced sensitization (Blednov and Harris, 2008; Kotlinska et al., 2006). Despite the fact that the exact mechanism of acamprosate remains unknown and a recent study which claims that calcium rather than homotaurine is the active component (Spanagel et al., 2014), acamprosate is postulated to balance neurotransmission disrupted by alcohol dependence, particularly that of glutamatergic neurotransmission. Copious studies showed that acamprosate suppressed alcohol withdrawal-related behaviors and alleviated the withdrawal-induced hyperglutamatergic state (e.g., Dahchour and De Witte, 1999; Dahchour and De Witte, 2003; De Witte, 2004; Hinton et al., 2012). In ethanol self-administration models of non-dependent animals, acamprosate also demonstrated a suppression effect on ethanol drinking behavior (Brager et al., 2011; Gupta et al., 2008; Lee et al., 2011).
Serotonergic transmission is negatively correlated with ethanol drinking behavior (reviewed by Lovinger, 1997). Selective serotonin reuptake inhibitors (SSRIs), which inhibit presynaptic serotonin reuptake by blocking serotonin transporter, may help to regulate drinking behavior. Serotonin transporter knockout mice showed lower alcohol consumption in a free-choice paradigm and were insensitive to the suppression of alcohol consumption by the SSRI fluoxetine, which was demonstrated in wild-type mice (Boyce-Rustay et al., 2006; Kelai et al., 2003). Treatment with other SSRIs have also been shown to reduce alcohol consumption (e.g., Ciccocioppo et al., 1997; Maurel et al., 1999; Murphy et al., 1985). In addition, chronic antidepressant treatments, including SSRIs and ketamine, have been shown to modulate glutamatergic neurotransmission by dampening presynaptic glutamate release, modulating functions of postsynaptic NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, as well as that of metabotropic glutamate receptors and glial-specific glutamate transporters (reviewed by Musazzi et al., 2013). These mechanisms may help relieve the heightened activity of the glutamatergic system observed in depressed individuals, in turn reversing the neurological damage caused by hyperglutamatergic toxicity such as dendritic atrophy (Musazzi et al., 2013). Therefore, the use of antidepressants as adjunctive therapy for AUD with comorbid depression might be considered since combining acamprosate and SSRIs such as escitalopram in theory could simultaneously downregulate glutamatergic and upregulate serotonergic neurotransmissions, in turn suppressing ethanol consumption. Clinical studies found SSRIs improved depressed mood and decrease alcohol use in some alcohol dependent individuals (Kranzler et al., 2012; Muhonen et al., 2011; Muhonen et al., 2008). Escitalopram is currently the most widely used SSRI (Owens et al., 2001). The combined use of acamprosate and escitalopram to treat AUD patients with comorbid major depressive disorder yielded a depression response rate of 50% and a remission rate of 42%, with significant reduction in alcohol consumption per week and per month, outcomes that were superior to those obtained with escitalopram alone (Witte et al., 2012). These data suggest that acamprosate and escitalopram combined therapy may have synergistic effects in treating AUD with comorbid depression.
Since studies on the joint effects of antidipsotropic agents and antidepressant are limited, we conducted this study to investigate whether the combined use of acamprosate and escitalopram would be more efficacious in reducing alcohol consumption than their sole administration in stressed-induced depressed mice. We hypothesize that this drug combination would yield a synergistic effect on alcohol consumption reduction compared to the use of either drug alone in mice demonstrating depression- and anxiety-like behaviors induced by chronic unpredictable stress.
MATERIALS AND METHODS
Animals
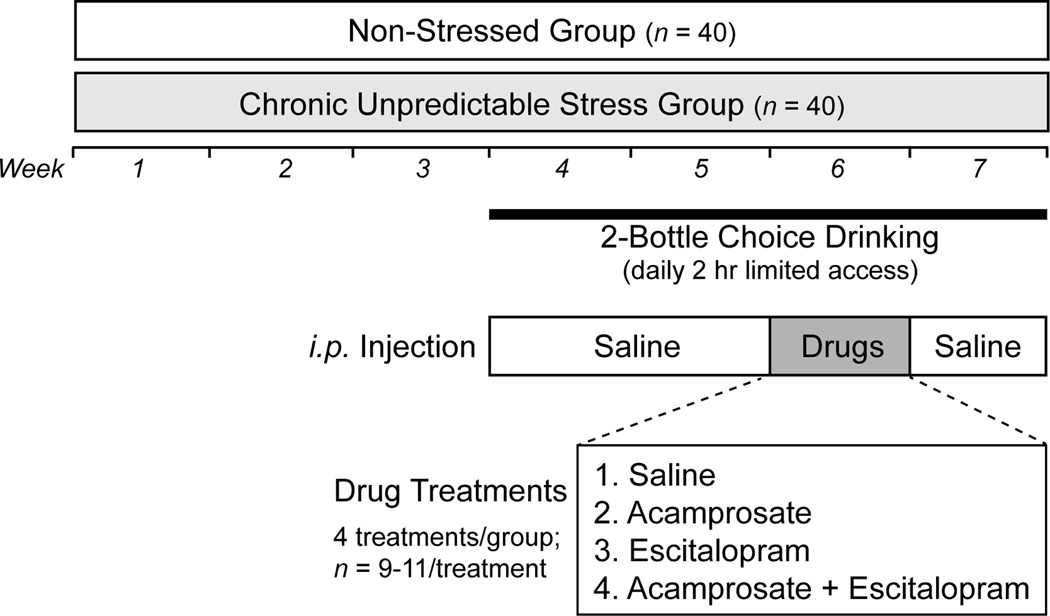
Male C57BL/6J mice (n = 80) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). We used C57BL/6J due to its extensive use in alcoholism research and its high voluntary consumption of unsweetened alcohol (e.g., Rhodes et al., 2007, Yoneyama et al., 2008). Upon arrival, they were kept in groups of 5 and were maintained under standard animal house conditions with food and water access available ad libitum (12 hour light/dark cycle; light on at 0600; 21± 1°C) for 2 weeks before the experiment started when mice reached 8 weeks of age. After acclamation, half of the mice were subjected to a chronic unpredictable stress regimen for 3 weeks and were singly housed on Day 1 of the regimen (stressed group; n = 40), while the rest were only subjected to normal handling and remained group-housed during the same period (non-stressed group; n = 40). Following 2 weeks of ethanol consumption training in a 2-bottle choice drinking setting, mice were separated into 4 drug groups (saline, acamprosate, escitalopram, acamprosate plus escitalopram) and were subjected to drug administration for a week during which the 2-bottle choice drinking continued. Mice were monitored for their 2-bottle choice drinking performance in the week after drug treatment (post-treatment). The study design overview is illustrated in Figure 1. Animal care and handling procedures were approved by Mayo Clinic Institutional Animal Care and Use Committee in accordance with NIH guidelines.
Fig. 1.
Study design overview.
Chronic Unpredictable Stress Paradigm
Forty mice were subjected to a chronic unpredictable stress paradigm modified from Dias-Ferreira et al. (2009) throughout the whole experiment. The paradigm consisted of three stressors: social defeat, forced swim, and restrain stress. Each stressed mouse was subjected to one of these stressors daily during one of the experiment sessions (0900–1200 or 1400–1700). The day-to-day order of stressor presented was randomized, but the total number of exposures to each stressor was ensured to be similar for all subjects. (1) Social defeat. Male CD-1 retired breeders (n = 10), which were known to be aggressive, were obtained from Charles-River Laboratory (Wilmingon, MA, USA) as aggressive residents. They were singly housed under the aforementioned standard animal house condition for a week to establish territorial defense behavior before use. They were screened for their attack propensity and aggressiveness in five test sessions. Residents were selected if in three consecutive sessions, they had attacked the intruder within 30 sec after introduction and initiated a series of fights in the first 10 min with each fight lasting for at least 5 sec and resulting in intruder fleeting and/or displaying a surrender posture. Five out of ten residents were excluded after the screen. The social defeat session began with the introduction of an intruder mouse into the resident’s cage. The intruder and the resident were allowed to fully interact for 10 min and then were separated by a plexiglass divider for another 20 min. At the end of the 30-min session, the intruder was returned to its cage and the resident was allowed to rest for an hour before the next session. Due to the small number of aggressive residents available, each subject had been presented multiple times to each resident, but it was ensured that each intruder had not met the same resident in consecutive sessions and that each intruder had been exposed to all residents. (2) Restrain stress. 50 ml falcon tubes with perforations on the lid were used as restrainers. A mouse was guided into the tube with head facing the entrance. Each restrain session lasted for 30 min. (3) Forced swim. Mice were placed in a 2000 ml glass beaker half-filled with 25±1°C tap water for 6 min, followed by thorough drying on heating pad to prevent hypothermia before returning to their home cage. Note that single housing (chronic isolation) of these mice was a fixed stressor in addition to the three variable stressors (Lopez et al., 2011; Nollet et al., 2013).
Behavioral Tests for Chronic Stress-induced Mood Disorder Model Validation
After the 3 weeks of the chronic unpredictable stress treatment, a subset of stressed and non-stressed mice (n = 10) were subjected to four behavioral tests, one test each day between 0900 and 1700 (apart from sucrose preference test), in the following sequence. (1)Sucrose preference test. On the day before the test day, subjects were singly housed in a cage and presented with food and two sipper bottles filled with 1% (w/v) sucrose (Sigma-Aldrich, MO, USA) in tap water for 24 hours for acclamation to the environment as well as to the taste of sucrose. At the end of the acclamation period at 9 a.m., both bottles were exchanged for tap water. The test began on the same day at 9 p.m. (3 hours into dark phase), with one water bottle exchanged for 1% sucrose. At 9 a.m. on the next day, the sucrose bottle was exchanged for tap water. Bottles were weighed at 9 p.m. and 9 a.m. before the aforementioned changes were made. This process was repeated once with bottle position switched. Liquid dripping and evaporation were accounted for by two control cages that did not have a mouse. (2) Elevated plus maze. A subject was placed in the center zone of a light gray elevated plus maze (width 80 cm; length 80 cm; platform 60 cm above ground) facing a designated open arm. Its movement during the 5-minute test period was video tracked and analyzed by EthoVision XT software (Noldus, Wageningen, Netherlands). The time spent in and the numbers of entry into the open or closed arms were measured only when the subject’s nose, body and tail points were all tracked within a region. (3) Open field test. Subjects were placed in an open field chamber (Med Associates, VT, USA) for one hour. Location and movement of the subjects during the test period were tracked by infrared sensors located at the base of the field and were analyzed by Activity Monitor v5.9 (28 cm × 28 cm × 20 cm; Med Associates Inc., VT, USA). The center zone was defined as a 13.5 cm × 13.5 cm area in the middle of the field. (4)Forced swim test. Procedures as described above in the force swim stress with addition of video recording. Latency to the first immobility was recorded and total immobility time was measured in the last four minutes of the test by a blinded researcher. Immobility was defined as limb and body movements other than balancing and keeping head above water as well as body displacement without limb movement.
Testing of Drug Effects on Two-bottle Choice Limited Access Ethanol Drinking
We adopted the drinking paradigm by Lopez et al. (2011), which showed self-administration of 15% ethanol in C57BL/6J mice was altered by chronic variable stress and isolation, but we adjusted the drinking session start time to 3 hours into dark phase (9 p.m. to 11 p.m.) as ethanol consumption was found to be higher compared to starting from the beginning of dark phase (e.g., Gupta et al., 2008, Rhode et al., 2005). During the drinking session, the mice were presented with two 50 ml sipper bottles installed with a stainless steel sipper tip which contained either tap water or ethanol in tap water. Bottles were weighed before and at the end of the drinking session for the calculation of ethanol consumption and preference. Control bottles were installed in four empty cages to adjust for leakage. Positions of ethanol and tap water tubes were swapped daily to account for side preference. Mice were presented with 10% ethanol in the first two days and then escalated to 15% for the rest of the experiment. Non-stressed mice were singly housed only during the ethanol drinking period and were returned to their home cage after the drinking session. In order to standardize the effect of stress caused by injection on ethanol drinking throughout the course of drinking period, an i.p. injection was always immediately followed by the drinking session. During the 14-day training period, saline (0.9% sodium chloride) was injected. In the subsequent 7 days of drug administration, each stress group was sub-divided into four drug groups (n = 9 – 11): saline (vehicle), acamprosate only (400 mg/kg/day), escitalopram only (10 mg/kg/day), and combined drug (400 mg/kg/day acamprosate and 10 mg/kg/day escitalopram). Saline was injected in the subsequent 7-day post-drug period. Both acamprosate (Estechpharma, Kyonggi-do, Korea) and escitalopram (Sigma-Aldrich, MO, USA) were dissolved in saline for injection. Acamprosate dose (400 mg/kg/day) was based on previously published literature illustrating that 400 mg/kg/day was the highest effective dose for reducing ethanol intake and preference (Gupta et al., 2008; Kotlinska et al., 2006; Olive et al., 2002). The escitalopram dose (10 mg/kg/day) was based on its effect of reducing ethanol withdrawal symptoms in rats (Saglam et al., 2006) and reversing depression symptoms induced by 1-(m-chlorophenyl)piperazine in mice (Rajkumar et al., 2009).
Statistical Analysis
GraphPad Prism 5 (GraphPad Software Inc., CA, USA) was used for statistical analysis and graphing. Two-tailed t test (adjusted for assumed unequal variance if necessary) was used to compare data of behavioral tests, ethanol consumption and preference between stress groups. For each mouse, baseline ethanol consumption and preference were represented by averaging the measurements in the last two days of the training period. These parameters in the subsequent drug administration and post-treatment weeks were represented by the averages over their corresponding seven days. Due to individual differences at baseline, ethanol consumption and preference were presented and compared as percentage to baseline, which was calculated by dividing average ethanol consumption during the drug administration or post-treatment week by the baseline average of each individual mouse. One-way ANOVA was applied to compare ethanol consumption and preference among drug groups at each time point independently within each stress group. Statistical significance was set at p < 0.05.
REUSLTS
Validation of depressive-like behavior after exposure to chronic unpredictable stress
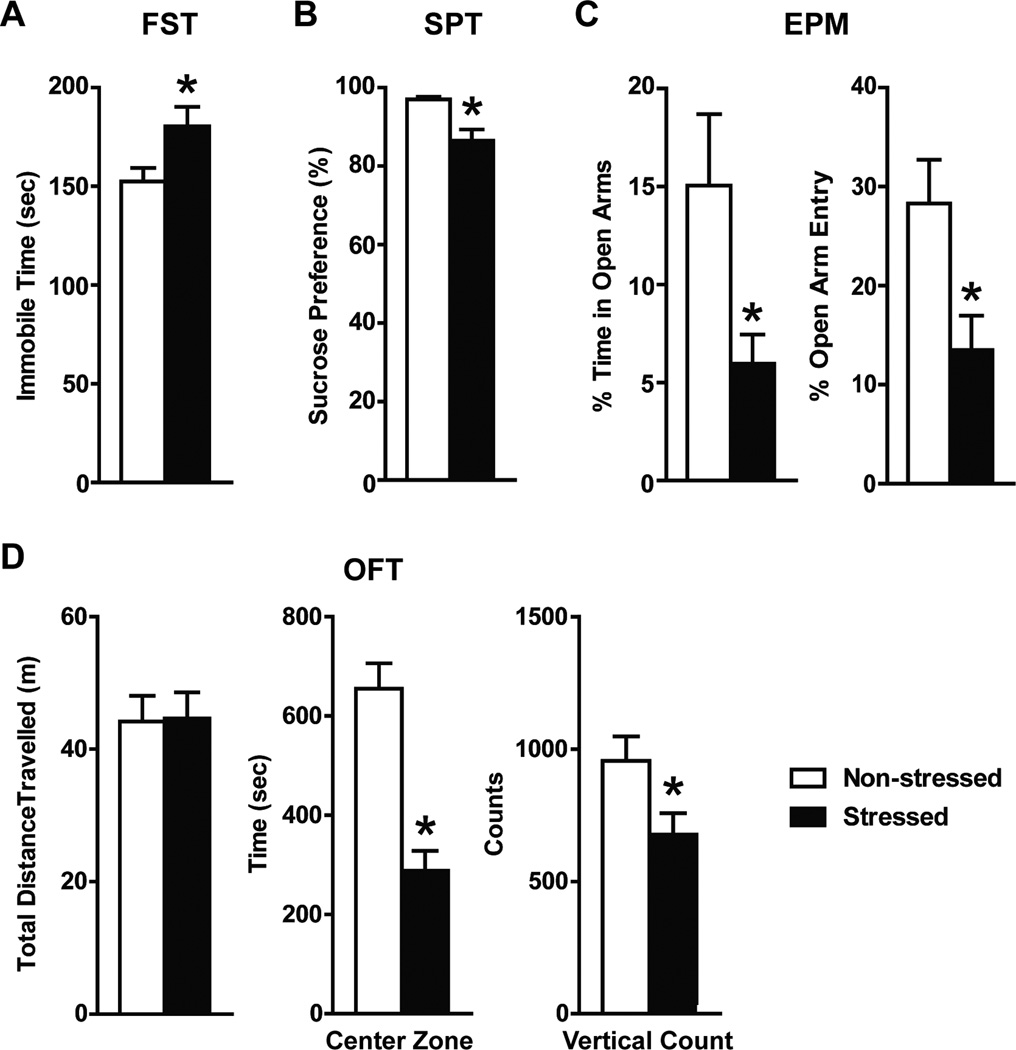
After 3 weeks of chronic unpredictable stress paradigm, stressed mice exhibited significantly longer immobility time in the forced swim test (180.4 ± 9.8 sec; t = 2.345, df = 18, p = 0.015) and lower sucrose preference (86.5 ± 2.9%; t =3.583, df = 9, p = 0.006) compared to the non-stress controls (152.4 ± 6.9 sec and 97.0 ± 0.7% respectively; Figure 2A and B), supporting that the former had a higher degree of behavioral despair and anhedonia. Stressed mice also demonstrated anxiety-like behaviors compared to non-stressed controls by entering the open arms less frequently (13.5 ± 4.4% vs 28.3 ± 4.4% of total zone entry; t = 2.216, df = 18, p = 0.041) and spending significantly shorter time in the open arms of the elevated plus maze (6.0 ± 1.5% vs 15.1 ± 3.6% of total time; t = 2.636, df = 18, p = 0.017; Figure 2C). They also spent significantly shorter time in the center zone of the open field arena (288.6 ± 39.6 sec; t = 5.670, df = 18, p <0.0001) compared to non-stressed controls (655.0 ± 51.1 sec; Figure 2D). In addition, stressed mice also showed reduced exploratory behaviors by having fewer vertical/rearing counts (657.7 ± 82.8; t = 2.408, df = 18, p = 0.027) during the open field test.
Fig. 2.
Behavioral test results after 3 weeks of the chronic unpredictable stress paradigm (n = 10): forced swim test (A), sucrose preference test (B), elevated plus maze (C), and open field test (D). Compared to the non-stress mice, stressed mice had a significantly lower sucrose preference and displayed a significantly longer forced swim immobility time (*unpaired t test p < 0.05). Stressed mice demonstrated significantly lower percentage time in the open arms of the elevated plus maze with lower percentage of total entry into these arms (*unpaired t tests p < 0.05). They also spent less time in the center zone of the open field (*unpaired t test p < 0.05).
Ethanol consumption and preference were higher in stressed mice
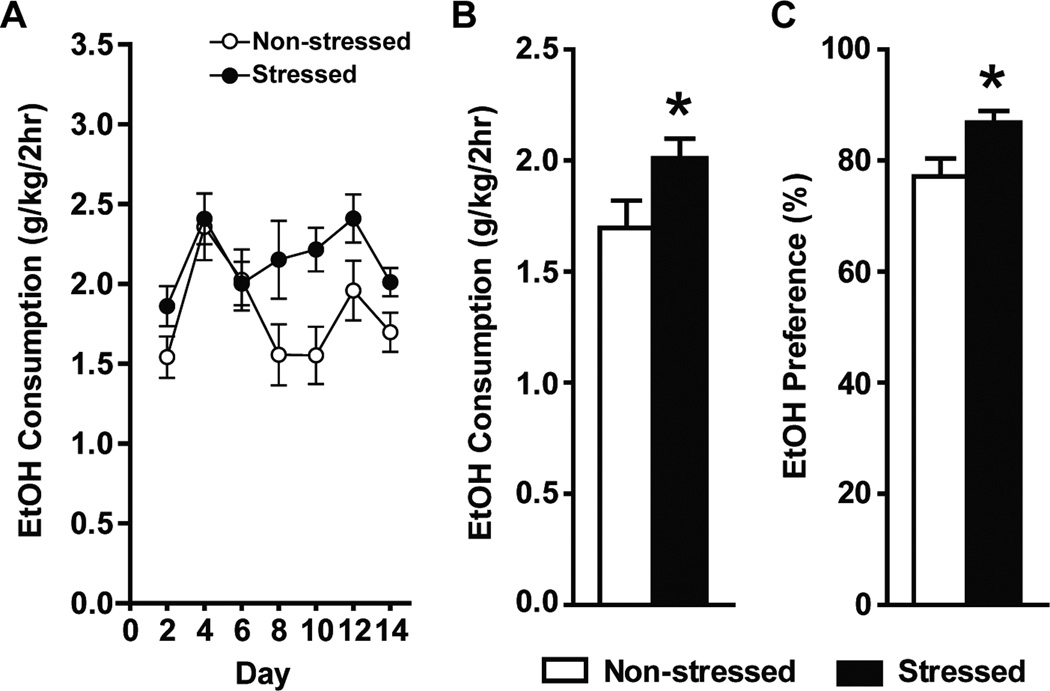
Compared to non-stressed mice, stressed mice demonstrated significantly higher ethanol consumption (1.697 ± 0.123 vs 2.010 ± 0.009; t = 2.076, df = 70, p = 0.042) and ethanol preference (77.3 ± 3.2% vs 86.9 ± 2.0%; t = 2.551, df = 66, p = 0.013; Figure 3B and C).
Fig. 3.
(A) Ethanol consumption during the training period. Note that 10% ethanol was presented for the first 2 days, and was then escalated to 15% for the rest of the experiment. Averages of the last two days of ethanol consumption and preference were used as baseline. Stressed mice demonstrated significantly higher ethanol consumption (B) and ethanol preference (C) compared to non-stressed mice (*unpaired t test p < 0.05).
Effects of acamprosate and escitalopram on ethanol consumption & preference
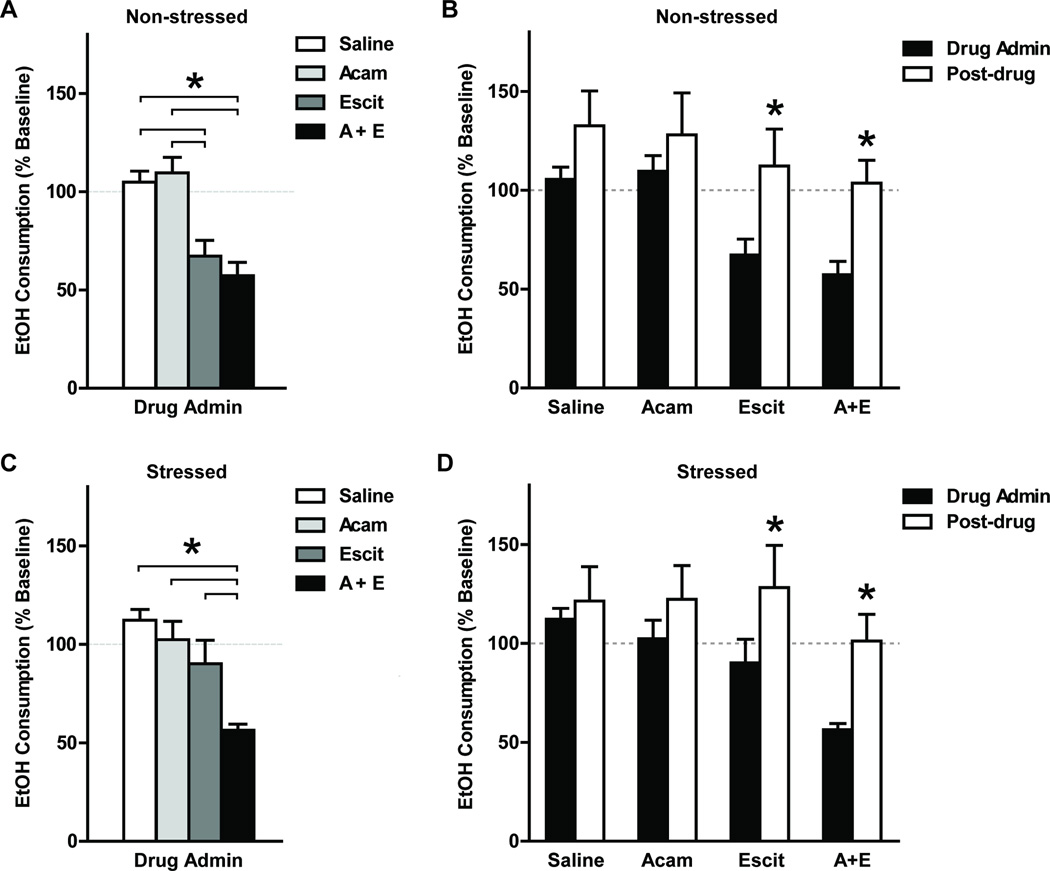
During the drug administration week, ethanol consumption ratios were significantly different among drug groups in non-stressed mice (one-way ANOVA: F3,36 = 10.13, p < 0.0001; Figure 4A). The escitalopram-only group and the combined drug group demonstrated significantly lower ethanol consumption percentage to baseline compared to the saline or acamprosate-only groups (post hoc Tukey’s tests p < 0.05). Similarly in stressed mice, ethanol consumption percentages to baseline were significantly different among drug groups during the drug administration week (F3,36 = 8.284, p = 0.0003; Figure 4C). However, only the combined drug group demonstrated a significantly lower ethanol consumption percentage to baseline compared to all three other drug groups (post hoc Tukey’s tests p < 0.05). Ethanol preference percentages to baseline were not significantly different among drug groups in either non-stressed or stressed groups (one-way ANOVA p > 0.05). In contrast, during the post-drug treatment week, ethanol consumption and preference percentage to baseline were not significantly different among drug groups in either non-stressed or stressed groups (one-way ANOVA p > 0.05).
Fig. 4.
Ethanol consumption during drug administration and post-treatment weeks are presented as percentage to baseline (mean ± SEM; n = 9 – 11 for each drug group). During the drug administration week, ethanol consumption percentage to baseline was significantly different among drug treatment groups in both non-stressed and stressed mice (one-way ANOVA p < 0.05). (A) In non-stressed mice, the escitalopram-only (Escit) and the combined drug treatment (A+E) groups showed significantly lower ethanol consumption percentage to baseline compared to the saline and acamprosate-only groups (*Tukey posthoc tests p < 0.05). (C) While in stressed mice, only the combined treatment group (A+E) showed significantly lower ethanol consumption percentage to baseline to all other drug treatment groups (*Tukey posthoc tests p < 0.05). (B and D) In the post-treatment week, ethanol consumption percentages to baseline had significantly increased compared to the drug administration week in mice previously treated with escitalopram-only (Escit) or with combined drugs (A+E) in both stress treatment groups (*paired t tests p < 0.05).
During the post-drug treatment week, we observed that ethanol consumption percentages of all treatment groups had escalated to around or above the baseline level (Fig. 4B and D), hence we performed one-tailed paired t test to determine whether ethanol consumption percentages to baseline had significantly increased in the post-treatment week compared to the drug administration week. In the non-stressed group, ethanol consumption percentage to baseline increased significantly in mice which were treated with escitalopram-only or with combined drugs (p = 0.024 and 0.006 respectively). Similarly, in the stressed group, a significant increase of ethanol consumption percentage to baseline was observed in the escitalopram-only and the combined drug treated mice (p = 0.011 and 0.003 respectively).
DISCUSSION
In this study, we investigated the effect of combining acamprosate and escitalopram on ethanol consumption in stress-induced depressed mice. We have applied a chronic unpredictable stress paradigm to induce depression- and anxiety-like behaviors and subsequently studied their response to the sole and combined use of acamprosate and escitalopram on limited-access ethanol drinking. We observed that the sole administration of acamprosate did not affect ethanol consumption, while the sole administration of escitalopram reduced ethanol consumption in non-stress mice but not in stressed mice, whereas the combined drug treatment could reduce ethanol intake in both groups. These results suggest that combining acamprosate and escitalopram may be more effective in controlling ethanol use for AUD individuals with or without comorbid depression.
We first used a chronic unpredictable stress paradigm, which was previously used in rats (Dias-Ferreira et al., 2009), to induce depression- and anxiety-like behaviors in male C57BL/6J mice. This paradigm consists of three social and physical stressful stimuli (social defeat, forced swim, and restrain) without day-light cycle interference and food or water restriction, hence it is similar to human day-to-day life stress exposure. After only 3 weeks of stress treatment, we showed that this paradigm was capable of inducing depression- and anxiety-like behaviors in C57BL/6J mice, which is a strain relatively less vulnerable to stress despite being most widely used (Monteiro et al., 2015). It is also less demanding in terms of resources and time (all stressors can be performed simultaneously) than other chronic unpredictable mild stress mice models which involved six to eight stressors extended up to 9 weeks (e.g., Mineur et al., 2006; Monteiro et al., 2015; Nollet et al., 2013). The short duration required by each stressor (≤ 30 min) allows ethanol drinking behavior or even alcohol dependence induced by chronic intermittent ethanol exposure to be studied in parallel with the stress paradigm, hence further imitates human alcohol use under continuous daily stress. Furthermore, mice that underwent this stress paradigm showed higher ethanol binging consumption and preference, which mirrors alcohol binging in depressed humans for mood elevation. Nonetheless, the overall impact of this stress treatment was significant but did not have a large effect compared to what was observed in rats (Dias-Ferreira et al., 2009), which could be due to the difference in stress vulnerability between species and C57BL/6 mice are known to be relatively less vulnerable to (Monteiro et al., 2015) and demonstrate high voluntary ethanol consumption (e.g., Rhodes et al., 2007). Moreover, repeated exposure to the same stressor may reduce its impact as the paradigm proceed, adding additional stressors may attenuate habituation towards the stressors. Finally, the inclusion of forced swim in the paradigm may influence forced swim test performance, but the tail suspension test can be used alternatively for assessing behavioral despair. Despite these limitations, we have demonstrated that this chronic unpredictable stress paradigm displays construct and face validity with logistical practicality for modelling depression comorbid with AUD.
In this study, we did not observe an effect of acamprosate on ethanol intake. In order to maintain constant exposure to acamprosate, we injected the drug twice daily, thus each dose was halved and the immediate effect during the two-bottle ethanol drinking would be equivalent to 200 mg/kg. This dose reduction could partially explain the ineffectiveness of sole acamprosate treatment since previous studies showed a dose dependent effect of i.p. injected acamprosate on ethanol intake and conditioned place preference for ethanol (Gupta et al., 2008; McGeehan and Olive, 2003) and the dose we administered was at the lower range. Next, we observed that the sole administration of escitalopram reduced ethanol consumption in non-stressed mice, which was not observed in the stressed mice. The observation in the non-stress mice agreed with previous reports of SSRIs such as fluoxetine, sertraline, paroxetine and citalopram reducing ethanol intake (e.g., Ciccocioppo et al., 1997; Maurel et al., 1999; Murphy et al., 1985). This effect may be due to the ability of SSRI to upregulate serotonin transmission which is correlated with reduced ethanol drinking behavior (reviewed by Lovinger, 1997). However, SSRIs had also been found to affect fluid consumption (e.g., Maurel et al., 1999; Strekalova et al., 2006). Maurel et al (1999) screened the impact of various SSRIs, including citalopram, the racemic mixture of escitalopram and its enantiomer, on the specificity of consummatory behavior in alcohol-preferring rats. They observed a dose-dependent subtle reduction of ethanol intake and preference as well as fluid and food intake at 3 and 10 mg/kg citalopram, but such reductions became most significant when the dose was increased to 30 mg/kg with ethanol preference showing the least reduction compared to ethanol, fluid, and food intake. Thus, our observation of reducing ethanol consumption by escitalopram in non-stressed mice agrees with Maurel et al (1999), but since 5 mg/kg escitalopram was a relatively low dose, its impact on ethanol preference was not detected, suggesting escitalopram may have affected general liquid consumption.
By combining these doses of acamprosate and escitalopram, ethanol consumption was significantly reduced in both stressed and non-stressed mice. In non-stressed mice, this effect may be attributed mainly to escitalopram since escitalopram alone demonstrated such capacity. However, stressed mice required both drugs to reduce ethanol consumption, demonstrating that escitalopram’s effect was insufficient under the chronic unpredictable stress condition. Chronic unpredictable stress treatment induces the loss of AMPA and NMDA receptors, limiting glutamate-glutamine cycling, and reducing glial progenitors, indicating a heightened glutamatergic system (see review by Popoli et al., 2012). This stress treatment also decreases the level of serotonin and its metabolites in frontal cortex and striatum (Ahmad et al., 2010). On top of these effects, repeated binge drinking exacerbates the increase of extracellular glutamate levels while further reducing brain serotonin levels (George et al., 2010; Szumlinski et al., 2007). Antagonizing NMDA receptors and type I metabotropic glutamatergic receptors had been shown to ameliorate the impact on synaptic structure induced by chronic unpredictable stress (Li et al., 2011) and reduce binge intake (Cozzoli et al., 2012; Cozzoli et al., 2014; Lum et al., 2014). Intra-hippocampal administration of serotonin was shown to reduce depressive-like symptoms induced by chronic unpredictable stress (Luo et al., 2008) and SSRIs capacity to reduce ethanol consumption behavior (reviewed by Lovinger, 1997). Since our results implicate that co-treatment of acamprosate and escitalopram may normalize glutamatergic and serotoninergic systems simultaneously, which results in a more potent effect in reducing alcohol consumption, further investigations are warranted on the expression of NMDA and AMPA receptors, glial progenitor and glutamate levels as well as serotonin and its metabolites levels under combined drug treatment as compared to control and mono-therapy groups. Taken together with our results, suppressing glutamatergic transmission while simultaneously potentiating serotonergic transmission may be an effective strategy to decrease ethanol intake under chronic stress exposure, hence explaining why combining acamprosate and escitalopram showed antidipsotropic efficacy in stressed mice.
The ethanol consumption suppression effect in certain drug groups during the drug administration week did not persist into the post-treatment week in either stressed or non-stressed mice. Ethanol consumption of the escitalopram and combined drug groups in both stressed and non-stressed mice had increased to a level similar to or above the baseline during the post-treatment week. These results might be attributed to the inadequacy of the current drug dosage and/or the drug administration duration to impose long-lasting changes in ethanol consumption. Moreover, owing to the inter-species differences in pharmacokinetics, mice might absorb, metabolize and excrete acamprosate and escitalopram before a stable blood concentration of the drugs could be attained, thus despite our effort to mimic daily multiple drug dosing in humans, the drugs imposed their effects most strongly within a narrow time window shortly after injection. For the current drug administration scheme at least, our results did not support a prolonged ethanol consumption suppression effect by these drugs or their combination after cessation of drug administration. Notably, these results infer an increased risk of relapse or retuning to previous drinking level when AUD patients stop using these medications, thus motivating them to sustain their medication compliance, albeit challenging, is crucial to maintain treatment effectiveness.
A few limitations of this study need to be noted. Firstly, mice were not dependent on ethanol and were only provided with ethanol two hours per day, thus the present study design was modelling the initial stage of alcohol misuse (binging) preceded by chronic stress induced mood abnormalities that may be observed in human. Thus, it could be possible that administration of an antidipsotropic agent and/or antidepressant could reduce the amount of excessive alcohol consumed during repeated exposure, which is a character of AUD, hence lowering the risk for progressing into more severe levels of AUD. Secondly, the non-stressed mice were group-housed before being exposed to ethanol during which they were singly housed. Thus, the non-stressed mice were subjected to extra handling during transferring between cages. In addition, these mice were singly housed during the 2-hour drinking session, to which these mice were not previously subjected. These stressors might contribute to a continuous increase of ethanol consumption during the post-treatment week in the saline group of the non-stressed mice which could potentially be alleviated by longer habituation time. Thirdly, we observed that some mice were relatively more resistant to the chronic unpredictable stress treatment than others. It is possible that future studies might need to exclude these animals before proceeding to further treatment to reduce behavioral heterogeneity. Finally, there could be gender differences in the response to our stress paradigm and drugs, which would require investigation on female mice.
In summary, we have applied a chronic unpredictable stress model to study the combined effects of acamprosate and escitalopram on ethanol consumption in mice demonstrating depression-like behaviors. We have confirmed that the chronic unpredictable stress model could induce depression- and anxiety-like behaviors in C57BL/6J mice. In non-stressed mice, administration of escitalopram alone or in combination with acamprosate reduced ethanol intake. However, combined medication was required to attain such effect in stressed mice. These results suggest that the combination of acamprosate and escitalopram may help AUD individuals with or without comorbid depression to reduce alcohol use.
Acknowledgments
We thank S. Choi for mouse husbandry. This work was supported by the Samuel C. Johnson for Genomics of Addiction Program at Mayo Clinic, the Ulm Foundation, the Godby Foundation, David Lehr Research Award from American Society for Pharmacology and Experimental Therapeutics, Mayo-Karolinska Institute (KI) Research Award and National Institute on Alcohol Abuse and Alcoholism (AA018779, AA017830) to DSC as well as the National Institute of General Medical Science GM28157 to RMW.
REFERENCES
- Adamson SJ, Sellman JD, Frampton CM. Patient predictors of alcohol treatment outcome: a systematic review. J Subst Abuse Treat. 2009;36(1):75–86. doi: 10.1016/j.jsat.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Rasheed N, Banu N, Palit G. Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress. 2010;13(4):355–364. doi: 10.3109/10253891003667862. [DOI] [PubMed] [Google Scholar]
- al Qatari M, Bouchenafa O, Littleton J. Mechanism of action of acamprosate. Part II. Ethanol dependence modifies effects of acamprosate on NMDA receptor binding in membranes from rat cerebral cortex. Alcohol Clin Exp Res. 1998;22(4):810–814. [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11(6):775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, van den Brink W, Smit JH, Veltman DJ, Beekman ATF, Penninx BWJH. Alcohol use disorders and the course of depressive and anxiety disorders. Brit J Psychiat. 2012;200(6):476–484. doi: 10.1192/bjp.bp.111.097550. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30(12):1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Brager A, Prosser RA, Glass JD. Acamprosate-responsive brain sites for suppression of ethanol intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1032–R1043. doi: 10.1152/ajpregu.00179.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L, Teesson M, O'Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100(6):787–796. doi: 10.1111/j.1360-0443.2005.001069.x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Dourish CT, Massi M. Blockade of pre- and post-synaptic 5-HT1A receptors does not modify the effect of fluoxetine or 5-hydroxytryptophan on ethanol and food intake in rats. Psychopharmacology. 1997;134(1):55–63. doi: 10.1007/s002130050425. [DOI] [PubMed] [Google Scholar]
- Conner KR, Pinquart M, Gamble SA. Meta-analysis of depression and substance use among individuals with alcohol use disorders. J Subst Abuse Treat. 2009;37(2):127–137. doi: 10.1016/j.jsat.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, Klugmann M, Metten P, Worley PF, Crabbe JC, Szumlinski KK. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res. 2012;36(9):1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK. Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology. 2014;39(2):435–444. doi: 10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Nolen-Hoeksema S, Zucker RA. Alcohol involvement as a function of co-occurring alcohol use disorders and major depressive episode: Evidence from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2011;117(2–3):145–151. doi: 10.1016/j.drugalcdep.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Acamprosate decreases the hypermotility during repeated ethanol withdrawal. Alcohol. 1999;18(1):77–81. doi: 10.1016/s0741-8329(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effects of acamprosate on excitatory amino acids during multiple ethanol withdrawal periods. Alcohol Clin Exp Res. 2003;27(3):465–470. doi: 10.1097/01.ALC.0000056617.68874.18. [DOI] [PubMed] [Google Scholar]
- De Witte P. Imbalance between neuroexcitatory and neuroinhibitory amino acids causes craving for ethanol. Addict Behav. 2004;29(7):1325–1339. doi: 10.1016/j.addbeh.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- George AK, Paul J, Kaimal SB, Paulose CS. Decreased cerebral cortex and liver 5-HT2A receptor gene expression and enhanced ALDH activity in ethanol-treated rats and hepatocyte cultures. Neurol Res. 2010;32(5):510–518. doi: 10.1179/174313209X385554. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32(11):1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Hinton DJ, Lee MR, Jacobson TL, Mishra PK, Frye MA, Mrazek DA, Macura SI, Choi DS. Ethanol withdrawal-induced brain metabolites and the pharmacological effects of acamprosate in mice lacking ENT1. Neuropharmacology. 2012;62(8):2480–2488. doi: 10.1016/j.neuropharm.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelai S, Aissi F, Lesch KP, Cohen-Salmon C, Hamon M, Lanfumey L. Alcohol intake after serotonin transporter inactivation in mice. Alcohol Alcoholism. 2003;38(4):386–389. doi: 10.1093/alcalc/agg095. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, Kessler RC. The familiar aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: A family history study. Brit J Psychiat. 1997;170:541–548. doi: 10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of dsm-iii-r alcohol abuse and dependence with other psychiatric disorders in the national comorbidity survey. Arch Gen Psychiatry. 1997;54(4):313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M, Danysz W. N-methyl-D-aspartate and group I metabotropic glutamate receptors are involved in the expression of ethanol-induced sensitization in mice. Behav Pharmacol. 2006;17(1):1–8. doi: 10.1097/01.fbp.0000181600.95405.c7. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Tennen H. Post-treatment outcomes in a double-blind, randomized trial of sertraline for alcohol dependence. Alcohol Clin Exp Res. 2012;36(4):739–744. doi: 10.1111/j.1530-0277.2011.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Hinton DJ, Wu J, Mishra PK, Port JD, Macura SI, Choi DS. Acamprosate reduces ethanol drinking behaviors and alters the metabolite profile in mice lacking ENT1. Neurosci Lett. 2011;490(2):90–95. doi: 10.1016/j.neulet.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45(4):355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Serotonin's role in alcohol's effects on the brain. Alcohol Health Res World. 1997;21(2):114–120. [PMC free article] [PubMed] [Google Scholar]
- Lum EN, Campbell RR, Rostock C, Szumlinski KK. mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology. 2014;79:679–687. doi: 10.1016/j.neuropharm.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo DD, An SC, Zhang X. Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Res Bull. 2008;77(1):8–12. doi: 10.1016/j.brainresbull.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Maurel S, De Vry J, Schreiber R. Comparison of the effects of the selective serotonin-reuptake inhibitors fluoxetine, paroxetine, citalopram and fluvoxamine in alcohol-preferring cAA Rats. Alcohol. 1999;17(3):195–201. doi: 10.1016/s0741-8329(98)00046-9. [DOI] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The anti-relapse compound acamprosate inhibits the development of a conditioned place preference to ethanol and cocaine but not morphine. Br J Pharmacol. 2003;138(1):9–12. doi: 10.1038/sj.bjp.0705059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175(1):43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Monteiro S, Roque S, de Sa-Calcada D, Sousa N, Correia-Neves M, Cerqueira JJ. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front Psychiatry. 2015;6:6. doi: 10.3389/fpsyt.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhonen LH, Lahti J, Alho H, Lonnqvist J, Haukka J, Saarikoski ST. Serotonin transporter polymorphism as a predictor for escitalopram treatment of major depressive disorder comorbid with alcohol dependence. Psychiatry Res. 2011;186(1):53–57. doi: 10.1016/j.psychres.2010.07.039. [DOI] [PubMed] [Google Scholar]
- Muhonen LH, Lahti J, Sinclair D, Lonnqvist J, Alho H. Treatment of alcohol dependence in patients with co-morbid major depressive disorder--predictors for the outcomes with memantine and escitalopram medication. Subst Abuse Treat Prev Policy. 2008;3:20. doi: 10.1186/1747-597X-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Waller MB, Gatto GJ, McBride WJ, Lumeng L, Li TK. Monoamine uptake inhibitors attenuate ethanol intake in alcohol-preferring (P) rats. Alcohol. 1985;2(2):349–352. doi: 10.1016/0741-8329(85)90073-4. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry. 2013;73(12):1180–1188. doi: 10.1016/j.biopsych.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Naassila M, Hammoumi S, Legrand E, Durbin P, Daoust M. Mechanism of action of acamprosate. Part I. Characterization of spermidine-sensitive acamprosate binding site in rat brain. Alcohol Clin Exp Res. 1998;22(4):802–809. [PubMed] [Google Scholar]
- Nollet M, Le Guisquet AM, Belzung C. Models of depression: unpredictable chronic mild stress in mice. Curr Protoc Pharmacol. 2013;Chapter 5(Unit 5):65. doi: 10.1002/0471141755.ph0565s61. [DOI] [PubMed] [Google Scholar]
- Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW. Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release. Eur J Pharmacol. 2002;437(1–2):55–61. doi: 10.1016/s0014-2999(02)01272-4. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50(5):345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar R, Pandey DK, Mahesh R, Radha R. 1-(m-Chlorophenyl)piperazine induces depressogenic-like behaviour in rodents by stimulating the neuronal 5-HT(2A) receptors: proposal of a modified rodent antidepressant assay. Eur J Pharmacol. 2009;608(1–3):32–41. doi: 10.1016/j.ejphar.2009.02.041. [DOI] [PubMed] [Google Scholar]
- Rammes G, Mahal B, Putzke J, Parsons C, Spielmanns P, Pestel E, Spanagel R, Zieglgansberger W, Schadrack J. The anti-craving compound acamprosate acts as a weak NMDA-receptor antagonist, but modulates NMDA-receptor subunit expression similar to memantine and MK-801. Neuropharmacology. 2001;40(6):749–760. doi: 10.1016/s0028-3908(01)00008-9. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6(1):1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Saglam E, Kayir H, Celik T, Uzbay T. Effects of escitalopram on ethanol withdrawal syndrome in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):1027–1032. doi: 10.1016/j.pnpbp.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Vengeliene V, Jandeleit B, Fischer WN, Grindstaff K, Zhang X, Gallop MA, Krstew EV, Lawrence AJ, Kiefer F. Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology. 2014;39(4):783–791. doi: 10.1038/npp.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 2006;17(3):271–287. doi: 10.1097/00008877-200605000-00008. [DOI] [PubMed] [Google Scholar]
- Szumlinski K, Diab M, Friedman R, Henze L, Lominac K, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology. 2007;190(4):415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Witte J, Bentley K, Evins AE, Clain AJ, Baer L, Pedrelli P, Fava M, Mischoulon D. A randomized, controlled, pilot study of acamprosate added to escitalopram in adults with major depressive disorder and alcohol use disorder. J Clin Psychopharmacol. 2012;32(6):787–796. doi: 10.1097/JCP.0b013e3182726764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3):149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]