Abstract
Arachidonic acid is metabolized to epoxyeicosatrienoic acids (EETs) by CYP-epoxygenases, and EETs are kidney protective in multiple pathologies. We determined the ability of an EET analog, EET-A, to mitigate experimental radiation nephropathy. The kidney expression of the EET producing enzyme CYP2C11 was lower in rats that received total body irradiation (TBI rat) compared to non-irradiated control. At 12 weeks after TBI, the rats had higher systolic blood pressure and impaired renal afferent arteriolar function compared to control, and EET-A or captopril mitigated these abnormalities. The TBI rats had 3-fold higher blood urea nitrogen compared to control, and EET-A or captopril decreased BUN by 40–60%. The urine albumin/creatinine ratio was increased 94-fold in TBI rats, and EET-A or captopril attenuated that increase by 60–90%. In TBI rats, nephrinuria was elevated 30-fold and EET-A or captopril decreased it by 50–90%. Renal interstitial fibrosis, tubular, and glomerular injury were present in the TBI rats, and each was decreased by EET-A or captopril. We further demonstrated elevated renal parenchymal apoptosis in TBI rats, which EET-A or captopril mitigated. Additional studies revealed that captopril or EET-A mitigated renal apoptosis by acting on p53/Fas/FasL apoptotic pathway. Overall, this study demonstrates a novel EET-analog based strategy for mitigation of experimental radiation nephropathy by improving renal afferent arteriolar function and by decreasing renal apoptosis.
Keywords: novel small lipid molecule, radiotherapy, afferent arteriole, apoptosis, Fas
INTRODUCTION
Progressive loss of kidney function is a debilitating feature of many chronic kidney diseases (CKD) and current therapies only slow this decline in CKD. A better mechanistic understanding of the pathophysiology of CKD and resulting improved treatments are clearly needed. In cancer patients, normal tissue injury from chemo- and radiotherapy accounts for CKD in many cancer survivors [1]. CKD is a known and life-threatening late sequel of hematopoietic stem cell transplantation (HSCT), and is often linked to total body irradiation (TBI) given just before the HSCT [2]. The US National Cancer Institute has identified late effects after cancer treatment and their mitigation as a priority for research and patient care [3]. It is particularly important to identify mitigators, i.e. agents that can be started after completion of chemotherapy or irradiation but before manifestation of injury. Moreover, a mitigator will not interfere with the treatment of cancer, but may attenuate or even eliminate late effects of cancer treatment.
Angiotensin-converting-enzyme inhibitors (ACEI) mitigate experimental radiation nephropathy, yet must be used at doses above their human equivalent to achieve maximum benefit [4,5]. This justifies a search for novel treatments for radiation renal injury. One such novel approach could be the epoxyeicosatrienoic acids (EETs). EETs are produced from arachidonic acid through epoxidation catalyzed by CYP-epoxygenase enzymes. In rat kidney, the members of CYP2C family including CYP2C11 and CYP2C23 produce EETs. Increasing bioavailability of EETs by inhibiting their degradation by soluble epoxide hydrolase (sEH) demonstrated kidney protective effects in multiple pathologies [6–10]. But the major drawbacks of sEH inhibitors are that they result in a generalized increase in EETs and that their effectiveness depends on CYP epoxygenase-mediated EET generation [11]. This is an important limitation because many renal and cardiovascular diseases are associated with impaired epoxygenase generation of EETs [12,13]. With this background, we have developed chemically and metabolically more robust synthetic analogs of the EETs and demonstrated their benefit in models of cis-platinum, Dahl salt-sensitive hypertension, and angiotensin II (ANG II)-induced hypertension [14–17]. These EET analogs also promote organ and tissue regeneration and wound healing [18]. They are thus optimal candidates to test in radiation nephropathy.
After renal irradiation in sufficient doses, there is a latent period of weeks to months before the development of proteinuria, azotemia, and hypertension [4,19]. In radiation nephropathy, renal endothelial dysfunction and altered hemodynamics are known features [20–22]. Inflammation is not prominent, but tubular cell loss and progressive interstitial scarring coincide with progressive loss of renal function [23]. There is some evidence for apoptosis as the mechanism of renal tubular cell loss in radiation nephropathy [24]. In this regard and considering EET analogs as a candidate mitigator for radiation nephropathy, we earlier demonstrated that our lead EET analog, EET-A, has beneficial actions as a vasodilating, anti-hypertensive, kidney protective, and anti-apoptotic agent [15–17]. We, therefore, hypothesize that EET-A would be an effective novel mitigator of radiation nephropathy. We determined the mitigating effect of EET-A in radiation nephropathy and investigated its mechanism with attention to its renal vascular and anti-apoptotic effects.
MATERIALS AND METHODS
Materials
All chemicals and assay kits were purchased from Sigma Aldrich (St. Louis, MO, USA) unless otherwise mentioned. The EET analog, EET-A was designed and synthesized in the laboratory of John R. Falck, Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX.
Animal model of radiation nephropathy
The studies were performed in syngeneic 7–8-week-old male WAG/RijCmcr rats that were bred and housed in a moderate security barrier. The rats were maintained in the Biomedical Resource Center of the Medical College of Wisconsin. The Institutional Animal Care and Use Committee of the Medical College of Wisconsin approved the animal protocols. The total-body irradiation (TBI) was performed using a X-RAD 320 orthovoltage unit (Precision X-Ray, North Branford, CT) as described earlier [25] Rats were immobilized in a Plexiglas jig that restricts their movement. Eleven Gy TBI was delivered posteriorly to anteriorly. The half value layer of the beam used for irradiation was 1.4 mm of Cu and it was given at a dose rate of 1.75 Gy/min. Bone marrow was partially spared by shielding a hind leg with a ¼ inch thick lead block.
Experimental design
The rats were divided into four groups (n=8–10/group) and these were; Group 1: Vehicle (drinking water) treated non-irradiated control (Vehicle), Group 2: Vehicle treated irradiated rats (11Gy+Vehicle), Group 3: irradiated rats placed on EET-A (10mg/kg/d) (11Gy+EET-A), and Group 4: irradiated rats placed on captopril (30mg/kg/d) (11Gy+Captopril). EET or captopril was given orally in drinking water started at 2 days after TBI and continued for 12 weeks. Systolic blood pressure was measured using tail-cuff method (Kent Scientific Corporation, Torrington, CT, USA) and urine was collected over a 24-h period on the 12th week after irradiation, Twelve weeks after irradiation, rats were anesthetized for blood sample collection followed by euthanasia and tissue collection. Urine and serum samples were kept frozen at −80°C until analyzed. The kidneys were removed, washed with physiological saline and stored at −80°C. A part of the kidney was preserved in 10% buffered formalin for histology and immunohistology.
Biochemical analysis
The levels of blood urea nitrogen (BUN) (BioAssay Systems, Hayward, CA, USA), urinary creatinine (Cayman Chemical Company, Ann Arbor, MI, USA), and protein (Cayman Chemical Company, Ann Arbor, MI, USA) were measured spectrophotometrically using commercial kits. Urinary albumin and nephrin were measured using ELISA kits (Exocell, Philadelphia, PA). Kidney cortex homogenate was prepared with a lysis Buffer (50 mM HEPES, pH 7.4, with 5 mM CHAPS and 5 mM DTT), centrifuged at 10,000 g for 10 min and then the resulting supernatant was used for the assay. Caspase 3 activity in the kidney homogenate was determined using a commercial fluorimetric assay kit. The caspase 3 fluorimetric assay is based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-ValAsp-7-amido-4-methylcoumarin (Ac-DEVD-AMC) by caspase 3, resulting in the release of the fluorescent 7-amino-4-methylcoumarin (AMC) moiety. The caspase 3 activity is expressed as nmol of AMC/min/µL. Kidney tissue protein content was measured using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). The activity of caspase 9 was measured using colorimetric assay that is based on detection of cleavage of substrate LEHD-AFC (AFC: 7-amino-4-trifluoromethyl coumarin) (Abcam, Cambridge, MA).
Real-Time PCR analyses
Real-Time PCR (RT-PCR) analysis was carried out to assess the renal mRNA expressions of the CYP-epoxgenase enzymes CYP2C23, CYP2C11, and soluble epoxide hydrolase enzyme (sEH). Renal mRNA expressions of the DNA repair checkpoint protein p53 and apoptotic signalling molecules Bcl-2 (B-cell lymphoma 2), Bcl-2 associated X protein (Bax), and Bcl-2 antagonist/killer protein (Bak) were studied using RT-PCR. The RT-PCR analysis was carried out to determine the renal mRNA expression of caspase 3, caspase 8, and caspase 9. Renal mRNA expressions of several members of the death receptor pathway of apoptosis such as Fas, FasL, tumour necrosis factor-α (TNF-α) and its receptor TNFR1 were also determined using RT-PCR analysis. Messenger RNA (mRNA) was isolated from kidney homogenate using RNeasy Mini Kit (QIAGEN, CA, USA) according to the manufacturer’s instructions. The mRNA samples were quantified by spectrophotometry at 260 nm and 1 µg of total RNA was reverse-transcribed to cDNA using iScript™ Select cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). The target gene expression was quantified by iScript One-Step RT-PCR Kit with SYBR green using MyiQ™ Single Color Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Each amplified sample in all wells was analyzed for homogeneity using dissociation curve analysis using iQ5 Optical System Software, Version 2.1 (Bio-Rad Laboratories, Hercules, CA, USA). After denaturation at 95°C for 2 min, 40 cycles were performed at 95°C for 10s and at 60°C for 30s. Each sample was run in triplicate, and the comparative threshold cycle (Ct) method was used to quantify fold increase (2–ΔΔCt) in the expression of the target genes compared to controls. In analyzing the relative expression of the target genes, the Ct values were normalized to two housekeeping genes (pgk1 and 18S). Statistical analyses were carried out for at least 5–7 experimental samples in each experimental group.
Western Immunoblotting
Fifty micrograms of homogenized kidney cortex protein samples were separated by SDS-PAGE on a 10% Tris-glycine gel, and proteins were transferred electrophoretically to a nitrocellulose membrane. Nonspecific binding sites were blocked by incubating the blots overnight at 4°C in a Tris NaCl buffer (TBS) containing 5% nonfat dry milk and 0.1% Tween 20. The primary antibodies used were for CYP2C23 (1:500) and CYP2C11 (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA) and for sEH (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA). The blots were then washed in TBS-0.1% Tween, and incubated with horseradish peroxidase conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA) for 1h. Detection was accomplished using enhanced chemiluminescence Western blot analysis, band intensity was measured densitometrically using ImageQuant TL 8.1 image analysis software (GE Healthcare, PA, USA) and the values were normalized to β-actin.
TUNEL assay
Terminal deoxynucleotidyltransferase-mediated triphosphate nick-end labeling (TUNEL) technique was used to determine apoptosis. De-paraffinized and gradually hydrated 3µm thick sections of kidney tissues collected after 12-week experimental protocol were used to assess apoptosis using the TUNEL assay. This was performed using the TUNEL Apoptosis Detection Kit (GenScript, Piscataway, NJ, USA).
Histopathology
After fixation of the kidneys with 10% buffered formalin, renal tissues were sectioned and stained with periodic Acid-Schiff (PAS) and Picrosirius Red (PSR) for histological examination. Histological injury was determined in PAS stained tissue sections at magnification of ×200 using image analyzing software by NIS Elements AR version 3.0 (Nikon instruments Inc., Melville, NY, USA). Histopathological changes were scored as published earlier. Renal interstitial fibrosis was measured in PSR stained tissue sections and the areas positive for collagen were analyzed using software by NIS Elements AR version 3.0. The renal tissue areas positive for collagen were expressed as the percentage area fraction relative to total area analyzed. To minimize observer bias, the cast area calculation was performed by two observers in a masked fashion without knowledge of the treatment group from which the tissues originated.
Renal afferent arteriolar response to acetylcholine
Afferent arteriolar responses to acetylcholine were studied at the twelve week time point using an ex vivo perfused juxtamedullary nephron preparation [26,27]. Briefly, after pentobarbital anesthesia (50 mg/kg, i.p.) and midline laparotomy, the right renal artery was cannulated through the superior mesenteric artery, and the kidney was immediately perfused with a Tyrode’s solution containing 6% albumin and a mixture of l-amino acids. After the microdissection procedures were completed the renal artery perfusion pressure was set to 100 mm Hg. The tissue surface was continuously superfused with a Tyrode’s solution containing 1% albumin. After a 20-minute equilibration period, an afferent arteriole was chosen for study, monitored continuously by video microscopy, and its baseline diameter was measured. The afferent arteriole was pre-constricted with norepinephrine (0.5µM) and subsequently exposed to increasing concentrations of acetylcholine (0.1–10 µM) in perfusate and diameter changes were monitored for 3 minutes at each concentration. Steady-state diameter after acetylcholine exposure was attained by the end of the second minute, and the average diameter at the third minute of each treatment period was used for statistical analysis.
Statistical analysis
Results are reported as mean ± S.E.M. Statistical significance between two measurements was determined by the two-tailed unpaired Student’s t test. Among groups it was determined by repeated measure one-way analysis of variance followed by Tukey’s post-hoc test using GraphPad Prism® Version 4.0 software (GraphPad Software Inc, La Jolla, CA, USA). Probability values of p <0.05 were considered significant where the critical value of p was two-sided.
RESULTS
Radiation decreases renal expression of CYP-epoxygenase enzyme
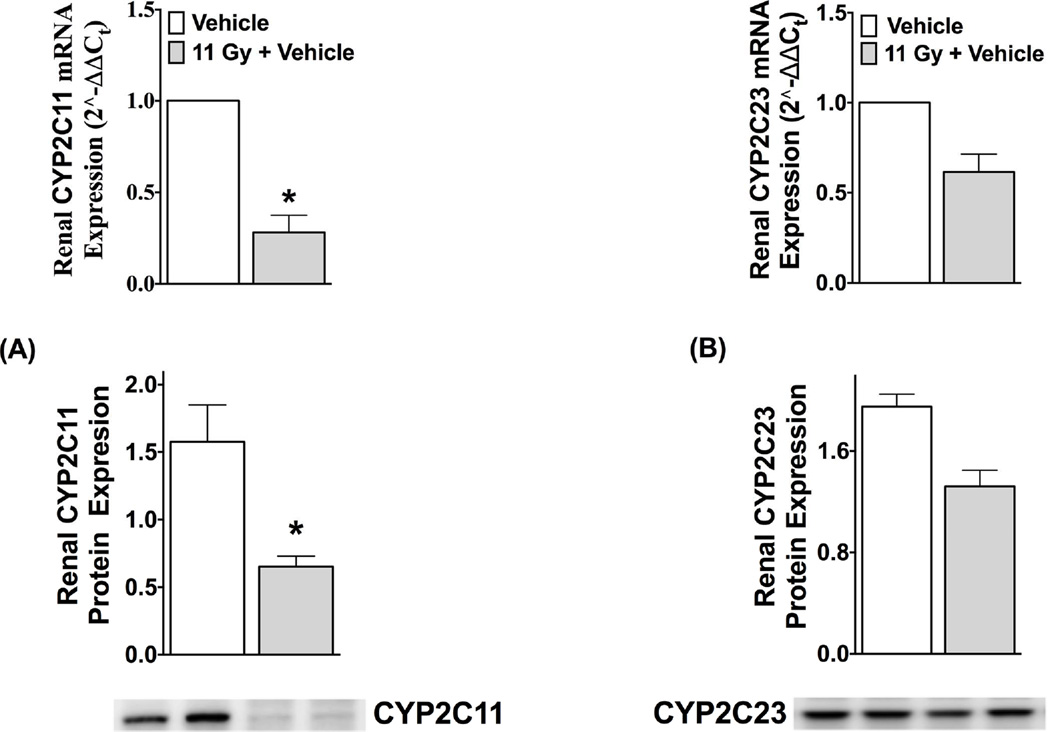
In the irradiated rats not on drug (TBI rats), both mRNA and protein expressions of EET-synthesizing CYP2C11 were 60–70% lower than non-irradiated control. The mRNA and protein expressions of CYP2C23 were also 30–40% lower in TBI rats compared to non-irradiated controls, but these latter changes did not reach statistical significance (Figure 1). Renal CYP epoxygenase mRNA and protein expression and urinary EET levels are decreased six weeks following TBI, a time point where BUN and systolic blood pressure are not elevated (Fig. S1 & S2, Online Supplement).
Figure 1.
Renal mRNA and protein expressions of CYP2C11 (A) and CYP2C23 (B) at 12-week after total-body radiation (TBI) compared to control (non-irradiated, vehicle-treated and age-matched). In immunoblotting experiments, protein expressions of CYP2C23 and CYP2C11 are normalized to the expression of β-actin. *P<0.05 vs. Control, data expressed as mean ± SEM, and n=5.
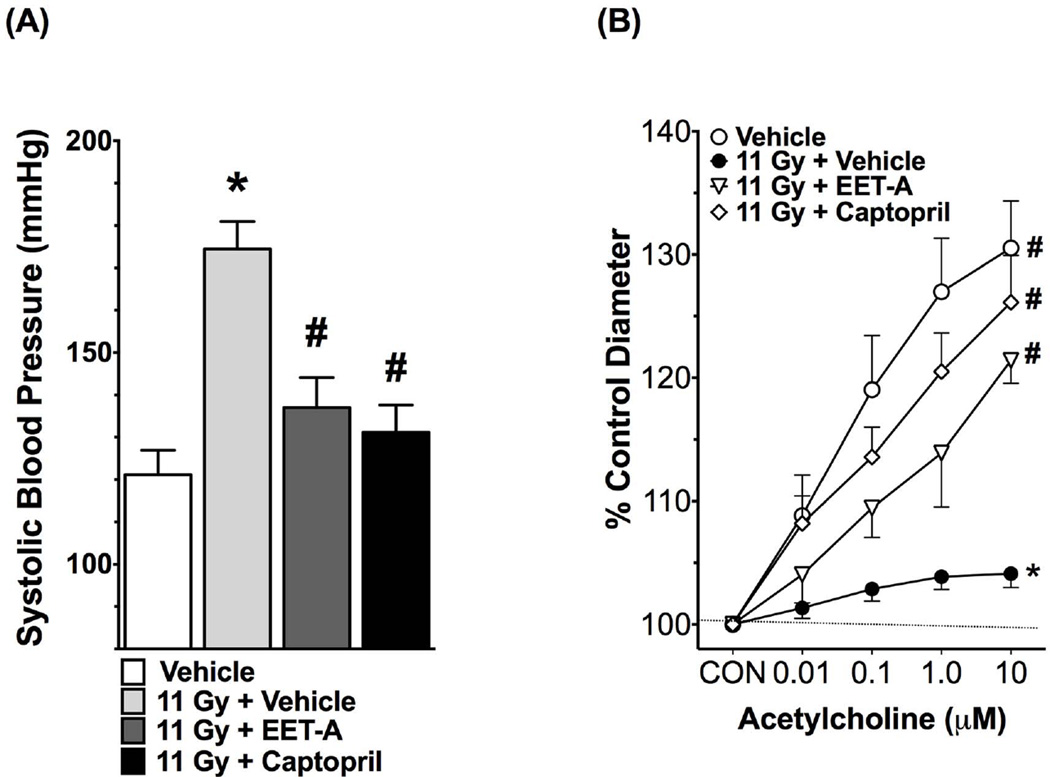
EET-A or captopril mitigate hypertension and improve renal afferent arteriolar function in radiation nephropathy
The irradiated rats developed hypertension with a markedly elevated systolic blood pressure compared to non-irradiated control, and EET-A or captopril mitigated this elevation of systolic blood pressure (Figure 2). We measured renal afferent arteriolar function using the ex vivo juxtamedullary preparation. We demonstrated that in TBI rats the renal afferent arteriolar function was impaired as demonstrated by markedly impaired renal afferent arteriolar response to acetylcholine (Figure 2). Both EET-A and captopril improved renal afferent arteriolar responses to acetylcholine in TBI rats (Figure 2). We also demonstrated that renal afferent arteriolar autoregulation is impaired in TBI rats, and EET-A or captopril treatments improved it (Figure S3, Online Supplement). Overall, these data demonstrate elevated blood pressure and impaired renal afferent arteriolar vasodilatory and vasoconstrictive function in TBI rats, and these abnormalities were mitigated by EET-A or captopril.
Figure 2.
Systolic blood pressure (A) and renal afferent arteriolar responses to acetylcholine (B) in the experimental groups at the end of 12-week protocol. In determining renal afferent arteriolar response to acetylcholine, the baseline afferent arteriolar diameters in Vehicle, 11Gy+Vehicle, 11Gy+EET-A and 11Gy+Captopril were 26.2±2.1 (n=7), 26.7±0.9 (n=8), 25.8±0.6 (n=5) and 22.4±0.6 µm (n=8), respectively. *P<0.05 vs. Vehicle; #P<0.05 vs. 11Gy+Vehicle. All data expressed as mean ± SEM, and in systolic blood pressure measurement n=8 for each group.
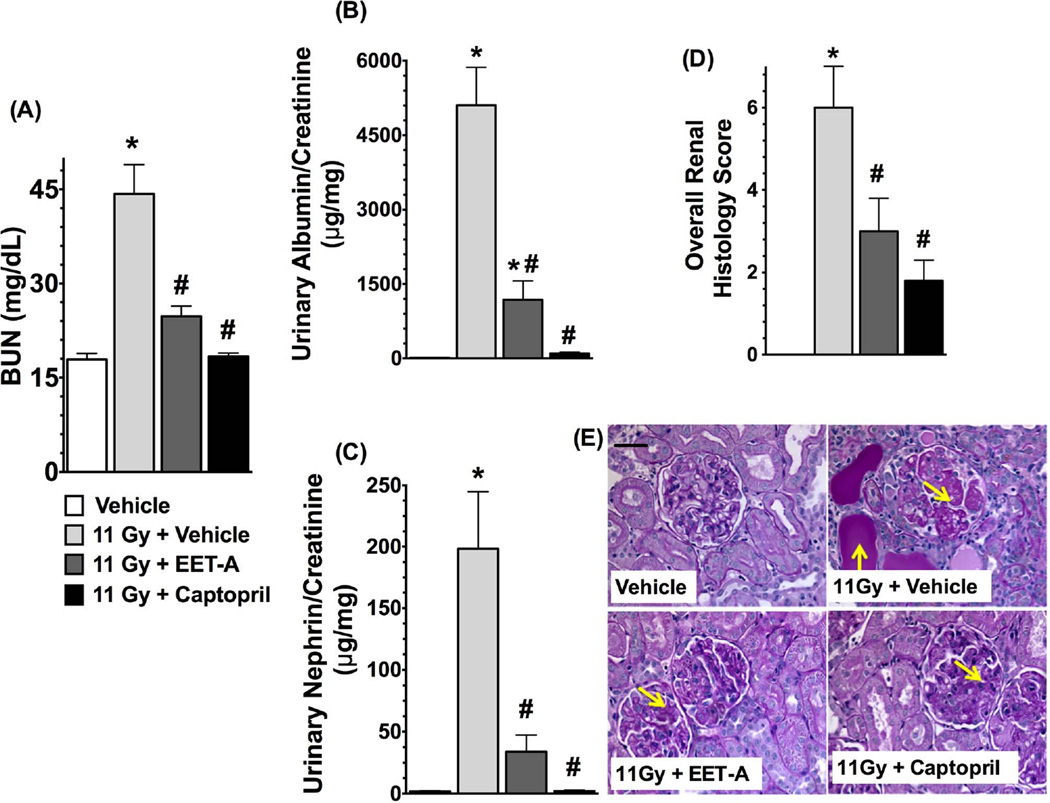
EET-A or captopril mitigates kidney injury in radiation nephropathy
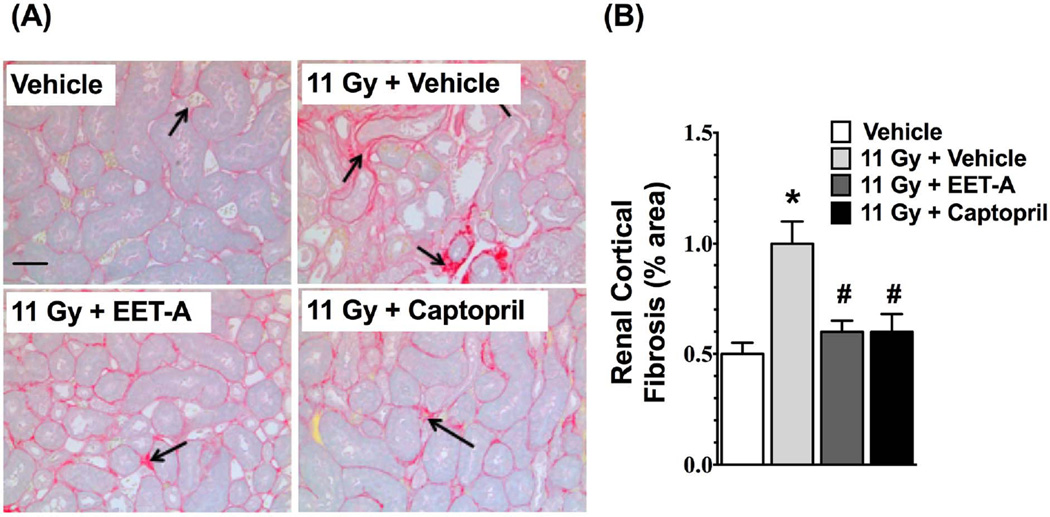
Radiation caused marked kidney injury with a 3-fold elevation in BUN levels (p<0.05). Both EET-A and captopril markedly mitigated kidney injury in TBI rats and reduced the BUN levels by 40–60% (Figure 3a-A). The TBI rats had a 90-fold higher urinary albumin/creatinine ratio compared to control rats, and EET-A or captopril mitigated this by 60–90% (Figure 3a-B). In TBI rats, there was also marked nephrinuria with 30-fold higher urinary nephrin excretion compared to control. This was significantly mitigated in TBI rats by EET-A or captopril treatments with 50–90% reductions in urinary excretion of nephrin (Figure 3a-C). The albuminuria and nephrinuria in TBI rats were associated with marked tissue injury. These histopathological changes were markedly mitigated by EET-A or captopril (Figure 3a-D, E). The TBI rats also had marked renal cortical interstitial fibrosis, which was mitigated by EET-A or captopril (Figure 3b-A,B).
Figure 3.
a: (A) Blood urea nitrogen (BUN), (B) albuminuria, (C) nephrinuria, and (D) histopathological injury score in the experimental groups at the end of 12-week protocol. (E) Representative photomicrographs of Periodic Acid-Schiff staining (200×) depicting renal tubular casts and damaged glomeruli (yellow arrows) in the kidney sections of different experimental groups. P<0.05 vs. Vehicle, and #P<0.05 vs. 11Gy+Vehicle. Data expressed as mean ± SEM, and n=8 for each group.
b: (A) Representative photomicrographs of Picrosirius Red staining (200×) depicting collagen formation (black arrows) in the kidney sections of the experimental groups. (B) Percentage of collagen positive area relative to total area of a kidney section analyzed (200×). Staining was done at the end of 12-week protocol. P<0.05 vs. Vehicle, and #P<0.05 vs. 11Gy+Vehicle. Data expressed as mean ± SEM, and n=8 for each group.
EET-A or captopril mitigate radiation-induced renal apoptosis
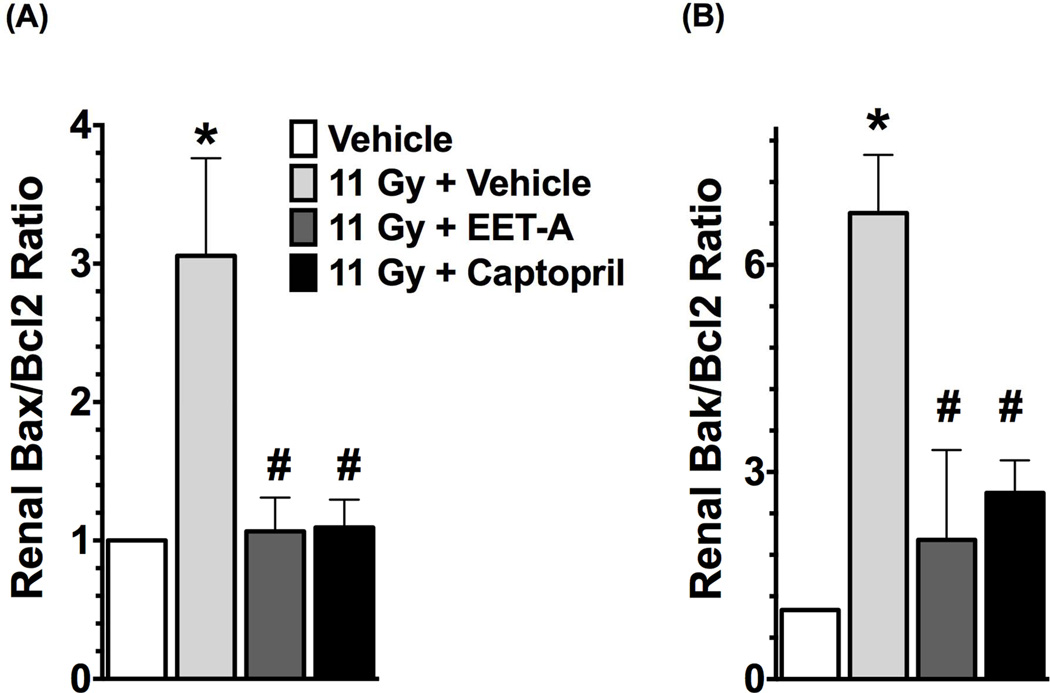
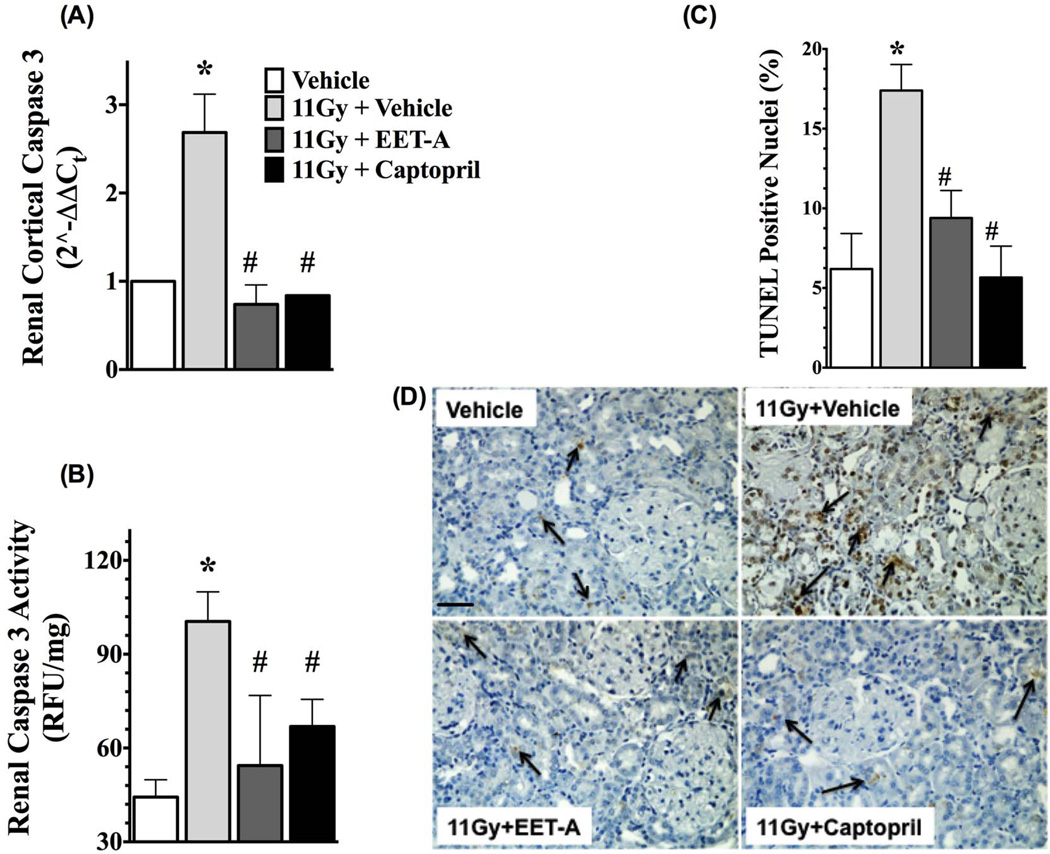
In the kidney cortex of TBI rats, we demonstrated increased apoptotic signalling with markedly elevated mRNA expressions of pro-apoptotic molecules Bax and Bak in relation to the anti-apoptotic molecule Bcl-2 (Bax/Bcl-2 and Bak/Bcl-2 ratios). We demonstrated a 3 to 7-fold increase in Bax/Bcl-2 and Bak/Bcl-2 ratios in the kidney of the TBI rats. Interestingly, both EET-A and captopril equally mitigated the kidney apoptotic signalling in the TBI rats with 60–70% reductions in Bax/Bcl-2 and Bak/Bcl-2 ratios compared to use of no mitigator (Figure 4). In accord with these findings, the kidney of irradiated rats also had markedly higher mRNA expression and activity of caspase 3. EET-A or captopril decreased mRNA expression and the activity of caspase 3 in the kidney of TBI rats, and both mitigators had comparable renal anti-apoptotic effects (Figure 5). The antiapoptotic effects of EET-A or captopril in the kidney of TBI rats were confirmed by the reduction of TUNEL positive renal apoptotic cells. In the kidney cortex of the TBI rats, we demonstrated 3-fold increase in the presence of TUNEL positive apoptotic cells. This was mainly localised to the tubular epithelium. Use of EET-A and captopril as mitigators reduced these cells by 45–60% compared to vehicle (Figure 5). These results clearly demonstrate marked renal apoptosis in TBI rats, and this was mitigated by EET-A or captopril.
Figure 4.
Renal mRNA expression ratio of pro-apoptotic Bax (A) and Bak (B) relative to the mRNA expression of anti-apoptotic Bcl2 in the experimental groups. All measurements were done at the end of 12-week protocol. P<0.05 vs. Vehicle, and #P<0.05 vs. 11Gy+Vehicle. Data expressed as mean ± SEM, and n=6–8 for each group.
Figure 5.
Renal mRNA expression (A) and the activity (B) of caspase 3, and (C) percentage (%) of TUNEL positive renal apoptotic cells in the kidney of different experimental groups. (D) Representative photomicrographs of TUNEL staining in the kidney of the experimental groups. All measurements were done at the end of 12-week protocol. P<0.05 vs. Vehicle, and #P<0.05 vs. 11Gy+Vehicle. Data expressed as mean ± SEM, and n=8 for each group.
Mitigation of radiation-induced renal apoptosis by EET-A or captopril is via the Fas/FasL pathway
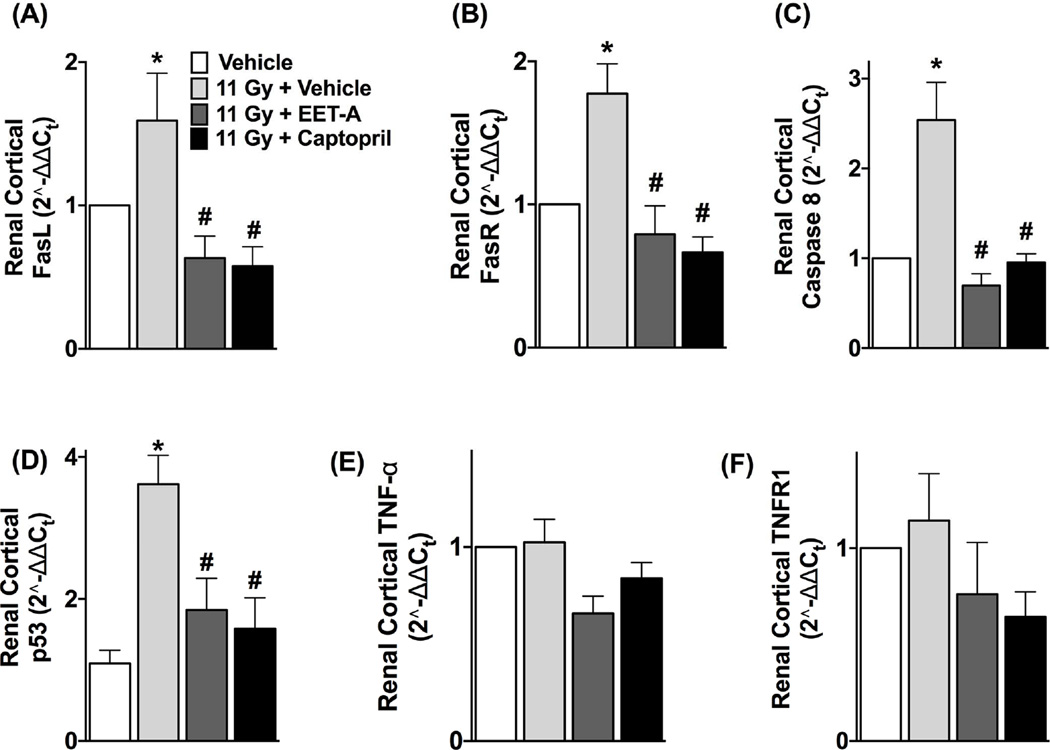
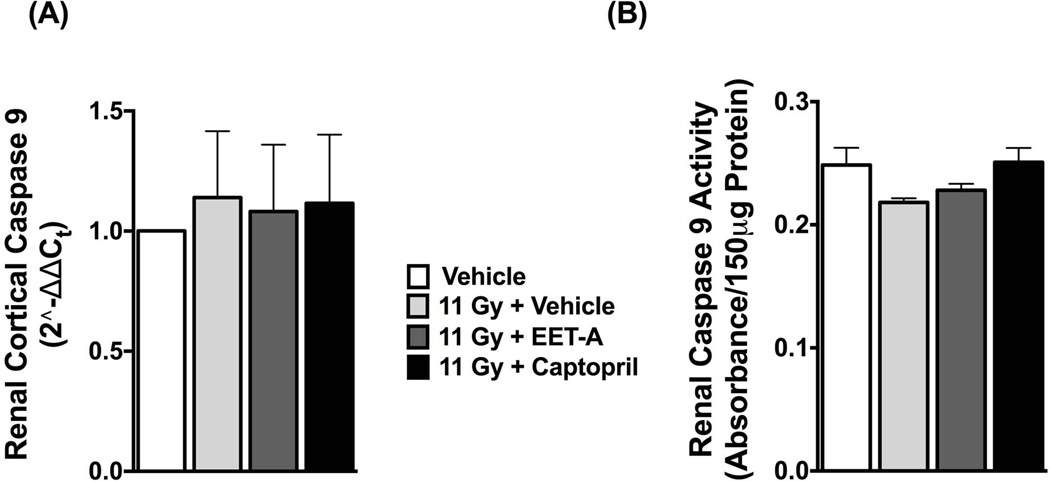
We demonstrated elevated mRNA expressions of FasL (Fas ligand) and its receptor FasR (also known as Fas) in the kidney of TBI rats compared to control. There was an increased level of caspase 8 mRNA in the kidney of TBI rats compared to control. Treatments with EET-A or captopril equally decreased the mRNA expression of FasL, Fas and caspase 8 in the kidney of the TBI rats (Figure 6). Fas expression is activated by p53, a key protein in the checkpoint pathways affected by ionizing radiation [28,29]. We determined renal mRNA expression of p53 in TBI rats and demonstrated its 3-fold increase compared to control. EET-A or captopril reduced renal p53 mRNA expression to a level similar to control (Figure 6). We also studied mRNA expression of TNF-α and its receptor TNFR1 that constitute another component of the death receptor mediated extrinsic apoptotic pathway other than the one mediated by Fas/FasL. In the kidney of the irradiated TBI rats, we did not find any difference in TNF-α and TNFR1 mRNA expressions (Figure 6). We further studied mRNA expression and activity of caspase 9 that constitute the caspase cascade of the intrinsic apoptotic pathway, and did not find any difference in either mRNA expression or the activity of caspase 9 between the TBI and control rats (Figure 7).
Figure 6.
Renal mRNA expressions of (A) FasL, (B) FasR, (C) caspase 8, (D) p53, (E) TNF-α, and (F) TNFR1 in the kidney of the experimental groups. All measurements were done at the end of 12-week protocol. P<0.05 vs. Vehicle, and #P<0.05 vs. 11Gy+Vehicle. Data expressed as mean ± SEM, and n=8 for each group.
Figure 7.
Renal mRNA expression (A) and the activity (B) of caspase 9 in the experimental groups. All measurements were done at the end of 12-week protocol. P<0.05 vs. Vehicle, and #P<0.05 vs. 11Gy+Vehicle. Data expressed as mean ± SEM, and n=8 for each group.
DISCUSSION
The kidneys are the dose-limiting organs for radiotherapy of gastrointestinal cancers, gynecologic cancers, lymphomas, and sarcomas of the upper abdomen. They are also at risk during total body irradiation (TBI) in patients undergoing HSCT. Indeed, as many as 20% of long-term survivors of HSCT develop chronic kidney disease and irradiation is a known factor in its development [19]. Thus the ability to mitigate the severity of radiation-induced kidney injury will have a significant clinical impact in cancer therapy. Further, the threat of radionuclear accidents and terrorism requires efforts to identify effective mitigators of normal tissue radiation injury. The present studies have immediate clinical relevance [30,31].
In the present study, both captopril and EET-A were used as mitigators. They were started two days after irradiation, and exerted significant long-term benefit. By starting these drugs well after irradiation, they are not acting as radioprotectors, but, rather, they are interfering with later injury pathways. In this mitigation regimen, EET-A and captopril are not acting to treat established renal injury, which occurs many weeks after irradiation. Use of pharmaceuticals in a mitigation regimen is very advantageous in the case of radiation exposure. For clinical radiotherapy, the mitigator can be started after irradiation and thus not interfere with cancer treatment. For unpredictable accidental or belligerent radiation exposures, mitigators can be started after those exposures and exert long-term benefit.
Approval of radiation injury mitigators for accidental or belligerent exposures requires taking into account the Food and Drug Administration animal rule, in which mechanisms of injury and also of mitigation must be understood. Radiation nephropathy presents clinically as proteinuria, hypertension, and azotemia. In our model these features occur in rats at seven to ten weeks following TBI [32]. Renal endothelial and vascular dysfunction occur before proteinuria, hypertension, and azotemia. We have observed attenuated afferent arteriolar dilator responses to acetylcholine as early as three weeks following TBI [33]. Renal epithelial cell damage also occurs following radiation and contributes to the chronic renal injury [23,34]. In the present study, we demonstrate that the TBI rats at 12 weeks have hypertension, impaired renal afferent arteriolar response to acetylcholine, impaired afferent arteriolar autoregulation, renal apoptosis and parenchymal injury. Most importantly, in the TBI rats these abnormalities developed along with reduced renal mRNA and protein expressions of CYP2C11, while the mRNA and protein expressions of the EET degrading enzyme sEH remain unaffected (Fig S4, Online Supplement). We further demonstrated that such decreased CYP epoxygenase expressions along with decreased urinary EET levels were evident at 6 weeks post TBI, and prior to the development of hypertension and azotemia in TBI rats (Fig S1 & S2, Online Supplement). The 30% reduction of urinary EETs may not be sufficient to explain all of the injury in this model, our findings suggest an important novel role for a markedly down-regulated EET producing enzyme system in the pathophysiology of renal radiation injury.
Earlier studies demonstrated a vascular component in the pathophysiology of hypertension and radiation nephropathy [20–22,35,36]. Morphologic vascular injury occurs within six weeks of irradiation in a sequentially evaluated porcine model [35]. Human studies suggested that radiation-induced hypertension is caused by injury to the small renal arteries [22]. Renal vascular injury in radiation is further supported by the reports that renal arteriolar rarefaction occurred at 20 weeks after irradiation in a rat model [37], and impaired renal hemodynamics is a known feature of human and experimental radiation nephropathy [20,21]. We believe that there is reduction of renal blood flow in the present studies although we have no direct measurement of that. Dysfunction in afferent arteriolar function reduce renal function. For instance, Juncos et al. [36] reported impaired renal function in irradiated kidney, and demonstrated that the expected increase in renal blood flow caused by intra-renal infusion of acetylcholine and bradykinin was impaired in the irradiated kidney. In line with the findings in these earlier studies, the current study demonstrated impaired afferent arteriolar dilator and autoregulatory responses 12 weeks following 11 Gy radiation. It is important to note that Juncos et al used a single fraction 30 Gy exposure, which is much higher than the doses used in our present studies. The 11 Gy dose used in our studies is close to a clinically used single fraction total body irradiation dose of 10 Gy and thus relevant to human scenarios [38]. Taken together, these data show an important role for vascular injury in radiation nephropathy.
We extended this knowledge by showing that EET-A or captopril mitigate the impaired acetylcholine vasodilatation in irradiated rats. We also showed that the afferent arteriolar autoregulatory response to increased perfusion pressure was also impaired in the irradiated kidney, and this is mitigated by EET-A or captopril (Fig. S3, Online Supplement). The contribution of ACEi in protecting the endothelium and improving vascular function is known in pathological conditions characterized with vascular dysfunction [39,40]. In regard to a role of EET-A in restoring renal vascular function in irradiated kidney, several earlier studies reported that EETs are an endothelial derived hyperpolarizing factor produced in the endothelium [41,42], and contribute to improve vascular functions in different pathological conditions and in multiple vascular beds including renal [15, 41–44]. Previous studies suggested that improving afferent arteriolar function was important to attenuate renal injury [45]. Overall, the present studies indicate a role for impaired afferent arteriolar function in the development of hypertension and renal injury after radiation exposure and that mitigation with EET-A or captopril mitigated renal injury by improving afferent arteriolar function. Matsuda et al. demonstrated in dogs that ACE inhibition resulted in a significant dilation of the juxtamedullary afferent arterioles. This renal hemodynamic action was independent of decreased angiotensin II levels. However, the renal vascular responses to ACE inhibition were dependent on activation of bradykinin receptors and the subsequent generation of nitric oxide and CYP450 metabolites. These data provide evidence for a shared benefit and site for ACE inhibition and EET analogs on renal hemodynamics. [46]
The kidney protective actions of EET-A were reported earlier in several preclinical disease models. We showed that EET-A treatment attenuated blood pressure elevation through vasodilation and tubular epithelial sodium channel inhibition, and protected the kidney from hypertensive injury [47]. Previously, we also demonstrated marked kidney protection by the use of an EET analog in salt-sensitive hypertension, and this protection was associated with marked reductions in renal oxidative, inflammatory and endoplasmic reticulum (ER) stress [17]. The EET analog EET-A reduced renal injury caused by the cancer chemotherapeutic, cisplatin [16]. Others and we reported that endogenous elevation of EETs and exogenous administration of EET analogs including EET-A protected kidneys from cisplatin nephrotoxicity by reducing renal oxidative stress, inflammation and apoptosis [8,16]. But in the present study we demonstrate that EET-A mitigated radiation kidney injury by mechanisms other than oxidative stress and inflammation. Indeed, a role for chronic persistent oxidative stress has not been established in renal radiation injury [48,49]. With biochemical and genomic methods we showed that persistent chronic oxidative stress does not play a role in the pathophysiology of radiation renal injury [49,50]. In the current study we determined that urinary nitric oxide and oxidative stress levels are not elevated at six weeks following TBI (Fig S5, Online Supplement). The contribution of inflammation to the pathophysiology of radiation renal injury is also controversial. The presence of infiltrating leukocytes has been reported in mouse kidney after 26–28 weeks after exposure to 12 Gy radiation [51]. But in the irradiated kidney of rhesus monkey the leukocyte numbers were unchanged between irradiated and control kidneys after 6–8 years of radiation [52]. In the present study, there was no change in the urinary level of an early inflammatory marker, monocyte chemotactic protein-1 (MCP-1) at 12 weeks after 11 Gy TBI (Fig. S6, Online Supplement). We further demonstrated that the renal mRNA expression of tumor necrosis factor-α (TNF-α) and its receptor (TNF-α receptor 1) were not affected by irradiation in the TBI rats and support our view on the absence of inflammation in radiation renal injury. Overall, based on the findings in the current study and several earlier findings led us to suggest that mitigation of radiation renal injury by captopril and EET-A is not via anti-inflammatory or anti-oxidant activities.
Radiation-induced apoptosis is thought to underlie the toxicity of radiation to normal tissues. Whole-body irradiation results in apoptosis of endothelial cells that could be a key pathway of the gastrointestinal syndrome or central nervous system injury [53,54], and renal epithelial apoptosis occurs in whole body irradiated mice [55]. In the present study, we demonstrated marked renal apoptosis following TBI. This was mainly localized to the tubular epithelium. Both captopril and EET-A mitigated the subsequent renal parenchymal apoptosis. Earlier we reported similar anti-apoptotic effects of EET-A in cisplatin nephropathy and demonstrated that this anti-apoptotic effect is associated with renal ER stress [16]. However, in the present study we did not find a role of ER stress in radiation renal injury (Fig. S7, Online Supplement). Our findings in the present study clearly indicate a role for renal apoptosis and its mitigation by EET-A or captopril, albeit not via ER stress pathways.
We extended our studies to further test the apoptotic pathways and the effects of our two mitigators. In radiation renal injury we did not find a role for TNFR1 pathway as the renal expressions of TNF-α and TNFR1 were not affected by TBI. We demonstrated that irradiated rats have elevated pro-apoptotic signaling in terms of increased Bax/Bcl-2 and Bak/Bcl-2 ratios. However, in exploring this finding, we did not find any change in the expression and activity of caspase 9 in the irradiated rats and led us to suggest that elevated pro-apoptotic signaling is not enough for apoptosis in radiation renal injury. Indeed, it has earlier been shown that changing the Bax/Bcl-2 ratio is not enough to induce apoptosis and could require involvement of other modulator of apoptosis such as Fas. Fas is an important promotor of apoptosis and in an event when Fas expression is elevated the anti-apoptotic molecule Bcl-2 is unable to inhibit apoptosis [56]. Interestingly, in the present study, we demonstrated up-regulations of Fas and its ligand FasL in the irradiated rats that received TBI.
Our finding that increased Fas and FasL contributes to radiation nephropathy is consistent with data from other models. FasL/Fas contributes to kidney injury in diverse renal pathologies including cisplatin nephropathy and renal ischemia-reperfusion [57,58]. Contribution of Fas/FasL in ionizing radiation induced cell death has been reported in radiation pneumonitis, which is a dose-limiting side effect of thoracic irradiation [59]. Moreover, an association was reported between Fas-FasL genes with rectal and urinary toxicity developed after prostate cancer radiotherapy [60]. However, to the best of our knowledge there are no reports on the role of Fas/FasL mediated apoptosis in radiation renal injury caused by TBI. Another novel finding in the present study is the identification of an upstream modulator of apoptosis that has a direct link with the expression of Fas. The DNA damage induced by ionizing radiation initiates signals which ultimately activate checkpoints and permit time for genetic repair or irreversible growth arrest that in turn results in apoptosis [61]. In this regard, we demonstrated increased expression of p53, one of the major proteins of DNA repair checkpoint pathways. Indeed, an important role of p53 on Fas/FasL mediated apoptosis has been reported as p53 directly regulates Fas expression [62]. Taken together, our results demonstrate a role for p53 and FasL/Fas in radiation-induced renal apoptosis. We also demonstrate that mitigation of radiation renal injury by captopril or EET-A is associated with reduced kidney mRNA expression of p53 as well as the mRNA expressions of Fas, FasL and caspase 8. In several in vitro studies, ACEi and EET have been shown to decrease Fas and p53 expression and exert anti-apoptotic effects [63,64]. Overall, our findings support the notion that in radiation renal injury renal apoptosis occurs by the Fas/FasL arm of extrinsic apoptotic pathway, and this was mitigated by captopril or EET-A. It remains possible that both the EET analog and captopril exert some of their mitigating action simply by control of the blood pressure. But the morphology of injury at the 12 week time point includes features of radiation nephropathy such as mesangiolysis, which is not a usual feature of hypertensive injury.
Supplementary Material
CLINICAL PERSPECTIVE.
Chronic kidney disease associated with radiation renal injury is a known and life-threatening effect of cancer radiotherapy. Radiation renal injury also is a late sequel of hematopoietic stem cell transplantation (HSCT), and is often linked to total body irradiation given just before the HSCT [2]. A large number of studies demonstrate kidney protective effects of EET and its analogs in other renal injury models [6–10, 14–18]. Moreover, a recent study demonstrated that EET enhance hematopoietic stem and progenitor cell homing and engraftment in experimental HSCT [65]. Testing an EET analog to mitigate radiation injury is a logical next step.
The present study shows the potential of an EET analog as a novel mitigator of radiation renal injury. Our findings indicate that the novel small molecule EET analog EET-A has promising mitigator effects comparable to the ACEi captopril in radiation renal injury.
Moreover, our study demonstrated that EET-A and captopril mitigate radiation renal injury by improving renal afferent arteriolar function, and also by reducing renal apoptosis by reducing Fas/FasL mediated apoptotic signaling.
Acknowledgments
This work is supported by a grant to John D. Imig sponsored by the National Center for Advancing Translational Sciences, National Institute of Health through a grant number 8UL1TR000055; grants NIH HL111392, DK38226 and the Robert A. Welch Foundation (GL625910) to John R. Falck, and by a Merit Review Award, 5I01BX002256-02, from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development, to Eric P. Cohen. Editorial assistance provided by Yvonne Morauski of Department of Radiation Biology, Medical College of Wisconsin, Milwaukee, USA is gratefully acknowledged. Expert technical assistance of Michael Thomas and the Shared Mass Spectrometry Facility, Department of Pharmacology & Toxicology, Medical College of Wisconsin, Milwaukee, WI, USA is gratefully acknowledged to determine urinary epoxygenase metabolite levels.
List of abbreviations
- ACEI
angiotensin-converting-enzyme inhibitor
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- EETs
epoxyeicosatrienoic acids
- Gy
Gray
- HSCT
hematopoietic stem cell transplantation
- MCP-1
monocyte chemoattractant protein 1
- sEH
soluble epoxide hydrolase
- TBI
total body irradiation
- TNF-α
tumour necrosis factor-α
- TNFR1
tumour necrosis factor-αreceptor 1
Footnotes
AUTHOR CONTRIBUTION
Md. Abdul Hye Khan, Brian Fish, John D. Imig, Eric P. Cohen, and John E. Moulder conceived the study, interpreted the data and wrote the manuscript. Md. Abdul Hye Khan, Brian Fish, Geneva Wahl, and Amit Sharma performed experiments and analyzed data. John R. Falck and Mahesh P. Paudyal designed and synthesized the EET analog.
DISCLOSURE
Drs. Imig and Falck have a patent application that covers the composition of matter for EET-A. There are no other conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Cohen EP, Krzesinski JM, Launay-Vacher V, Sprangers B. Onco-Nephrology: Core curriculum. Am J Kidney Dis. 2015;66:869–883. doi: 10.1053/j.ajkd.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen EP, Bedi M, Irving AA, Jacobs E, Tomic R, Klein J, Lawton CA, Moulder JE. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2012;83:292–296. doi: 10.1016/j.ijrobp.2011.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Movsas B, Vikram B, Hauer-Jensen M, Moulder JE, Basch E, Brown SL, Kachnic LA, Dicker AP, Coleman CN, Okunieff P. Decreasing the adverse effects of cancer therapy: National Cancer Institute guidance for the clinical development of radiation injury mitigators. Clin Cancer Res. 2011;17:222–228. doi: 10.1158/1078-0432.CCR-10-1402. [DOI] [PubMed] [Google Scholar]
- 4.Cohen EP, Fish BL, Moulder JE. The renin-angiotensin system in experimental radiation nephropathy. J Lab Clin Med. 2002;39:251–257. doi: 10.1067/mlc.2002.122279. [DOI] [PubMed] [Google Scholar]
- 5.Cohen EP, Joines MM, Moulder JE. Prevention and treatment of radiation injuries – the role of the renin-angiotensin system. In: Rubin P, Constine LS, Mark LB, Okunieff P, editors. Late effects of cancer treatment on normal tissues. Heidelberg: Springer-Verlag; 2008. pp. 69–76. 2008. [Google Scholar]
- 6.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F740–F748. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olearczyk JJ, Quigley JE, Mitchell BC, Yamamoto T, Kim IH, Newman JW, Luria A, Hammock BD, Imig JD. Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci (Lond) 2009;116:61–70. doi: 10.1042/CS20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrish AR, Chen G, Burghardt RC, Watanabe T, Morisseau C, Hammock BD. Attenuation of cisplatin nephrotoxicity by inhibition of soluble epoxide hydrolase. Cell Biol Toxicol. 2009;25:217–225. doi: 10.1007/s10565-008-9071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Lu X, Nguyen S, Olson JL, Webb HK, Kroetz DL. Epoxyeicosatrienoic acids prevent cisplatin-induced renal apoptosis through a p38 mitogen-activated protein kinase-regulated mitochondrial pathway. Mol Pharmacol. 2013;84:925–934. doi: 10.1124/mol.113.088302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Yoon SP, Toews ML, Imig JD, Hwang SH, Hammock BD, Padanilam BJ. Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2015;308:F131–F139. doi: 10.1152/ajprenal.00531.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol. 2010;56:329–335. doi: 10.1097/FJC.0b013e3181e96e0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma YH, Schwartzman ML, Roman RJ. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 1994;267:R579–R589. doi: 10.1152/ajpregu.1994.267.2.R579. [DOI] [PubMed] [Google Scholar]
- 13.Kaergel E, Muller DN, Honeck H, Theuer J, Shagdarsuren E, Mullally A, Luft FC, Schunck WH. P450-dependent arachidonic acid metabolism and angiotensin II-induced renal damage. Hypertension. 2002;40:273–279. doi: 10.1161/01.hyp.0000029240.44253.5e. [DOI] [PubMed] [Google Scholar]
- 14.Imig JD, Elmarakby A, Nithipatikom K, Wei S, Capdevila JH, Tuniki VR, Sangras B, Anjaiah S, Manthati VL, Sudarshan Reddy D, Falck JR. Development of epoxyeicosatrienoic acid analogs with in vivo anti-hypertensive actions. Front Physiol 2010. 2010;1:157. doi: 10.3389/fphys.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AH, Falck JR, Manthati VL, Campbell WB, Imig JD. Epoxyeicosatrienoic acid analog attenuates angiotensin II hypertension and kidney injury. Front Pharmacol. 2014;5:216. doi: 10.3389/fphar.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MA, Liu J, Kumar G, Skapek SX, Falck JR, Imig JD. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 2013;27:2946–2956. doi: 10.1096/fj.12-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hye Khan MA, Neckár J, Manthati V, Errabelli R, Pavlov TS, Staruschenko A, Falck JR, Imig JD. Orally active epoxyeicosatrienoic acid analog attenuates kidney injury in hypertensive Dahl saltsensitive rat. Hypertension. 2013;62:905–913. doi: 10.1161/HYPERTENSIONAHA.113.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panigrahy D, Kalish BT, Huang S, Bielenberg DR, Le HD, Yang J, Edin ML, Lee CR, Benny O, Mudge DK, Butterfield CE, Mammoto A, Mammoto T, Inceoglu B, Jenkins RL, Simpson MA, Akino T, Lih FB, Tomer KB, Ingber DE, Hammock BD, Falck JR, Manthati VL, Kaipainen A, D'Amore PA, Puder M, Zeldin DC, Kieran MW. Epoxyeicosanoids promote organ and tissue regeneration. Proc Natl Acad Sci USA. 2013;110:13528–13533. doi: 10.1073/pnas.1311565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen EP. Radiation nephropathy after bone marrow transplantation. Kidney Int. 2000;58:903–918. doi: 10.1046/j.1523-1755.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 20.Avioli LV, Lazor MZ, Cotlove E, Brace KC, Andrews JR. Early effects of radiation on renal function in man. Am J Med. 1963;34:329–337. doi: 10.1016/0002-9343(63)90120-7. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira VP, Boim MA, Segreto HR, Schor N. Acute, subacute, and chronic x-ray effects on glomerular hemodynamics in rats. Ren Fail. 1994;16:457–70. doi: 10.3109/08860229409045077. [DOI] [PubMed] [Google Scholar]
- 22.Verheij M, Dewit LG, Valdés Olmos RA, Arisz L. Evidence for a renovascular component in hypertensive patients with late radiation nephropathy. Int J Radiat Oncol Biol Phys. 1994;30:677–683. doi: 10.1016/0360-3016(92)90955-h. [DOI] [PubMed] [Google Scholar]
- 23.Withers HR, Mason KA, Thames HD., Jr Late radiation response of kidney assayed by tubule-cell survival. Br J Radiol. 1986;59:587–595. doi: 10.1259/0007-1285-59-702-587. [DOI] [PubMed] [Google Scholar]
- 24.Gobé GC, Axelsen RA, Harmon BV, Allan DJ. Cell death by apoptosis following X-irradiation of the foetal and neonatal rat kidney. Int J Radiat Biol. 1988;54:567–576. doi: 10.1080/09553008814552011. [DOI] [PubMed] [Google Scholar]
- 25.Moulder JE, Cohen EP, Fish BL. Mitigation of experimental radiation nephropathy by reninequivalent doses of angiotensin converting enzyme inhibitors. Int. J. Radiat. Biol. 2014;90:762–768. doi: 10.3109/09553002.2014.938375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br. J. Pharmacol. 1999;127:1399–405. doi: 10.1038/sj.bjp.0702662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11, 12-EET analogs involves PP2A activity and Ca2+-activated K+ Channels. Microcirculation. 2008;15:137–150. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen-Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, Fujiwara T, Roth JA, Deisseroth AB, Zhang WW, Kruzel E, Radinsky R. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol. 1995;15:3032–3040. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fei P, El-Deiry WS. p53 and radiation responses. Oncogene. 2003;22:5774–5783. doi: 10.1038/sj.onc.1206677. [DOI] [PubMed] [Google Scholar]
- 30.Cohen EP, Lawton CA, Moulder JE. Bone marrow transplant nephropathy: radiation nephritis revisited. Nephron. 1995;70:217–222. doi: 10.1159/000188587. [DOI] [PubMed] [Google Scholar]
- 31.Cohen EP, Fish BL, Moulder JE. Late-onset effects of radiation and chronic kidney disease. Lancet. 2015;386:1737–8. doi: 10.1016/S0140-6736(15)00697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen EP, Fish BI, Moulder JE. The renin-angiotensin system in experimental radiation nephropathy. J Lab Clin Med. 2002;139:251–257. doi: 10.1067/mlc.2002.122279. [DOI] [PubMed] [Google Scholar]
- 33.Imig JD, Sharma A, Khan MAH, Fish B, Cohen EP. Afferent Arteriolar Endothelial-dependent Dilation is Impaired Prior to the Development of Radiation-induced Nephropathy and Hypertension. Hypertension. 2015;66:AMP15. [Google Scholar]
- 34.Robbins MEC, Wooldridge MJA, Jaenke RS, Whitehouse E, Golding SJ, Rezvani M, Hopewell JW. A morphological study of radiation nephropathy in the pig. Radiat Res. 1991;126:317–327. 1991. [PubMed] [Google Scholar]
- 35.Jaenke RS, Robbins ME, Bywaters T, Whitehouse E, Rezvani M, Hopewell JW. Capillary endothelium. Target site of renal radiation injury. Lab Invest. 1993;68:396–405. [PubMed] [Google Scholar]
- 36.Juncos LI, Cornejo JC, Gomes J, Baigorria S, Juncos LA. Abnormal endothelium-dependent responses in early radiation nephropathy. Hypertension. 1997;30:672–676. doi: 10.1161/01.hyp.30.3.672. [DOI] [PubMed] [Google Scholar]
- 37.Cohen EP, Molteni A, Hill P, Fish BL, Ward WF, Moulder JE, Carone FA. Captopril preserves function and ultrastructure in experimental radiation nephropathy. Lab Invest. 1996;75:349–60. [PubMed] [Google Scholar]
- 38.Antignac C, Gubler MC, Leverger G, Broyer M, Habib R. Delayed renal failure with extensive mesangiolysis following bone marrow transplantation. Kidney Int. 1989;35:1336–1344. doi: 10.1038/ki.1989.132. [DOI] [PubMed] [Google Scholar]
- 39.Donnini S, Terzuoli E, Ziche M, Morbidelli L. Sulfhydryl angiotensin-converting enzyme inhibitor promotes endothelial cell survival through nitric-oxide synthase, fibroblast growth factor-2, and telomerase cross-talk. J Pharmacol Exp Ther. 2010;332:776–784. doi: 10.1124/jpet.109.159178. [DOI] [PubMed] [Google Scholar]
- 40.Lankhorst S, Kappers MH, van Esch JH, Smedts FM, Sleijfer S, Mathijssen RH, Baelde HJ, Danser AH, van den Meiracker AH. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: preclinical study. Hypertension. 2014;64:1282–1289. doi: 10.1161/HYPERTENSIONAHA.114.04187. [DOI] [PubMed] [Google Scholar]
- 41.Campbell WB, Falck JR, Gauthier K. Role of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factor in bovine coronary arteries. Med Sci Monit. 2001;7:578–584. [PubMed] [Google Scholar]
- 42.Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension. 2005;45:666–671. doi: 10.1161/01.HYP.0000153462.06604.5d. [DOI] [PubMed] [Google Scholar]
- 43.Zhang LN, Vincelette J, Chen D, Gless RD, Anandan SK, Rubanyi GM, Webb HK, MacIntyre DE, Wang YX. Inhibition of soluble epoxide hydrolase attenuates endothelial dysfunction in animal models of diabetes, obesity and hypertension. Eur J Pharmacol. 2011;654:68–74. doi: 10.1016/j.ejphar.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxideindependent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- 45.Elmarakby AA, Faulkner J, Al-Shabrawey M, Wang MH, Maddipati KR, Imig JD. Deletion of soluble epoxide hydrolase gene improves renal endothelial function and reduces renal inflammation and injury in streptozotocin-induced type 1 diabetes. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1307–R1307. doi: 10.1152/ajpregu.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuda H, Hayashi K, Wakino S, Kubota E, Honda M, Tokuyama H, Takamatsu I, Tatematsu S, Saruta T. Role of endothelium-derived hyperpolarizing factor in ACE inhibitor-induced renal vasodilation in vivo. Hypertension. 2004;43:603–609. doi: 10.1161/01.HYP.0000118053.42262.71. [DOI] [PubMed] [Google Scholar]
- 47.Hye Khan MA, Pavlov TS, Christain SV, Neckář J, Staruschenko A, Gauthier KM, Capdevila JH, Falck JR, Campbell WB, Imig JD. Epoxyeicosatrienoic acid analogue lowers blood pressure through vasodilation and sodium channel inhibition. Clin Sci (Lond) 2014;127:463–474. doi: 10.1042/CS20130479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen SR, Cohen EP. Chronic oxidative stress after irradiation: an unproven hypothesis. Med Hypotheses. 2013;80:172–175. doi: 10.1016/j.mehy.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenarczyk M, Cohen EP, Fish BL, Irving AA, Sharma M, Driscoll CD, Moulder JE. Chronic oxidative stress as a mechanism for radiation nephropathy. Radiat Res. 2009;171:164–172. doi: 10.1667/RR1454.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen EP, Lenarczyk M, Fish BL, Jia S, Hessner MJ, Moulder JE. Evaluation of Genomic Evidence for Oxidative Stress in Experimental Radiation Nephropathy. J Genet Disord Genet Rep. 2013;2 doi: 10.4172/2327-5790.1000101. pii: 1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart FA, Te Poele JA, Van der Wal AF, Oussoren YG, van Kleef EM, Kuin A, Verheij M, Dewit LG. Radiation nephropathy the link between functional damage and vascular mediated inflammatory and thrombotic changes. Acta Oncol. 2001;40:952–957. doi: 10.1080/02841860152708233. [DOI] [PubMed] [Google Scholar]
- 52.van Kleef EM, Zurcher C, Oussoren YG, Te Poele JA, van der Valk MA, Niemer-Tucker MM, van der Hage MH, Broerse JJ, Robbins ME, Johnston DA, Stewart FA. Long-term effects of totalbody irradiation on the kidney of Rhesus monkeys. Int J Radiat Biol. 2000;76:641–648. doi: 10.1080/095530000138303. [DOI] [PubMed] [Google Scholar]
- 53.Deng W, Viar MJ, Johnson LR. Polyamine depletion inhibits irradiation-induced apoptosis in intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G599–G606. doi: 10.1152/ajpgi.00564.2004. [DOI] [PubMed] [Google Scholar]
- 54.Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–327. [PubMed] [Google Scholar]
- 55.Ghassah M, Labejof Berry JP, Galle P. Early ultrastructural lesions of apoptosis induced in vivo in two varieties of tissues (thymus and kidney) after a single whole body gamma irradiation of adult mice. Cell Mol Biol. 1997;43:1197–1204. [PubMed] [Google Scholar]
- 56.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 57.Linkermann A, Himmerkus N, Rölver L, Keyser KA, Steen P, Bräsen JH, Bleich M, Kunzendorf U, Krautwald S. Renal tubular Fas ligand mediates fratricide in cisplatin-induced acute kidney failure. Kidney Int. 2011;79:169–178. doi: 10.1038/ki.2010.317. [DOI] [PubMed] [Google Scholar]
- 58.Nogae S, Miyazaki M, Kobayashi N, Saito T, Abe K, Saito H, Nakane PK, Nakanishi Y, Koji T. Induction of apoptosis in ischemia-reperfusion model of mouse kidney: possible involvement of Fas. J. Am Soc Nephrol. 1998;9:620–631. doi: 10.1681/ASN.V94620. [DOI] [PubMed] [Google Scholar]
- 59.Heinzelmann F, Jendrossek V, Lauber K, Nowak K, Eldh T, Boras R, Handrick R, Henkel M, Martin C, Uhlig S, Köhler D, Eltzschig HK, Wehrmann M, Budach W, Belka C. Irradiation-induced pneumonitis mediated by the CD95/CD95-ligand system. J Natl Cancer Inst. 2006;98:1248–1251. doi: 10.1093/jnci/djj335. [DOI] [PubMed] [Google Scholar]
- 60.Thurner EM, Krenn-Pilko S, Langsenlehner U, Renner W, Gerger A, Kapp KS, Langsenlehner T. Association of genetic variants in apoptosis genes FAS and FASL with radiation-induced late toxicity after prostate cancer radiotherapy. Strahlenther Onkol. 2014;190:304–309. doi: 10.1007/s00066-013-0485-0. [DOI] [PubMed] [Google Scholar]
- 61.Samuel T, Weber HO, Funk JO. Linking DNA damage to cell cycle checkpoints. Cell Cycle. 2002;1:162–168. [PubMed] [Google Scholar]
- 62.Müller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M, Krammer PH. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033–2045. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang C, Pan S, Yan S, Li Z, Yang J, Wang Y, Xiong Y. Inhibitory effect of 14,15-EET on endothelial senescence through activation of mTOR complex 2/Akt signaling pathways. Int J Biochem Cell Biol. 2014;50:93–100. doi: 10.1016/j.biocel.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Q, Lin S, Tang R, Veeraragoo P, Peng W, Wu R. Role of Fosinopril and Valsartan on klotho gene expression induced by angiotensin II in rat renal tubular epithelial cells. Kidney Blood Press Res. 2010;33:186–192. doi: 10.1159/000316703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.