Abstract
Background
Dimethoxy curcumin (DMC) is a kind of lipophilic analog of curcumin with great improvement in chemical and metabolic stability. DMC has been studied in breast and renal cancer, but no research in colon cancer has been found yet.
Material/Methods
Two colon cancer cells (HT-29 and SW480) and one normal human colon mucosal epithelial cell (NCM460) were used in this study. We studied the effect of DMC on the proliferation in vitro and in vivo. Transwell migration assay was used to estimate the inhibition of DMC on invasion. Moreover, the expressions of PARP, caspase-3, survivin and E-cadherin were detected to uncover the related signaling pathways by western blotting assay both in vitro and in vivo.
Results
DMC significantly inhibited the growth of colon cancer cells in dose-dependent manner; IC50 for DMC was calculated to be 43.4, 28.2 and 454.8μM on HT-29, SW480 and NCM460. DMC significantly increased the apoptosis in both HT-29 (p=0.0051) and SW480 (p=0.0013) cells in vitro, and significantly suppressed the growth of both cell lines in vivo. Moreover, DMC reduced the number of migrated cells in both HT-29 (p=0.007) and SW480 (p=0.004) cells. By western blotting analysis, the cleavage of pro-caspases-3 and PARP were clearly induced by DMC to their active form, while the expression of survivin was reduced and E-cadherin was enhanced in both cells in vitro and in vivo.
Conclusions
DMC may exert an effective anti-tumor effect in colon cancer cells by down-regulating survivin and upregulating E-cadherin.
MeSH Keywords: Apoptosis, Cell Migration Assays, Colonic Neoplasms
Background
Colon cancer remains the third most common cancer and third leading cause of cancer deaths in men and women [1]. Although curative surgery remains the mainstay of treatment for early-stage colon cancer, chemotherapy is already being considered an important way to improve survival for most colon cancer patients [2]. The discovery and application of new drug is a great benefit for colon cancer patients, such as Vascular Endothelial Growth Factor Receptor (VEGFR) targeted therapy for late-stage colon cancer patients [3], and additional compounds may be helpful for treatment of colon cancer.
Some natural compounds have recently been shown to be effective in cancer chemoprevention, such as resveratrol, Burgund Mare grapes, and curcumin [4–6]. Curcumin (diferuloylmethane) is a major active ingredient of turmeric (curcuma longa), with no discernable toxicity [7]. It governs numerous intracellular targets, including proteins involved in antioxidant response, immune response, apoptosis, cell cycle regulation, and tumor progression [8]. Existing data have suggested that curcumin in combination with chemotherapy is a superior strategy for treatment of gastrointestinal cancer [7,9]. Moreover, curcumin was suggested to effectively overcome chemoresistance during treatment of cancers [10,11].
However, the clinical application of curcumin has been very limited, mainly due to its undesirable physicochemical and pharmacokinetic properties, such as low water solubility, metabolic instability, and poor bioavailability [12]. During the past few decades, great attention has been paid to structural modification to overcome these problems, and various novel structural analogs of curcumin have been designed for screening [12].
Dimethoxy curcumin (DMC) is a kind of lipophilic analog of curcumin, with all the phenolic hydroxyl groups methylated [13]. A variety of experimental studies based on cells and animals have showed that such structural modifications brought about great improvement of DMC in chemical and metabolic stability [12], especially: (a) more stable in cultured cells, (b) more potent in the ability to kill cancer cells by apoptosis, (c) less extensively metabolized in microsomal systems, and (d) more stable in vivo [14,15].
To date, the studies of DMC in anti-tumor effects are few. DMC was demonstrated to induce cell death in breast carcinoma MCF7 cells through S-phase arrest and apoptosis [16] or trigger a strong proteasome inhibition and high induction of CHOP to achieve potent anti-cancer effects on malignant breast cancer cells [17]. In human renal carcinoma Caki cells, DMC induces apoptosis, possibly through the production of ROS, the release of mitochondrial Cytc, and the subsequent activation of caspase-3 [18]. Moreover, a variety of studies demonstrated that DMC does better than its original form of curcumin in exerting anti-cancer effects through inducing apoptosis, or having more comparable pro-oxidant activity than curcumin [17–19]. However, to the best of our knowledge, there have been no reports about the anti-tumor effect of DMC in colon cancer up to now.
In the present study, we examined the anti-cancer efficacy of DMC in HT-29 and SW480 cells, which are two representative colon cancer cells with distinctive genotypic differences, in vitro and in vivo.
Material and Methods
Cell culture and reagents
The colon cancer cell lines HT-29 and SW480, as well as normal human colon mucosal epithelial cell line (NCM460), provided by the Department of Pathology, College of Medicine, Zhejiang University (Hangzhou, China), were cultured in RPMI1640 with 10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin at 37°C in a 5% CO2 incubator. All the cell culturing reagents were purchased from Invitrogen (Shanghai, China).
DMC was supplied by the Department of Applied Chemistry, Lanzhou University (Lanzhou, China). Purity of DMC was detected by high-performance liquid chromatography to be >90%. DMC was prepared as stock solutions in DMSO and stored −20°C. For each experiment, the stock solutions were diluted with cell culture medium to get a final DMSO concentration of 0.5% (v/v).
CCK-8 assay
HT-29 or SW480 cells were seeded into 96-well plates at a density of 5×103 cells/well, grown at 37°C overnight, and then treated with DMC at various concentrations (12.5, 25, 50, 75, and 100 μM) for 24, 48, or 72 h. The NCM460 cell line was treated with DMC at concentrations of 25, 50, 100, 200, and 300 μM. After incubation, CCK-8 (Beyotime Biotechnology, Jiangsu, China) was added to each well. The optical density (OD) was measured at 490 nm using an ultra-microplate reader (SpectraMax; Molecular Devices Corp., Sunnyvale, CA). Relative cell growth inhibition was calculated as the formula: (OD490 control cells – OD490 treated cells)/OD490 control cells ×100%. The IC50 was defined as the concentration of DMC produced 50% inhibition of colon cancer cell growth in comparison with control culture. This process was independently repeated 5 times.
Annexin V apoptosis assay
Cell apoptosis was determined by using of an annexin V apoptosis kit according to the manufacturer’s instructions (BD Biosciences, Franklin Lakes, NJ). In brief, HT-29 and SW480 cells were treated with or without DMC of IC50 for 72 h before cells were trypsinized, resuspended in 500 μL binding buffer, and incubated with 5 μL annexin V-FITC and 10 μL propidium iodide working solution at room temperature for 5 min in the dark. Stained cells were then analyzed by flow cytometry (FAC Scan, BD Co. Ltd). This process was independently repeated 3 times.
In vivo anti-tumor activity
Fourteen female BALB/cA-nu/nu mice (4 to 5 weeks old) were supplied by the Laboratory Animal Center of Zhejiang Academy of Medical Sciences (Hangzhou, China). Two mice were subcutaneously implanted with 5×105 HT-29 or SW480 cells in 0.1 mL in one flank per mouse using a 22-gauge needle. The mice were killed when the xenografts grew to an approximately 1-cm diameter tumor. Well-developed tumors were cut into 1-mm3 fragments and implanted subcutaneously into one flank of each nude mouse. When the tumor volume reached 50~100 mm3 (day 0), the mice were randomly divided into control and treatment groups. The mice in the treatment group (each 3 mice of HT-29 cells and SW480 cells implanted) received DMC administration (50 mg/kg, p.o.) 5 days/week for 3 weeks. In the control group, instead of DMC, an equal volume of normal saline was administrated. Tumor growth and the body weight of mice were examined twice a week after implantation, and the tumor mass volume was measured with an electronic caliper and calculated as 1/2×length×width2 in mm3. Tumors were dissected after 3-week treatment and were photographed. The relative tumor volume (RTV) was calculated using the formula: TV=Vt/V0. V0 indicates the tumor volume before the drugs given to the mice and Vt indicates the tumor volume at each measurement.
All animals used in the study were housed and cared for in accordance with the Chinese Pharmacological Society Guidelines for Animal Use. The work was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Permit Number: 2016-132).
Transwell migration assay
Transwell migration assay was carried out using 8.0-μm cell culture inserts (BD Biosciences) to test the migratory ability of HT-29 and SW480 cells. Cells were seeded at 2×105 cells/well in 200 μL serum-free RPMI-1640 in the upper chamber of 24-well plates supplied with or without DMC. Culture media containing 20% FBS were added in the lower chamber. Cells were allowed to migrate for 48 h toward the underside of the membrane. After 48-h culturing, the cells in the upper chamber were removed by wiping the upper side of the membrane with a cotton swab and the migrated cells in the lower surface of the membrane were fixed with ice-cold methanol and stained with crystal violet in 20% ethanol for 1 h. Images were acquired under light microscopy and the cells that migrated to the underside of the membrane were quantitated by cell counting in 5 predetermined fields. This process was independently repeated 3 times.
Western blotting
HT-29 and SW480 cells were treated with or without DMC of IC50 for 72 h before cells were lysed in lysis buffer with 1 mM sodium fluoride, 2 mM sodium orthovanadate, and a cocktail of protease inhibitors (Santa Cruz, CA). For in vivo assay, fresh tumor tissues in RIPA lysis buffer containing 1 ug/ml PMSF were manually homogenized on ice using a glass homogenizer, then centrifuged at 10 000 g for 10 min to remove cellular and nuclear debris. Protein concentration was measured by modified Bradford assay. We electrophoresed 60 μg of total proteins on 10% SDS-PAGE gel and then transferred it to nitrocellulose membrane. The membranes were blocked with PBS containing 5% non-fat milk for 1 h at room temperature and then incubated overnight at 4°C with the primary antibodies against poly (ADP-ribose) polymerase (PARP), caspase-3, survivin, E-cadherin, and β-actin (Santa Cruz, CA). After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Beijing Zhongshan Co. Ltd.) at room temperature for 1 h, followed by chemiluminescence detection using a SuperSignal West Femto Maximum sensitivity substrate kit (Pierce). This process was independently repeated 3 times.
Statistical analysis
All quantitative data are expressed as mean ± standard deviation (SD), or median with range if non-normal distribution was found. The t test or Mann-Whitney U test was further applied using SPSS 17.0 software. IC50 was calculated using the “Analysis/Regression/Probit” application of SPSS 17.0 based on data from the CCK-8 assay. A p<0.05 was considered as statistically significant.
Results
DMC inhibits colon cancer cells growth in vitro
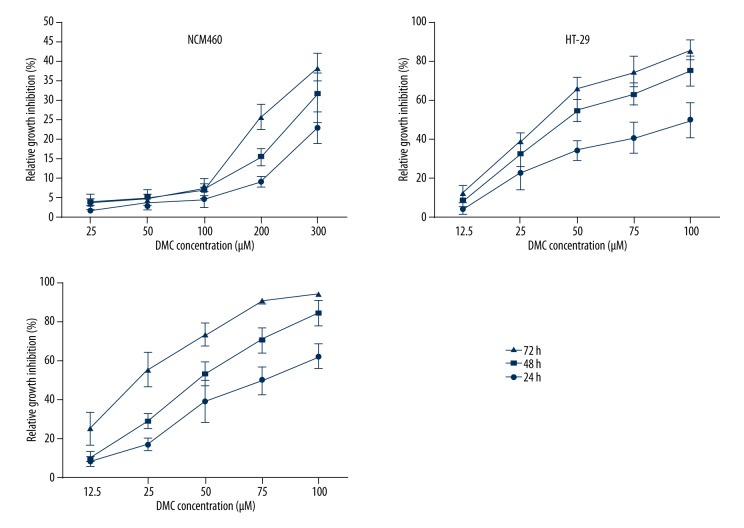
To study the direct effect of DMC on colon cancer cell growth, we first performed CCK-8 assay on HT-29 and SW480 cells treated with DMC at various concentrations. As showed in Figure 1, DMC significantly inhibited growth of colon cancer cells in a dose-dependent manner, and the maximum effect was observed at the time point of 72 h. IC50 for DMC was calculated to be 43.4 (Lower Bound of 37.7 and Upper Bound of 48.7) μM on HT-29 and 28.2 (Lower Bound of 22.8 and Upper Bound of 32.9) μM on SW480 cells at 72 h. This result demonstrated DMC suppressed colon cancer cells. In contrast, the inhibition rate of DMC in NCM460 cells at these concentrations was about 5%, and IC50 for NCM460 was 454.8 μM, which is much higher than in the colon cancer cell line, suggesting that DMC has low toxicity in normal colon epithelial cells.
Figure 1.
Dimethoxy curcumin (DMC) inhibits HT-29, SW480, and NCM460 cell growth. HT-29 and SW480 cells were treated with DMC at varied concentrations (0, 12.5, 25, 50, 75, and 100 μM) for 24, 48, or 72 h. NCM460 cells were treated with DMC at varied concentrations (0, 25, 50, 100, 200, and 300 μM) for 24, 48, or 72 hours. Cell viability was analyzed by CCK-8 assay. The relative growth inhibition rate was calculated as percentage of inhibition of the control cells. Results were obtained from 3 independent experiments and are expressed as means ±SD, n=3.
DMC induces apoptosis in colon cancer cells
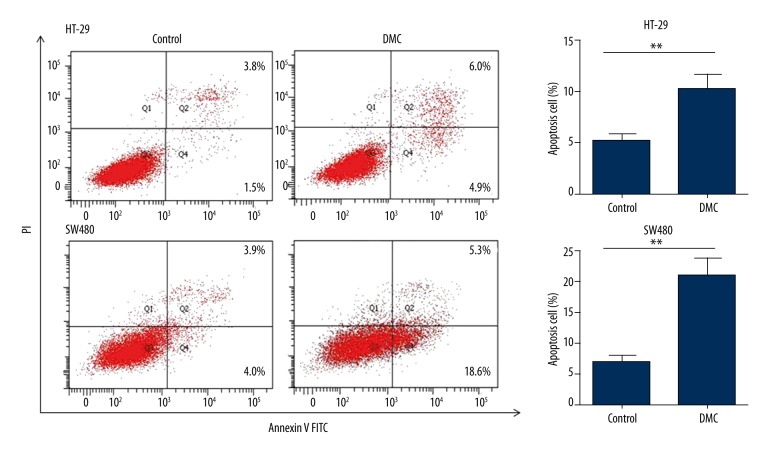
We next determined whether DMC-induced cell viability inhibition is caused by increased apoptosis. HT-29 and SW480 cells were treated with DMC at IC50 for 72 h and analyzed by Annexin V apoptosis assay using flow cytometry. For HT-29 cells, DMC treatment resulted in the change of apoptotic cells percentage from 5.1±0.4% to 10.23±0.8% (p=0.0051), while for SW480 cells it was from 7.0±0.5% to 20.8±1.7% (p = 0.0013). Representative results are shown in Figure 2. Our results suggest that DMC inhibits cells proliferation by regulating apoptosis.
Figure 2.
DMC induces apoptosis in colon cancer cells. HT-29 and SW480 cells were treated with 43.4 and 28.2 μM DMC, respectively, for 72 h. Cells were stained with Annexin V/PI and analyzed by flow cytometry. Results were obtained from 3 independent experiments and are expressed as means ±SD, ** p<0.01.
Anti-tumor activity of DMC in human colon cancer xenografts in vivo
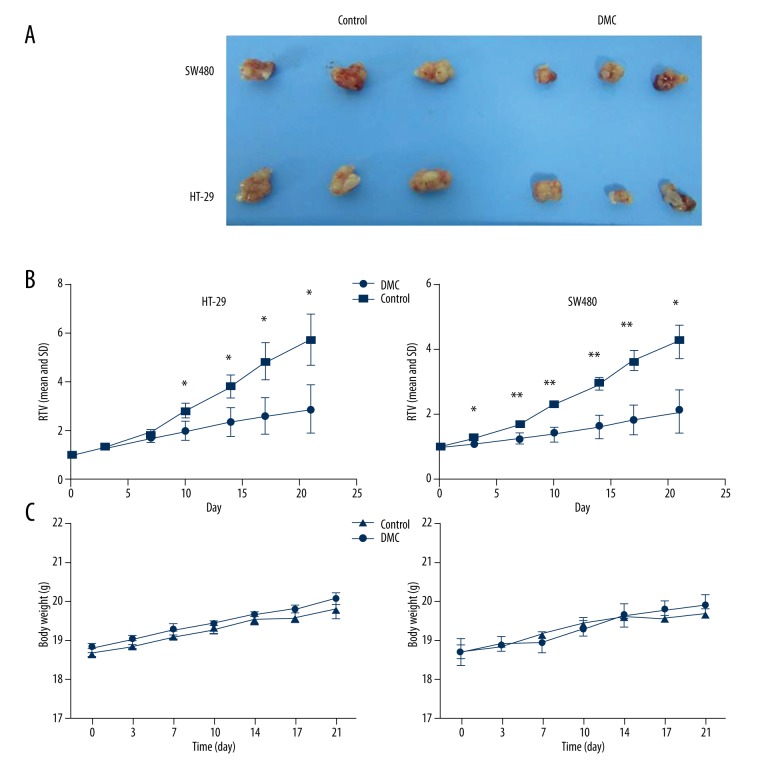
The anti-tumor activity of DMC was also evaluated in vivo. HT-29 or SW480 cells were inoculated into nude mice and resulted in tumor generation at the injection site. The tumor volume was measured locally. As shown in Figure 3A, the tumors of SW480 and HT29 cells treated with DMC were significantly smaller than that in mice treated with normal saline. The relative tumor volume (RTV) of the control group markedly increased in a time-dependent manner. In the DMC group, significant inhibitory effect was found on days 10, 14, 17, and 21 in HT-29 cells, and on days 3, 7, 10, 14, 17, and 21 in SW480 cells (Figure 3B). No significant body weight change of nude mice was detected in the HT-29 and SW480 groups (Figure 3C).
Figure 3.
Anti-tumor activity of DMC in human colon cancer xenografts in vivo. Mice were randomly divided into the DMC group or control group on day 0. The mice implanted with tumors (HT-29 or SW480 cells) were treated with normal saline or DMC (50 mg/kg, p.o.) once daily, 5 days/week for 3 weeks. (A) Tumors were dissected 3 weeks after treatment and were photographed. (B) Tumor volume changes in HT-29 or SW480 cells. (C) Body weight changes during the DMC treatments. The values indicate the means ±SD of the RTV (n=3). * p<0.05, ** p<0.01. RTV: relative tumor volume.
DMC inhibits colon cancer cells migration in vitro
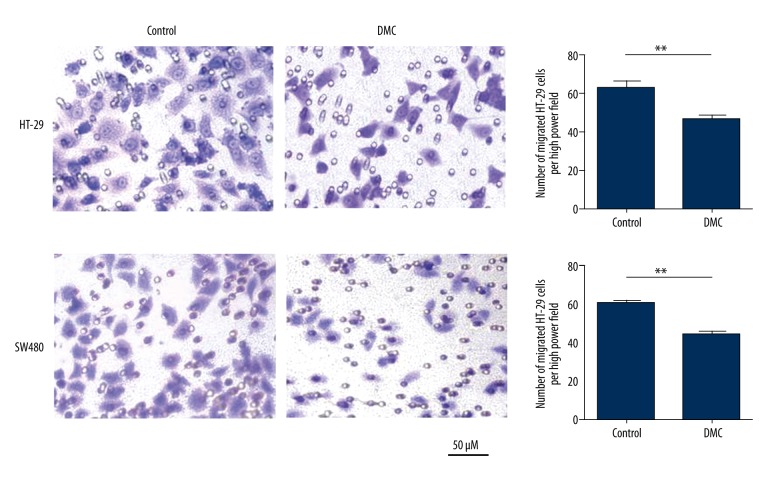
We also investigated whether DMC affected the migration of HT-29 and SW480 cells in a transwell assay. As shown in Figure 4, the presence of DMC decreased the number of migrated cells from 63.7±4.9 to 46.7±3.2 (p=0.007) in HT-29 cells and from 60.3±3.8 to 44.7±2.5 (p=0.004) in SW480 cells.
Figure 4.
Dimethoxy curcumin (DMC) inhibits colon cancer cells migration in vitro. HT-29 and SW480 cells in the upper chamber of transwell plates were treated with 43.4 and 28.2 μM DMC, or were untreated, for 48 h. The cells that migrated to the underside of the membrane were quantitated. Results were obtained from 3 independent experiments and are expressed as means ±SD. Representative images of migration are presented, ** p<0.01.
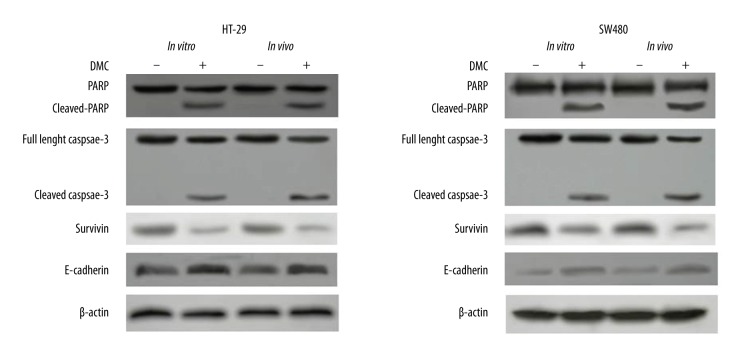
Effects of DMC on the expression of caspase-3, PARP, survivin, and E-cadherin in vitro and in vivo
The activities of caspase-3, PARP, survivin, and E-cadherin were tested in colon cancer cell lines and in xenografts. As shown in Figure 5, in the DMC-treated group, the cleavage of pro-caspases-3 and PARP, a specific substrate of caspase-3, were clearly induced to their active form both in vitro and in vivo. Moreover, DMC also induced reduction of survivin expression and enhancement of E-cadherin expression.
Figure 5.
Effects of DMC on the expression of caspase-3, PARP, survivin, and E-cadherin. HT-29 and SW480 cells were treated with DMC, or were untreated, in vitro before proteins were extracted or tumor tissues were collected after 3-week treatment for protein extraction. Proteins (60 μg) were separated by SDS-PAGE and immunoblotted with antibodies against poly-(ADP ribose) polymerase (PARP), caspase 3, survivin, and E-cadherin. Results were representative of 3 independent experiments.
Discussion
Curcumin has been shown to participate in antioxidant response, immune response, apoptosis, cell cycle regulation, tumor progression, and epithelial mesenchymal transition (EMT) through different signaling pathways in numerous cancers [8,20–25]. However, there have been few preclinical studies on the anti-tumor effect of DMC, an analog of curcumin with higher chemical and metabolic stability. Moreover, there is no report about the anti-tumor effect of DMC in colon cancer cells up to now. Our study demonstrates for the first time that DMC inhibits proliferation by inducing apoptosis in two different cell lines of colon cancer both in vitro and in vivo, and one of the mechanisms might be through the down-regulation of survivin. Furthermore, we found that DMC inhibited colon cancer cell migration, possibly through upregulating E-cadherin expression, which is a hallmark of EMT.
It is well known that apoptotic processes are mediated through various pathways and regulated by multiple mechanisms [26]. Survivin (BIRC5) is the smallest member of the inhibitor of apoptosis (IAP) gene family, and is thought to be able to directly inhibit caspase-3, -7, and-9 activities to prevent apoptosis in various cancer tissues and cancer cell lines [27–29]. Caspase-3 is the most important effect or protease in apoptosis: being inactive inside the cell, it undergoes enzymatic cleavage and subsequent activation once the apoptotic cascade is triggered [30]. In the present study, survivin was down-regulated, while caspase-3 and PARP were cleaved to its active fragment in the DMC group. These results indicate that DMC may activate caspase-3 by down-regulating survivin to induce apoptosis.
EMT, a key developmental regulatory program, has been recently reported to play critical and intricate roles in promoting tumor invasion and metastasis in epithelium-derived carcinomas. Its process is very complex and controlled by various families of transcriptional regulators through various signaling pathways [31]. The suppression of E-cadherin transcription, which acts as a gate-keeper of the epithelial state in carcinomas, is a key step in the EMT process. The presence of DMC in both HT-29 and SW480 cells in this study not only reduced the number of migrated cells in transwell assays, but also enhanced the expression of E-cadherin in Western blot assay in vitro and in vivo, suggesting that DMC inhibits invasion through upregulating E-cadherin expression.
Conclusions
In conclusion, we demonstrated that DMC exerts an effective anti-tumor effect in colon cancer cells by down-regulating survivin and upregulating E-cadherin. These results lay the foundation for the future study of DMC use, alone or synergetic, in colon cancer chemotherapy.
Abbreviations
- DMC
dimethoxy curcumin
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transition
- PARP
poly (ADP-ribose) polymerase
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Source of support: This work was supported by grants from Traditional Chinese Medicine Science and Technology Plan of Zhejiang Province (No. 2015ZA053)
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Provenzale D, Jasperson K, Ahnen DJ, et al. Colorectal Cancer Screening, Version 1.2015. Journal of the National Comprehensive Cancer Network: JNCCN. 2015;13:959–68. doi: 10.6004/jnccn.2015.0116. quiz 968. [DOI] [PubMed] [Google Scholar]
- 3.Khan K, Wale A, Brown G, Chau I. Colorectal cancer with liver metastases: Neoadjuvant chemotherapy, surgical resection first or palliation alone? World J Gastroenterol. 2014;20:12391–406. doi: 10.3748/wjg.v20.i35.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scrobota I, Bolfa P, Filip AG, et al. Natural chemopreventive alternatives in oral cancer chemoprevention. J Physiol Pharmacol. 2015;67:161–72. [PubMed] [Google Scholar]
- 5.Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M. Curcumin and health. Molecules. 2016;21(3):264. doi: 10.3390/molecules21030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng M, Zhong LX, Zhan ZY, et al. Resveratrol treatment inhibits proliferation of and induces apoptosis in human colon cancer cells. Med Sci Monit. 2015;22:1101–8. doi: 10.12659/MSM.897905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel BB, Majumdar AP. Synergistic role of curcumin with current therapeutics in colorectal cancer: Minireview. Nutr Cancer. 2009;61:842–46. doi: 10.1080/01635580903285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar G, Mittal S, Sak K, Tuli HS. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci. 2016;148:313–28. doi: 10.1016/j.lfs.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Wang W, Li P, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol Res. 2016;23:29–34. doi: 10.3727/096504015X14452563486011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey A, Vishnoi K, Mahata S, et al. Berberine and curcumin target survivin and STAT3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-fluorouracil. Nutr Cancer. 2015;67:1293–304. doi: 10.1080/01635581.2015.1085581. [DOI] [PubMed] [Google Scholar]
- 11.Turrini E, Ferruzzi L, Fimognari C. Natural compounds to overcome cancer chemoresistance: Toxicological and clinical issues. Expert Opin Drug Metab Toxicol. 2014;10(12):1677–90. doi: 10.1517/17425255.2014.972933. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Xu H, Jiang Y, et al. Preparation, characterization, in vivo pharmacokinetics, and biodistribution of polymeric micellar dimethoxycurcumin for tumor targeting. Int J Nanomedicine. 2015;10:6395–410. doi: 10.2147/IJN.S91961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohtsu H, Xiao Z, Ishida J, et al. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J Med Chem. 2002;45:5037–42. doi: 10.1021/jm020200g. [DOI] [PubMed] [Google Scholar]
- 14.Tamvakopoulos C, Dimas K, Sofianos ZD, et al. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin Cancer Res. 2007;13:1269–77. doi: 10.1158/1078-0432.CCR-06-1839. [DOI] [PubMed] [Google Scholar]
- 15.Hassan HE, Carlson S, Abdallah I, et al. Curcumin and dimethoxycurcumin induced epigenetic changes in leukemia cells. Pharm Res. 2015;32:863–75. doi: 10.1007/s11095-014-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunwar A, Jayakumar S, Srivastava AK, Priyadarsini KI. Dimethoxy curcumin-induced cell death in human breast carcinoma MCF7 cells: Evidence for pro-oxidant activity, mitochondrial dysfunction, and apoptosis. Arch Toxicol. 2012;86:603–14. doi: 10.1007/s00204-011-0786-y. [DOI] [PubMed] [Google Scholar]
- 17.Yoon MJ, Kang YJ, Lee JA, et al. Stronger proteasomal inhibition and higher CHOP induction are responsible for more effective induction of paraptosis by dimethoxycurcumin than curcumin. Cell Death Dis. 2014;5:e1112. doi: 10.1038/cddis.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JW, Hong HM, Kwon DD, et al. Dimethoxy curcumin, a structural analogue of curcumin, induces apoptosis in human renal carcinoma caki cells through the production of reactive oxygen species, the release of cytochrome C, and the activation of caspase-3. Korean J Urol. 2010;51:870–78. doi: 10.4111/kju.2010.51.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunwar A, Barik A, Sandur SK, Indira Priyadarsini K. Differential antioxidant/pro-oxidant activity of dimethoxycurcumin, a synthetic analogue of curcumin. Free Radic Res. 2011;45:959–65. doi: 10.3109/10715762.2011.571681. [DOI] [PubMed] [Google Scholar]
- 20.Jordan BC, Mock CD, Thilagavathi R, Selvam C. Molecular mechanisms of curcumin and its semisynthetic analogues in prostate cancer prevention and treatment. Life Sci. 2016;152:135–44. doi: 10.1016/j.lfs.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan H, Liang Y, Jiang B, et al. Curcumin inhibits intracellular fatty acid synthase and induces apoptosis in human breast cancer MDA-MB-231 cells. Oncol Rep. 2016;35:2651–56. doi: 10.3892/or.2016.4682. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Chen H, Xu C, et al. Curcumin inhibits tumor epithelialmesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol Rep. 2016;35:2615–23. doi: 10.3892/or.2016.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang YT, Lin YW, Chiu HM, Chiang BH. Curcumin induces apoptosis of colorectal cancer stem cells by coupling with CD44 Marker. J Agric Food Chem. 2016;64:2247–53. doi: 10.1021/acs.jafc.5b05649. [DOI] [PubMed] [Google Scholar]
- 24.Yoysungnoen B, Bhattarakosol P, Changtam C, Patumraj S. Effects of tetrahydrocurcumin on tumor growth and cellular signaling in cervical cancer xenografts in nude mice. Biomed Res Int. 2016;2016:1781208. doi: 10.1155/2016/1781208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YH, Niu YB, Sun Y, et al. Role of phytochemicals in colorectal cancer prevention. World J Gastroenterol. 2015;21:9262–72. doi: 10.3748/wjg.v21.i31.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celik B, Yalcin AD, Bisgin A, et al. Level of TNF-related apoptosis-inducing-ligand and CXCL8 correlated with 2-[18F]Fluoro-2-deoxy-D-glucose uptake in anti-VEGF treated colon cancers. Med Sci Monit. 2013;19:875–82. doi: 10.12659/MSM.889605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesri M, Wall NR, Li J, et al. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest. 2001;108:981–990. doi: 10.1172/JCI12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Xu F, Xie T, et al. beta-elemene induces glioma cell apoptosis by downregulating survivin and its interaction with hepatitis B X-interacting protein. Oncol Rep. 2012;28:2083–90. doi: 10.3892/or.2012.2022. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T, Tanigawa N. The role of survivin as a new target of diagnosis and treatment in human cancer. Med Electron Microsc. 2001;34:207–12. doi: 10.1007/s007950100017. [DOI] [PubMed] [Google Scholar]
- 30.Lossi L, Cocito C, Alasia S, Merighi A. Ex vivo imaging of active caspase 3 by a FRET-based molecular probe demonstrates the cellular dynamics and localization of the protease in cerebellar granule cells and its regulation by the apoptosis-inhibiting protein survivin. Mol Neurodegener. 2016;11:34. doi: 10.1186/s13024-016-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao H, Xu E, Liu H, et al. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol Res Pract. 2015;211:557–69. doi: 10.1016/j.prp.2015.05.010. [DOI] [PubMed] [Google Scholar]