Abstract
Background
In our clinical experience we discovered that EEG band power may be correlated with corneal nerve injury in retinoblastoma patients. This study aimed to investigate biomarkers obtained from electroencephalography (EEG) recordings to reflect corneal nerve injury in retinoblastoma patients.
Material/Methods
Our study included 20 retinoblastoma patients treated at the Department of Ophthalmology, Affiliated Hospital of Weifang Medical University between 2010 and 2014. Twenty normal individuals were included in the control group. EEG activity was recorded continuously with 32 electrodes using standard EEG electrode placement for detecting EEG power. A cornea confocal microscope was used to examine corneal nerve injury in retinoblastoma patients and normal individuals. Spearman rank correlation analysis was used to analyze the correlation between corneal nerve injury and EEG power changes. The sensitivity and specificity of changed EEG power in diagnosis of corneal nerve injury were also analyzed.
Results
The predominantly slow EEG oscillations changed gradually into faster waves in retinoblastoma patients. The EEG pattern in retinoblastoma patients was characterized by a distinct increase of delta (P<0.01) and significant decrease of theta power P<0.05). Corneal nerves were damaged in corneas of retinoblastoma patients. Corneal nerve injury was positively correlated with delta EEG spectra power and negatively correlated with theta EEG spectra power. The diagnostic sensitivity and specificity by compounding in the series were 60% and 67%, respectively.
Conclusions
Changes in delta and theta of EEG appear to be associated with occurrence of corneal nerve injury. Useful information can be provided for evaluating corneal nerve damage in retinoblastoma patients through analyzing EEG power bands.
MeSH Keywords: Corneal Diseases, Electroencephalography Phase Synchronization, Retinoblastoma
Background
Retinoblastoma is the most common intraocular malignancy in childhood, and represents about 3% of all pediatric disease [1]. Retinoblastoma, a diagnosis that affects not only vision but also survival for many individuals, is also the most common intraocular tumor of childhood [2]. The incidence of retinoblastoma is generally the same worldwide, at 1 case per 15 000 to 20 000 live births, corresponding to about 9000 cases every year [3]. The mortality rate of retinoblastoma in children is about 40–70% in Asia and African countries, and 3–5% in Europe, Canada, and the USA [4]. Early retinoblastoma damage to the visual system may include pathophysiologic changes in the retina and corneal nerve head, and orbital corneal nerve damage [5]. Therefore, we speculate that the change in or injuries of the cornea may reflect or correlate with retinoblastoma status.
In this study we focused on biomarkers obtained from electroencephalography (EEG) recordings to reflect corneal nerve injury in retinoblastoma in the eyes-closed resting state. Previous studies [6,7] have also investigated corneal nerve injury in eyes of humans and animals. EEG power bands are easily obtained, relative cheap, and non-invasive. Several classical EEG biomarkers have recently been identified for predicting prognosis of retinoblastoma patients, including 5 different frequencies and powers [8]. According to our clinical observations, EEG band power may be correlated with corneal nerve injury in retinoblastoma patients. Thus, in this study we compared clinical EEG recordings with cornea confocal microscope results, and explored the use of EEG in retinoblastoma patients.
Material and Methods
Subjects
This research was approved by the Ethics Committee of Weifang Medical University. Written informed consent was obtained from parents or legal guardians for participation in the study. Twenty consecutive patients who had been referred due to retinoblastoma to the Department of Ophthalmology, Affiliated Hospital of Weifang Medical University between 2010 and 2014 participated in this study.
These 20 patients all had nonhereditary unilateral retinoblastoma presenting as intraocular tumor requiring primary enucleation. Retinoblastoma in this study was diagnosed according to the method of Dean et al. [9].
EEG recordings
We used EEG to investigate the dynamics of bioelectrical brain activity in retinoblastoma patients. The EEG activity was recorded continuously with 32 electrodes using standard EEG electrode placement. The visual analysis was performed on standard bipolar montages with electrode positions according to the 10 to 20 international systems. The detailed processes of the EEG examination were performed as reported by Zeng et al. [10].
Cornea confocal microscope observation
To observe the status of the subbasal never plexus, more than 3 representative images were evaluated for analysis. Three masked and independent investigators evaluated the morphology of the corneal nerve and the subbasal nerve plexus, performed according to a previous report [11]. The evaluation of the total length of nerve fibers represents the nerve density in the micrometers/frame (158, 700 μm2). We designated the number of main nerves under a microscope as the main nerve trunks. We designated the number of nerve branches as the nerve branching under a microscope. Using a microscope, we designated all of the nerves as the total nerves, including nerve branches and the main nerve trunks [12].
Statistical analysis
SPSS 17.0 was used for statistical analysis. The data analysis was performed by utilizing the Student’s t test. Spearman rank correlation was used in correlation analysis.
Results
Patients characteristics and histologic features
A total of 20 patients (11 girls and 9 boys) were included, with a median age of 58 months (range, 43 to 127 months). According to the intraocular international retinoblastoma classification (IIRC), there were 0 patients with extreme low risk (Group A), 1 patient with low risk (Group B), 1 patient with intermediate risk (group C), 16 patients with high risk (Group D), and 2 patients with extreme high risk (Group E).
EEG delta and theta power changes in retinoblastoma patients
Figure 1 shows the changes of the dominant power bands within delta and theta bands. The EEG pattern of the retinoblastoma patients was characterized by a distinct increase in the delta (Figure 1, P<0.01) and significant decrease in the theta power (Figure 1, P<0.05).
Figure 1.

Comparison of EEG power (delta and theta power) spectral density of power bands of retinoblastoma patients and normal controls. * P<0.05, ** P<0.01 represent the delta or theta power in retinoblastoma patients compared to the normal control group.
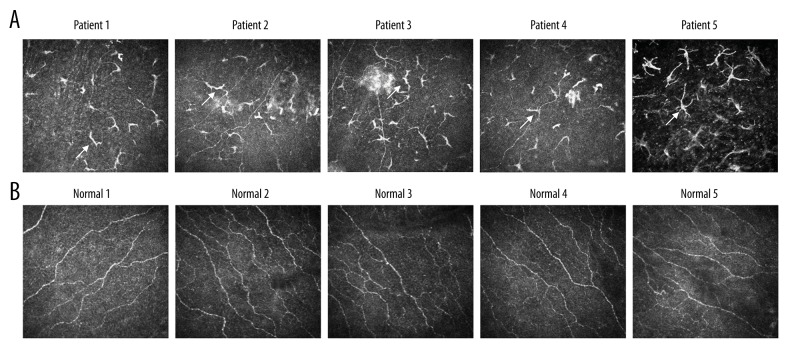
Corneal nerves were damaged in corneas of retinoblastoma patients
The results of cornea confocal observation showed that the corneal branch nerve fiber density was decreased significantly compared to the normal individuals. However, the density of the tumid and dendritic nerves was significantly increased compared to the normal individuals. The basal epithelium curving nerves were also significantly increased in the eyes of retinoblastoma patients (Figure 2A). However, the subbasal nerve plexus remained normal (Figure 2B). These results show that the corneal nerve was significantly damaged in eyes of retinoblastoma patients.
Figure 2.
Comparison for the cornea confocal microscope examination images in retinoblastoma patients and normal control individuals (randomly selected from the 20 patients). (A) The confocal images of 5 retinoblastoma patients. (B) The confocal images of 5 normal individuals. The arrows represent the tumid nerves, dendritic nerves, and curving nerves.
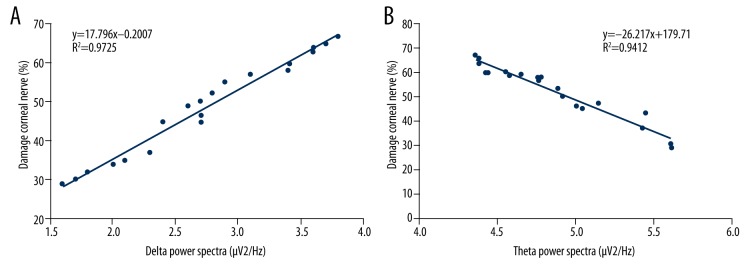
Correlation between the corneal nerve injury and the EEG spectra power
In order to investigate the correlation between corneal nerve injury and the EEG spectra power, Spearman rank correlation analysis was performed. The results indicated that corneal nerve injury was positively correlated with the delta EEG spectra power (Figure 3A, P<0.05) and the corneal nerve injury was negatively correlated with the theta EEG spectra power (Figure 3B, P<0.05). This suggests that the EEG spectra power changes may reflect corneal nerve injury in retinoblastoma patients.
Figure 3.
Spearman rank correlation analysis of corneal nerve injury compared with EEG power bands. (A) Correlation analysis for corneal nerve injury and delta power changes. (B) Correlation analysis for nerve injury and theta power changes.
The valves of increased delta power and decreased theta power in the diagnosis of corneal nerve injury in retinoblastoma patients
We found that the sensitivity and specificity of the increased delta power and decreased theta power in the diagnosis of retinoblastoma patients were different. In increased delta power (delta >3.8), sensitivity and specificity were 75% and 85%, and in decreased theta power (theta <4.3) sensitivity and specificity were 80% and 80%, respectively (Table 1). If compounded in parallel, the sensitivity of detecting corneal nerve injury was 75% and the specificity was 88%. If compounded in series, the sensitivity and specificity were 85% and 67%, respectively (Table 1), which is appropriate for the diagnosis of corneal nerve injury in retinoblastoma patients.
Table 1.
The values of EEG delta and theta power spectra in the diagnosis of corneal nerve in retinoblastoma patients.
| Normal individuals | Retinoblastoma patients | Sensivity | Spedificity | |||
|---|---|---|---|---|---|---|
| + | − | + | − | |||
| Delta>3.8 | 3 | 17 | 15 | 5 | 75% | 85% |
| Theta<4.3 | 4 | 16 | 18 | 2 | 80% | 80% |
| Compounding in parallel | 2 | 15 | 15 | 1 | 75% | 88% |
| Compounding in series | 6 | 16 | 17 | 3 | 85% | 67% |
Discussion
The EEG method of prognosis used in this study agrees with previous reports on use of the corneal nerve for disease diagnosis and prognosis [13–15]. However, using EEG to diagnose corneal nerve injury in retinoblastoma patients has not been explored in previous studies.
Retinoblastoma has the highest rate of survival among pediatric cancers [16]. The 3 goals of retinoblastoma diagnosis, treatment, and improvement are saving the patient’s life, eyes, and vision [17]. In our previous study, we found that retinoblastoma patients always had obviously changed corneal nerve status. However, the cornea usually was neglected in clinical practice. In the present study, we examined the EEG power bands and analyzed their relationship with corneal nerve injury.
We observed not only increased power in the prevailing EEG power bands, but also a moderate shift of the dominant frequency peak toward faster theta and delta. This might be because retinoblastoma afflicts the corneal nerve, which is related with visual activity. Our results show that the delta power was increased and theta power was decreased in retinoblastoma patients compared to normal individuals.
In clinical ophthalmology, the pathogenesis of many diseases, such as herpes simplex keratitis, glaucoma, and retinoblastoma, may affect the corneal nerves [5,18–20]. The cornea is the most densely innervated part of the human body, containing myelinated Aδ and unmyelinated C fibers derived from the ophthalmic division of the trigeminal nerve [21,22]. Therefore, corneal confocal microscope analysis is always used in the diagnosis of corneal nerve damage. Our results indicate decreased corneal branch nerve fiber density, as well as the appearance of the dendritic particles and tumid neurons, which suggests that retinoblastoma might induce corneal nerve injury. Corneal nerve damage was calculated by analyzing the percentage of nerve injury. Therefore, we analyzed the correlation between corneal nerve injury and the delta and theta power of EEG. The results indicated that the corneal nerve injury was positively correlated with the delta power and negatively correlated with the theta power of EEG.
Actually, the changes in EEG theta and delta power was very slight and short-term. Our study indicated that the theta and delta power were mainly observed in the high-risk stage (Group D) of retinoblastoma, according to the IIRC classification and that the changes in EEG powers cannot be obtained in many patients or at other stages. Therefore, we speculate that the changes in delta and theta power are transient and are related to IIRC stage for the retinoblastoma.
In our study, the sensitivity of the increased delta power and decreased theta power in the diagnosis of corneal nerve injury were 75% and 80%, respectively and the specificity was 85% and 80%, respectively. If compounded in parallel, the specificity of corneal nerve injury was 88% and if compounded in series, the sensitivity was 85%, and it was obviously higher than in other groups. The compounding of delta and theta achieved the best diagnosis for corneal nerve injury. If this method can help determine whether the corneal nerve is already injured in retinoblastoma patients, it will provide useful a reference for follow-up, adjunctive therapy, and the prognosis of retinoblastoma patients.
Conclusions
The changes in delta and theta of EEG appear to be associated with occurrence of corneal nerve injury. Useful information can be provided for evaluating corneal nerve damage of retinoblastoma patients through analyzing EEG power bands.
Footnotes
Conflict of interest
We declare that we have no conflict of interest.
Source of support: Departmental sources
References
- 1.Mastrangelo D, De Francesco S, Di Leonardo A, et al. The retinoblastoma paradigm revisited. Med Sci Monit. 2008;14(12):RA231–40. [PubMed] [Google Scholar]
- 2.Weaver MS, Lam CG. Improving retinoblastoma outcomes through a stage-based. Lancet Glob Health. 2014;2:e143. doi: 10.1016/S2214-109X(14)70001-4. [DOI] [PubMed] [Google Scholar]
- 3.Kivela T. The epidemiological challenge of the most frequent eye cancer: Retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93:1129–31. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 4.Dimaras H, Kimani K, Dimba EA, et al. Retinoblastoma. Lancet. 2012;379:1436–46. doi: 10.1016/S0140-6736(11)61137-9. [DOI] [PubMed] [Google Scholar]
- 5.Shetye NG, Mataftsi A, Maeder P, et al. Post-chemoreduction cryptic optic nerve relapse in a patient with bilateral retinoblastoma. Br J Ophthalmol. 2013;97:233, 245–46. doi: 10.1136/bjophthalmol-2012-302384. [DOI] [PubMed] [Google Scholar]
- 6.Yun H, Rowe AM, Lathrop KL, et al. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J Virol. 2014;88:7870–80. doi: 10.1128/JVI.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaguchi T, Calvacanti BM, Cruzat A, et al. Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55:7457–66. doi: 10.1167/iovs.14-15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang XH, Sun YX, Zhao JH, et al. Grand total EEG as a predictive biomarker cognitive severity in cerebral infarcts of Chinese. Clin EEG Neurosci. 2014;45:158–62. doi: 10.1177/1550059413496778. [DOI] [PubMed] [Google Scholar]
- 9.Dean M, Bendfeldt G, Lou H, et al. Increased incidence and disparity of diagnosis of retinoblastoma patients in Guantemala. Cancer Lett. 2014;351:59–63. doi: 10.1016/j.canlet.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng K, Wu XD, Cai HD, et al. Relationship between EEG beta power abnormality and early diagnosis of cognitive impairment post cerebral hemorrhage. Clin EEG Neurosci. 2013;44:203–8. doi: 10.1177/1550059412471336. [DOI] [PubMed] [Google Scholar]
- 11.Calvillo MP, McLaren JW, Hodge DO, et al. Corneal reinnervation after LASIK: prospective 3-years longitudinal study. Invest Ophthalmol Vis Sci. 2004;45:3991–96. doi: 10.1167/iovs.04-0561. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–84. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Yang K, Su J, Lang Y, et al. Contradictory imaging and EEG results in resection surgery of bitemporal lobe epilepsy: A case report. Exp Ther Med. 2014;7:731–33. doi: 10.3892/etm.2013.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiba T, Takahashi M, Sato Y, et al. Relationship between severity of obstructive sleep apnea sysdrome and retinal nerve fiber layer thickness. Clin EEG Neurosci. 2014;157:1202–8. doi: 10.1016/j.ajo.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Sergeeva EG, Fedorov AB, Henrich-Noack P, et al. Transcorneal alternating current stimulation induces EEG “aftereffects” only in rats with an intact visual system but not after severe corneal nerve damage. J Neurophysiol. 2012;108:2494–500. doi: 10.1152/jn.00341.2012. [DOI] [PubMed] [Google Scholar]
- 16.Abramson DH. Retinoblastoma in the 20th century: Past success and future challenges the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2005;46:2683–91. doi: 10.1167/iovs.04-1462. [DOI] [PubMed] [Google Scholar]
- 17.da Rocha-Bastos R, Araujo J, Silva R, et al. Retinoblastoma: Experience of a referral center in the North region of Portugal. Clin Ophthalmol. 2014;8:993–97. doi: 10.2147/OPTH.S59601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu K, Liu XN, Zhang HB, et al. Replication-defective HSV-1 effectively targets trigeminal ganglion and inhibits viral pathopoiesis by mediating interferon gamma expression in SH-SY5Y cells. J Mol Neurosci. 2014;53:78–86. doi: 10.1007/s12031-013-0199-x. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Cull G, Burgoyne CF, et al. Longitudinal alterations in the dynamic autoregulation of corneal nerve head blood flow revealed in experimental glaucoma. Invest Ophthalmol Vis Sci. 2014;55:3509–16. doi: 10.1167/iovs.14-14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao T, Xing Y, Yang Y, et al. Correlation between matrix metalloproteinase expression and activation of the focal adhesion kinase signaling pathway in herpes stromal keratitis. Exp Ther Med. 2014;7:280–86. doi: 10.3892/etm.2013.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallinikos P, Morgan P, Efron N. On the etiology of keratocyte loss during contact lens wear. Invest Ophthalmol Vis Sci. 2004;45:3011–20. doi: 10.1167/iovs.04-0129. [DOI] [PubMed] [Google Scholar]
- 22.Tavakoli M, Hossain P, Malik RA. Clinical applications of corneal confocal microscopy. Clin Ophthalmol. 2008;2:435–45. doi: 10.2147/opth.s1490. [DOI] [PMC free article] [PubMed] [Google Scholar]