Abstract
Background
Diabetic nephropathy (DN) is a common complication of diabetes, caused by diabetic microvascular lesions. The pathogenesis of DN is complicated, involving genetics, physics, chemistry, and environmental factors. Chemerin is a fat cell factor that participates in regulating inflammation. Vascular endothelial growth factor (VEGF) promotes vascular endothelial cell proliferation, differentiation, and angiogenesis. The relationship role of Chemerin and VEGF in DN is not fully understood.
Material/Methods
SD rats were randomly divided into 2 groups: the control group and the DN group. Streptozotocin was used to construct the DN model. Serum creatinine (Scr), blood urea nitrogen (BUN), and urine microalbumin (UAlb) were detected. Real-time PCR and Western blot were used to test Chemerin and VEGF mRNA and protein expression in kidney tissue. ELISA was performed to test TGF-β1, TNF-α, and INF-γ levels. The correlation of Chemerin and VEGF with renal function and inflammatory factors was analyzed.
Results
DN group rats showed obviously increased Scr and BUN levels, and elevated TGF-β1, TNF-α, and INF-γ secretion (P<0.05). Compared with controls, Chemerin and VEGF were clearly overexpressed in the DN group (P<0.05). Chemerin and VEGF expression were positively correlated with inflammatory factors and renal function.
Conclusions
Chemerin and VEGF play important roles in DN by regulating inflammatory factors and renal function. They may be treated as indicators of DN.
MeSH Keywords: Adipokines; Diabetic Ketoacidosis; Vascular Endothelial Growth Factor, Endocrine-Gland-Derived
Background
With widespread changes in lifestyle, the incidence of diabetes is steadily increasing. There are nearly 300 million people with diabetes worldwide, and China has a high incidence [1,2]. Research shows a trend towards younger age of diabetes onset [3]. The leading cause of diabetes disability or death are its various complications. The leading diabetes complications are microangiopathy and macroangiopathy. Microvascular complications include diabetic retinopathy, diabetic nephropathy (DN), and nervous system diseases, while macroangiopathy involves the cardiovascular, cerebrovascular, and peripheral vascular systems [4,5]. DN is a common clinical complication. Diabetes and other factors cause renal vascular damage, resulting in extracellular matrix hyperplasia, glomerular basement membrane thickening, and glomerular sclerosis. Diabetes can lead to DN and end-stage renal failure [6]. Although there are various methods to treat diabetes and related complications, many DN patients eventually progress to renal failure, requiring dialysis or renal transplantation [7,8].
The pathogenesis of DN is complex, involving genetics, physics, chemistry, environment, and many other factors. Research has suggested that inflammatory cytokines, oxidative stress, and growth factors were involved in DN occurrence and development [9]. Recent studies reported that a newly discovered fat cell factor, Chemerin, is expressed in adipose tissue, liver, pancreas, lung, and other tissues. Its receptor is G protein-coupled receptor ChemR23 [10,11]. Chemerin participates in inflammation, obesity, and insulin resistance by regulating glucolipid metabolism and promoting adipocyte differentiation [12]. Vascular endothelial growth factor (VEGF) can stimulate vascular endothelial cell division and enhance vascular endothelial cell permeability, thus promoting vascular endothelial cell proliferation, differentiation, and angiogenesis [13,14]. However, the correlation of Chemerin and VEGF with DN has not been fully elucidated. This study aimed to investigate it through establishing a rat DN model.
Material and Methods
Experimental animals
We purchased 24 healthy male SD rats age 3 months and weight 250±30 g from the Experimental Animal Center of Shandong University. The rats were kept in an SPF lab with constant temperature (21±1ºC), constant humidity (50–70%), and 12-h day/night cycle.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of Shandong University.
Main materials and instruments
Surgical instruments were bought from Suzhou Medical Instruments. The operating microscope was from Zhenjiang Optical Instrument Co., LTD. STZ was purchased from Sigma and Trizol reagent was from Invitrogen. The serum creatinine detection kit was from Roche and the PVDF membrane was from Pall Life Sciences. Western blot-related chemical reagent was from Beyotime and ECL reagent was from Amersham Biosciences. Mouse anti-rat Chemerin and VEGF monoclonal antibody, goat anti-mouse HRP-tagged IgG secondary antibody were from Cell Signaling. RNA extraction kit, reverse transcription kit, TGF-β1, TNF-α, and IFN-γ ELISA kits were from R&D. Rat-specific urinary protein detection reagent was from Beijing Furui Biological Engineering Co., LTD. The microplate reader was from BD and the PE Gene DNA amplifier was a PE Gene Amp PCR System 2400. The electronic glucometer was from Advantage and the automatic biochemical analyzer was from Beckman. Glucose test paper was from Zhujiang Chemical Reagent Co., LTD. Other common reagents were purchased from Sangon.
Methods
Grouping
The rats were randomly and equally divided into 2 groups: the control (n=12) and the DN group (n=12). The DN group received STZ to establish the rat DN model.
Modeling
The rats were prepared for modeling after 1 week of adaptation. The rats were fasted for 12 h before treatment. STZ in 0.5% citric acid-sodium citrate buffer was intravenously injected at 40 mg/kg. The rats in the control group only received an equal amount of citric acid-sodium citrate buffer. Blood glucose, urine glucose, and urine volume were tested after 2 weeks. A 2-fold increase in blood glucose > 16.7 mmol/L, urine glucose < ++, and urine volume was considered DN modeling success [15].
Specimen collection
An abdominal aortic blood sample was collected in a vacuum biochemical tube using negative-pressure acquisition method at 2 weeks after modeling. After standing at room temperature for 30 min, the blood was centrifuged at 4°C and 3600 rpm for 10 min. The supernatant was cryopreserved at −20°C. Left kidney tissue was stored at −80°C.
Renal function detection
Scr and BUN were detected by automatic biochemical analyzer. UAlb was determined by radioimmunoassay method. Kidney weight ratio was calculated.
Real-time PCR
Total RNA was extracted using Trizol and reverse transcribed to cDNA according to the manual. The primers used were designed by Primer 6.0 and synthesized by Invitrogen (Table 1). Real-time PCR was performed at 55°C for 1 min, followed by 35 cycles of 92°C for 30 s, 58°C for 45 s, and 72°C for 35 s. GAPDH was selected as internal reference. Relative gene expression was calculated by 2−ΔCt method.
Table 1.
Primer sequence.
| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| GADPH | ACCAGGTATCTGCTGGTTG | TAACCATGATGTCAGCGTGGT |
| Chemerin | GCATGACCTGCTTATGACTG | TTCGTTCCGCTCAACTCTTA |
| VEGF | TGCTTATGCATGATGCCGACT | CGCTTCTTCGTCAACTCTTATC |
Western blot
Kidney tissue was ground in liquid nitrogen and treated by RIPA for 15~30 min. Then the tissue was ultrasonicated at 5 s 4 times and centrifuged at 4°C and 10 000× g for 15 min. The supernatant was stored at −20°C. The protein was separated by 10% SDS-PAGE and transferred to a PVDF membrane. After blocking by 5% skim milk for 2 h, the membrane was incubated in primary antibody (1:1000) at 4°C overnight. Next, the membrane was further incubated in secondary antibody (1:2000) at room temperature for 30 min after washing in PBST. We added a chemiluminescent agent to the membrane for 1 min and developed it. Protein image processing system software was used for scanning and Image J software was used for the quantification of the intensity of positive bands. Protein expressions were quantified as the ratio to β-actin. All the experiments were repeated 4 times (n=4).
ELISA
TGF-β1, TNF-α, and IFN-γ levels in rat serum were tested by ELISA according to the product manual. The sample concentration was calculated based on the standard curved of the OD value.
Statistical analysis
SPSS19.0 was used for data analysis. Measurement data are presented as mean ± standard deviation. The LSD test was used for comparison. Spearman’s rank-order correlation was performed for correlation analysis. P<0.05 was considered as statistical significance.
Results
Survival state and renal function
Rat survival, condition, kidney weight ratio, and renal function were observed. The rats in the control group showed good mental condition, lustrous hair, normal eating, drinking, activity, and urine output. The rats in the DN group appeared dispirited, lost hair, had polydipsia, and had increased urine output. Compared with control rats, the DN group had significant weight loss and elevated kidney weight ratio, Scr, BUN, and UAlb (P<0.05) (Table 2).
Table 2.
General indicators and renal function.
| Indicator | Control | DN group |
|---|---|---|
| Weight (g) | 421.4±52.7 | 237.9±36.1* |
| Blood glucose (mmol/L) | 6.3±0.5 | 27.6±2.3 |
| Kidney weight ratio (mg/g) | 2.5±0.3 | 5.7±0.9* |
| Scr (μmol/L) | 89.2±12.3 | 1777.6±41.5* |
| BUN (mmol/L) | 7.1±0.8 | 11.2±1.1* |
| UAlb (mg/24 h) | 0.3±0.1 | 0.8±0.2* |
P<0.05, compared with control.
Chemerin mRNA and protein expression in kidney
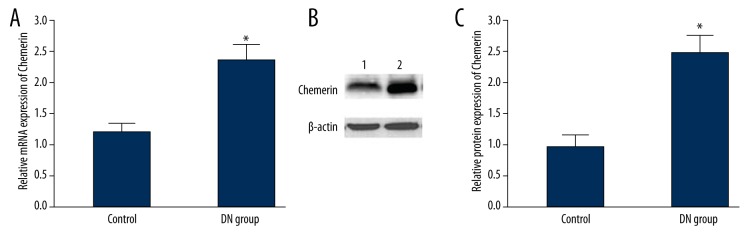
Real-time PCR and Western blot were used to detect Chemerin mRNA and protein expression in renal tissue. Compared with controls, Chemerin mRNA was significantly overexpressed in the DN group (P<0.05) (Figure 1A). Similar to the mRNA expression changes, Chemerin protein level in the DN group was also markedly elevated (P<0.05) (Figure 1B, 1C).
Figure 1.
Chemerin mRNA and protein expression in renal tissue. (A) Chemerin mRNA and protein expression in renal tissue; (B) Chemerin protein expression in renal tissue; (C) Chemerin protein expression analysis in renal tissue. 1, control; 2, DN group. * P<0.05, compared with control.
VEGF mRNA and protein expression in kidney
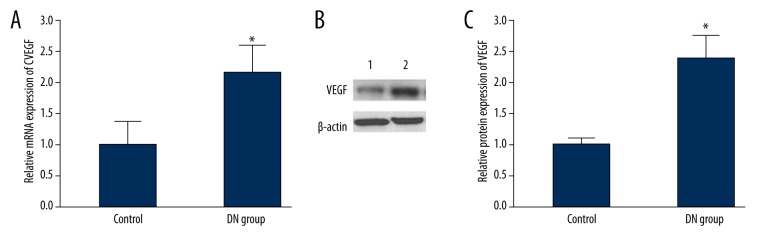
Real-time PCR and Western blot were used to detect VEGF mRNA and protein expression in renal tissue. Compared with controls, VEGF mRNA was significantly overexpressed in the DN group (P<0.05) (Figure 2A). Similar to the mRNA expression changes, the VEGF protein level in the DN group was also markedly elevated (P<0.05) (Figure 2B, 2C).
Figure 2.
VEGF mRNA and protein expression in renal tissue. (A) VEGF mRNA and protein expression in renal tissue; (B) VEGF protein expression in renal tissue; (C) VEGF protein expression analysis in renal tissue. 1, control; 2, DN group. * P<0.05, compared with control.
Inflammatory factors expression in serum
ELISA was used to test TGF-β1, TNF-α, and IFN-γ levels in rat serum. The results showed that TGF-β1, TNF-α, and IFN-γ levels in the DN group were obviously elevated compared with controls (P<0.05) (Figure 3).
Figure 3.
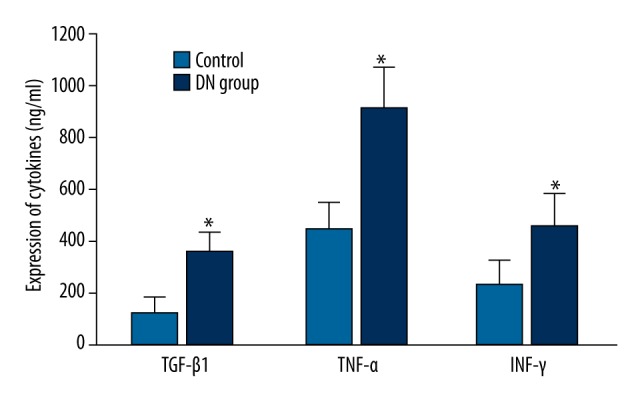
Inflammatory factors expression in serum. * P<0.05, compared with control.
Correlation analysis between Chemerin and DN
The relationship between Chemerin mRNA and protein expression with inflammatory factors and renal function in DN was analyzed. We found that Chemerin mRNA and protein were positively correlated with TGF-β1, TNF-α, IFN-γ, Scr, BUN, and UAlb (P<0.05) (Table 3).
Table 3.
Correlation analysis between Chemerin and DN.
| Indicator | Chemerin mRNA | Chemerin protein | ||
|---|---|---|---|---|
| r | P | r | P | |
| Scr | 0.751 | 0.032 | 0.727 | 0.041 |
| BUN | 0.874 | 0.005 | 0.869 | 0.006 |
| UAlb | 0.892 | 0.003 | 0.921 | 0.002 |
| TGF-β1 | 0.718 | 0.045 | 0.723 | 0.042 |
| TNF-α | 0.732 | 0.039 | 0.855 | 0.007 |
| INF-γ | 0.819 | 0.009 | 0.829 | 0.008 |
Correlation analysis between VEGF and DN
The relationship between VEGF mRNA and protein expression with inflammatory factors and renal function in DN was analyzed. we found that VEGF mRNA and protein were positively correlated with TGF-β1, TNF-α, IFN-γ, Scr, BUN, and UAlb (P<0.05) (Table 4).
Table 4.
Correlation analysis between VEGF and DN.
| Indicator | VEGF mRNA | VEGF protein | ||
|---|---|---|---|---|
| r | P | r | P | |
| Scr | 0.642 | 0.047 | 0.721 | 0.043 |
| BUN | 0.923 | 0.002 | 0.863 | 0.005 |
| UAlb | 0.897 | 0.005 | 0.821 | 0.009 |
| TGF-β1 | 0.735 | 0.037 | 0.723 | 0.044 |
| TNF-α | 0.856 | 0.007 | 0.923 | 0.002 |
| INF-γ | 0.826 | 0.008 | 0.869 | 0.004 |
Discussion
A recent study confirmed that adipose tissue is closely correlated with diabetes [16]. Adipose tissue is not just the main storage area for triglycerides and thermal energy, it is also an endocrine organ [17]. Adipose tissue can secrete and release bioactive adipose cytokines. Chemerin, also known as TIG2, is one of the fat cell cytokines that was first found in psoriasis. Chemerin can hydrolyze hydrophobic signal peptide through protease. The rest of the precursor protein has low activity and is further hydrolyzed to Chemerin. Chemerin has chemotactic activity, and when hydrolyzed by caspase in macrophages, it has anti-inflammatory activity [18,19]. It has been reported that Chemerin plays an important role in energy metabolism regulation, appetite, blood lipid, blood pressure, inflammation, and angiogenesis [20]. Chemerin expression level in the serum was significantly increased in patients receiving long-term kidney dialysis and was negatively correlated with glomerular filtration rate, suggesting that Chemerin has a role in regulating renal disease [21]; however, the correlation between Chemerin and DN has not been clarified. Through the establishment of rat DN model, the present study analyzed inflammatory factors and renal function changes in DN rats. The results confirmed that inflammatory factors, BUN, Scr, and 24 h proteinuria expression levels were obviously elevated following renal function damage in DN rats. It was further found that Chemerin mRNA and protein were overexpressed in DN rats, suggesting that Chemerin is closely associated with DN. Correlation analysis demonstrated that Chemerin mRNA and protein were positively correlated with TGF-β1, TNF-α, IFN-γ, Scr, BUN, and UAlb.
The correlation between VEGF level and DN is a popular research topic because VEGF increases the permeability of vascular endothelial cells and changes the glomerular filtration barrier [22]. Our study confirmed that VEGF mRNA and protein expression were significantly enhanced in DN rats, and were positively correlated with TGF-β1, TNF-α, IFN-γ, Scr, BUN, and UAlb, perhaps because VEGF overexpression increased glomerular filtration barrier and elevated permeability, promoting TGF-β1, TNF-α, and IFN-γ secretion and exudation. It further aggravates inflammatory reaction and induces renal hypoxia, resulting in 24-h proteinuria and increased Scr and BUN secretion. VEGF can also aggravate renal tubular basement membrane thickening, glomerular sclerosis, and renal interstitial fibrosis by promoting extracellular matrix deposition, leading to renal function damage [23–25].
Conclusions
Chemerin and VEGF play important roles in DN by regulating inflammatory factors and renal function. They may be useful as indicators of DN.
Footnotes
Disclosure of conflict of interest
The authors declare no competing financial or commercial interests.
Source of support: Departmental sources
References
- 1.Li X, Zhang J, Zhao W, Yang H, et al. Effect of Tongxinluo on nerve regeneration in mice with diabetic peripheral neuropathy. Cell Mol Biol. 2015;61:103–7. [PubMed] [Google Scholar]
- 2.Frustaci A, Ciccosanti F, Chimenti C, et al. Histological and proteomic profile of diabetic versus non-diabetic dilated cardiomyopathy. Int J Cardiol. 2015;203:282–89. doi: 10.1016/j.ijcard.2015.10.119. [DOI] [PubMed] [Google Scholar]
- 3.Augustin AJ, Kuppermann BD, Lanzetta P, et al. Dexamethasone intravitreal implant in previously treated patients with diabetic macular edema: Subgroup analysis of the MEAD study. BMC Ophthalmol. 2015;15:150. doi: 10.1186/s12886-015-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Lu C, Li X, et al. Synthesis and biological evaluation of novel gigantol derivatives as potential agents in prevention of diabetic cataract. PLoS One. 2015;10:e0141092. doi: 10.1371/journal.pone.0141092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding WJ, Ji Q, Shi YQ, et al. Incidence of deep sternal wound infection in diabetic patients undergoing off-pump skeletonized internal thoracic artery grafting. Cardiology. 2016;133:111–18. doi: 10.1159/000441137. [DOI] [PubMed] [Google Scholar]
- 6.Makhlough A, Makhlough M, Shokrzadeh M, et al. Comparing the levels of trace elements in patients with diabetic nephropathy and healthy individuals. Nephrourol Mon. 2015;7:e28576. doi: 10.5812/numonthly.28576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Ji Y, Lv W, et al. Protective effects of leflunomide on renal lesions in a rat model if diabetic nephropathy. Ren Fail. 2015;38(1):124–30. doi: 10.3109/0886022X.2015.1105024. [DOI] [PubMed] [Google Scholar]
- 8.Najafian B, Fogo AB, Lusco MA, Alpers CE. AJKD atlas of renal pathology: diabetic nephropathy. Am J Kidney Dis. 2015;66:e37–38. doi: 10.1053/j.ajkd.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Bonomo JA, Palmer ND, He JC, et al. Association analysis of the reticulon 1 gene in end-stage kidney disease. Am J Nephrol. 2015;42:259–64. doi: 10.1159/000441199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar JD, Holmberg C, Balabanova S, et al. Mesenchymal stem cells exhibit regulated exocytosis in response to Chemerin and IGF. PLoS One. 2015;10:e0141331. doi: 10.1371/journal.pone.0141331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao D, Bi G, Feng J, et al. Association of serum Chemerin levels with acute ischemic stroke and carotid artery atherosclerosis in a Chinese population. Med Sci Monit. 2015;21:3121–28. doi: 10.12659/MSM.895866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd JW, Zerfass KM, Heckstall EM, Evans KA. Diet-induced increases in chemerin are attenuated by exercise and mediate the effect of diet on insulin and HOMA-IR. Ther Adv Endocrinol Metab. 2015;6:189–98. doi: 10.1177/2042018815589088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carranza K, Veron D, Cercado A, et al. Cellular and molecular aspects of diabetic nephropathy; the role of VEGF-A. Nefrologia. 2015;35:131–38. doi: 10.1016/j.nefro.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Dabhi B, Mistry KN, Patel H, Lal S. Vascular endothelial growth factor insertion/deletion gene polymorphism in West Indian patients of type 2 diabetes and diabetic nephropathy. Indian J Biochem Biophys. 2015;52:209–12. [PubMed] [Google Scholar]
- 15.Tripathi AS, Timiri AK, Mazumder PM, Chandewar A. Does glimepiride alter the pharmacokinetics of sildenafil citrate in diabetic nephropathy animals: Investigating mechanism of interaction by molecular modeling studies. J Mol Model. 2015;21:276. doi: 10.1007/s00894-015-2823-x. [DOI] [PubMed] [Google Scholar]
- 16.Kunimoto H, Kazama K, Takai M, et al. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am J Physiol Heart Circ Physiol. 2015;309:H1017–28. doi: 10.1152/ajpheart.00820.2014. [DOI] [PubMed] [Google Scholar]
- 17.Ferland DJ, Watts SW. Chemerin: A comprehensive review elucidating the need for cardiovascular research. Pharmacol Res. 2015;99:351–61. doi: 10.1016/j.phrs.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu P, Cheng M, Hui X, et al. Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J Hypertens. 2015;33:1624–32. doi: 10.1097/HJH.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 19.Blaszak J, Szolkiewicz M, Sucajtys-Szulc E, et al. High serum chemerin level in CKD patients is related to kidney function, but not to its adipose tissue overproduction. Ren Fail. 2015;37:1033–38. doi: 10.3109/0886022X.2015.1040707. [DOI] [PubMed] [Google Scholar]
- 20.Szczepanska M, Machura E, Adamczyk P, et al. Evaluation of adipocytokines in children with chronic kidney disease. Endokrynol Pol. 2015;66:100–7. doi: 10.5603/EP.2015.0015. [DOI] [PubMed] [Google Scholar]
- 21.Bonomini M, Pandolfi A. Chemerin in renal dysfunction and cardiovascular disease. Vascul Pharmacol. 2016;77:28–34. doi: 10.1016/j.vph.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Sun CY, Lee CC, Hsieh MF, et al. Clinical association of circulating VEGF-B levels with hyperlipidemia and target organ damage in type 2 diabetic patients. J Biol Regul Homeost Agents. 2014;28:225–36. [PubMed] [Google Scholar]
- 23.Cheng H, Harris RC. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc Hematol Disord Drug Targets. 2014;14:22–33. doi: 10.2174/1871529x14666140401110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma L, Gao Y, Chen G, et al. Relationships of urinary VEGF/CR and IL-6/CR with glomerular pathological injury in asymptomatic hematuria patients. Med Sci Monit. 2015;21:356–62. doi: 10.12659/MSM.892085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das UN. Is lipoxins A(4) a better alternative to anti-VEGF and anti-TNF-alpha antibody to prevent and treat age-related macular degeneration, diabetic macular edema and retinopathy? Med Sci Monit. 2012;18(1):LE1–2. doi: 10.12659/msm.882187. [DOI] [PubMed] [Google Scholar]