Abstract
Nek2 (NIMA‐related kinase 2) is a serine‐threonine kinase and human homolog of the mitotic regulator NIMA of Aspergillus nidulan. We reported the efficiency of Nek2 siRNA in several cancer xenograft models using cholangiocarcinoma, breast cancer and colorectal cancer. Pancreatic cancer is difficult to treat due to its rapid progression and resistance to chemotherapy. Novel treatments are urgently required to improve survival in pancreatic cancer, and siRNA are a promising therapeutic option. However, finding an in vivo drug delivery system of siRNA remains a major problem for clinical application. In this study, the overexpression of Nek2 was identified in pancreatic cancer cell lines. Nek2 siRNA inhibited tumor growth in a subcutaneous xenograft mouse model of pancreatic cancer, prolonged the survival time in an intraperitoneal xenograft mouse model and efficiently prevented the progression of liver metastasis using a portal venous port–catheter system. Taken together, Nek2 is an effective therapeutic target in pancreatic cancer. An adequate delivery system is considered important in treating advanced pancreatic cancer, such as peritoneal dissemination and liver metastasis. Further investigations are required on the safety and side effects of the portal venous port–catheter system. We hope that Nek2 siRNA will be a novel therapeutic strategy for pancreatic cancer with liver metastasis and peritoneal dissemination.
Keywords: Liver metastasis, Nek2, pancreas cancer, portal venous port–catheter system, siRNA
Pancreatic cancer is one of the most refractory cancers due to its rapid progression and resistance to chemotherapy.1, 2 Despite progress in early diagnosis and curative surgical treatments for pancreatic cancer, there has been little improvement in the clinical outcomes for patients.2 Novel strategies are required to treat pancreatic cancer, especially for local recurrence, peritoneal dissemination and liver metastases.
Nek2 is a serine‐threonine kinase and the human homolog of the mitotic regulator NIMA of Aspergillus nidulans.3, 4 Nek2 localizes in the centrosome and has a critical role in the splitting of the centrosome and spindle formation in mammalian cells.5 Nek2 overexpression has been identified in various cancers, including cholangiocarcinoma, breast cancer, colorectal cancer, ovarian cancer and leukemia.6 Nek2 is also associated with chromosome instability and aneuploidy in various cancers.7 We previously reported that Nek2 is a novel therapeutic target for cholangiocarcinoma,8 breast cancer9 and colorectal cancer.10 Although siRNA is an effective tool to suppress gene expression, designing a drug delivery system, especially an in vivo delivery system, is an important challenge for clinical application.
In this study, we first examined how Nek2 silencing suppressed tumor growth and prolonged survival time in pancreatic cancer with peritoneal dissemination. Subsequently, the efficiency of the Nek2 siRNA treatment using a venous port–catheter system to treat liver metastasis was investigated to determine whether this system has therapeutic potential as a drug delivery system of siRNA.
Materials and Methods
Patients and samples
Pancreatic cancer tissue and normal pancreatic tissues were obtained from seven patients who underwent pancreaticoduodenectomy at Nagoya University Hospital. An informed consent form, which was approved by the Institutional Review Board at Nagoya University, was obtained from all patients.
Cell lines and cell culture
Seven human pancreatic cancer cell lines (Panc1, MIAPaCa‐2, KLM1, PK45H, PK59, PK8 and PK9) were obtained from the Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer Tohoku University. One human pancreatic cancer cell line, KP4, was obtained from the Cell Bank, RIKEN BioResource Center. The normal human foreskin fibroblast cell line (HFF) was kindly supplied by Dr T. Tsurumi (Aichi Cancer Center, Nagoya, Japan). PK9, PK8, KLM1, PK45H, Panc1, KP4 and PK59 cell lines were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) with 10% heat‐inactivated FBS (Equitech‐Bio, Kerrville, TX, USA) at 37°C in a humidified atmosphere with 5% CO2. MIAPaCa‐2 cell lines and the HFF cell line were cultured in DMEM (Sigma‐Aldrich, St. Louis, MO, USA) with 10% heat‐inactivated FBS at 37°C in a humidified atmosphere with 5% CO2.
Real time RT‐PCR
RNA was extracted from the tissue samples using QIAcube (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. The cDNA was generated from total RNA samples using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA). Real‐time RT‐PCR analysis was performed using a 7300 Fast Real Time PCR System (Applied Biosystems). Each reaction was performed in a 10‐μL reaction mixture containing TaqMan Universal PCR Master Mix according to the manufacturer's instructions (Applied Biosystems). The TaqMan probe and primer for Nek2 (assay identification no. Hs00601227_mH) were purchased from Applied Biosystems, and 18S rRNA (assay identification no. Hs99999901_s1; Applied Biosystems) was used as an internal control. In each experiment, the relative expression of the gene of interest was normalized to the 18S control using standard curves prepared for each gene, and the average values were used for quantification. The average value obtained for normal pancreatic tissue was set as one‐fold induction, and the remaining data were adjusted to that baseline.
Western blot analysis
Whole cell extracts were prepared by Laemmli sample buffer. Cell lysate was electrophoresed on SDS‐polyacrylamide gels, transferred to PVDF membranes (Immobilon; Millipore, Billerica, MA, USA) and probed with antibody. Mouse monoclonal anti‐Nek2 antibody (1:500; BD Biosciences, San Jose, CA, USA) and mouse monoclonal anti‐β‐actin antibody (1:10,000; Sigma‐Aldrich) were applied as primary antibodies. Rabbit anti‐mouse monoclonal IgG HRP conjugated antibody (1:4,000; Biosource, Camarillo, CA, USA) was applied as a secondary antibody. Signals were detected using Pierce Western Blotting Substrate (Thermo, Rockford, IL, USA).
RNA interference
Seven different Nek2 siRNA were designed and chemically synthesized by Sigma Aldrich. The sequences of the strands were Nek2‐1 siRNA, Nek2‐2, Nek2‐3, Nek2‐4, Nek2‐5, Nek2‐6 and Nek2‐7.
Nek2‐1 siRNA; sense: 5′‐GGAAUGCCACAGACGAAGUdTdT‐3′, antisense: 5′‐ACUUCGUCUGGCAUUCC dTdT‐3′, Nek2‐2 siRNA; sense: 5′‐GAGGGCGACAAUUAGGAGAdTdT‐3′, antisense: 5′‐UCUCCUAAUUGUCGCCCUCdTdT‐3′, Nek2‐3 siRNA; sense: 5′‐GGAGGGGAUCUGGCUAGUGdTdT‐3′, antisense: 5′‐CACUAGCCAGAUCCCCUCCdTdT‐3′, Nek2‐4 siRNA; sense: 5′‐CUUUGGGCUAGCUAGAAUAUU‐3′, antisense: 5′‐UAUUCUAGCUAGCCCAAAGUC‐3′, Nek2‐5 siRNA; sense: 5′‐GUGAUUAAUACCAUGACAUCU‐3′, antisense: 5′‐AUGUCAUGGUAUUAAUGACCA‐3′, Nek2‐6 siRNA; sense: 5′‐GAUGCAAUUUGGUCAUUAAUA‐3′, antisense: 5′‐UUAAUGACCAAAUUGCAUCUA‐3′ and Nek2‐7 siRNA; sense: 5′‐CUGAGUGGUAUGCUUACAAUU‐3′, antisense: 5′‐UUGUAAGCAUACCACUCAGUC‐3′.
Control siRNA was used as a control (QIAGEN, Valencia, CA, USA).
Control siRNA; sense: 5′‐UUCUCCGAACGUGUCACGUdTdT‐3′, antisense: 5′‐ACGUGACACGUUCGGAGAAdTdT‐3′.
Cancer cell lines were transfected using Lipotrust (Hokkaido System Science, Hokkaido, Japan) according to the manufacturer's instructions. Seven Nek2 siRNA (Nek2‐1 siRNA, Nek2‐2, Nek2‐3, Nek2‐4, Nek2‐5, Nek2‐6, and Nek2‐7) and control siRNA at 50 nM were transfected into the cells using transfection reagents after 48 h of incubation and 30–40% confluence.
In vitro cell‐proliferation assay
Cell growth was determined using the WST‐1 cell proliferation assay system (Cell Proliferation Reagent WST‐1; Roche, Indianapolis, IN, USA). The cells (2 × 103 cells) were seeded in 96‐well plates after transfection with siRNA. After incubation overnight at 37°C in an atmosphere of 5% CO2, the medium was removed and replaced with fresh medium; this time point was set as 0 h. At the indicated time points (0, 48 h), 10 μL of WST‐1 Reagent was added to each well, and the cells were incubated for 1.5 h. The absorbance of each well was measured at 450 and 630 nm.
In vitro cell‐migration assay
Cell migration was assessed using a scratch wound healing assay. Cells were plated in 6‐well plates. A scratch was made on the cell layer with a micropipette tip. Cells were incubated in medium with 10% serum at 37°C in 5% CO2. Wound healing was evaluated using photographs taken at 48 h. The average distance of the scratch was measured at the six points.
In vitro cell‐invasion assay
Invasion was determined with an invasion assay using modified Boyden chambers (Corning, NY, USA). The membrane of the upper chamber was coated with Matrigel (BD, Franklin Lakes, NJ, USA). A total of 200 μL of medium with 0.1% serum was placed in the upper chamber, and 600 μL of medium without serum was placed in the lower chamber. Cells (1 × 105) were added into the upper chamber and incubated at 37°C in 5% CO2 for 18 h. Invaded cells were stained with HE. The area of the invaded cells was evaluated in five randomly selected fields.
In situ intravital microscopy
Animals were anesthetized with pentobarbital sodium (50 mg/kg body weight, i.p.). As the liver preparation was stabilized on a fluorescent microscope, the liver surface was illuminated with an epifluorescent LED excitation system (Cool LED pE excitation system) using 532–554‐nm excitation and 573–613‐nm emission band‐pass filters to visualize fluorescent rhodamine labeled siRNA in the liver. The images were recorded by a Digital Camera C10600 ORCA‐R2 and processed using Aquacosmos software (Hamamatsu Photonics, Hamamatsu, Japan).
Subcutaneous xenograft mouse model
All animal experiments were handled in compliance with the guidelines of the Institute for Laboratory Animal Research, Nagoya University Graduate School of Medicine. Mice and rats were kept in a temperature and humidity controlled environment under a 12‐h light–dark cycle and had free access to water and food at all times. Male BALB/c nude mice (7 weeks old and weighing 20–25 g) were obtained from SLC Japan (Nagoya, Japan). KLM1 cells (1 × 107) were inoculated into the femoral area of mice. Mice were randomly divided into two groups, and each group consisted of five mice. Nek2‐1 siRNA (50 μmol/L) dissolved in 50 μL of biomaterial collagen (AteloGene; KOKEN, Tokyo, Japan) was administered directly into the tumor once a week for 3 weeks. Control siRNA was used as a control. Tumor growth was assessed by its volume (in mm3), calculated as (L × W 2)/2, where L is tumor length (in mm) and W is tumor width (in mm). After treatment, the thoracic and peritoneal cavities were checked for metastatic or disseminated lesions.
Intraperitoneal xenograft mouse model
KLM1 cells (1 × 107) were injected into the intraperitoneal cavity of mice. Mice were randomly divided into two groups, and each group consisted of three mice. Nek2‐1 siRNA (50 μmol/L) was administered into the peritoneal cavity once a week for 3 weeks. Control siRNA was used as a control. Lipotrust (Hokkaido System Science, Sapporo, Japan) was used for the delivery of siRNA. The number and weight of peritoneal dissemination was assessed and compared between Nek2‐1 siRNA‐treated mice and control siRNA‐treated mice 7 weeks after the KLM1 injection.The survival period was evaluated between Nek2‐1 siRNA‐treated mice and control siRNA‐treated mice.
Liver xenograft rat model
KP4 cells (1 × 107) were injected into the portal vein through the ileocolic vein of male F344/Njcl nude rats (6 weeks old and weighing 200–210 g). An intraportal catheter was inserted into the splenic vein, and a blood access port was implanted subcutaneously after 6 days of inoculation. Rats were randomly divided into two groups, and each group consisted of five rats. Seven days after implantation, Nek2‐1 siRNA or control siRNA were administered to the liver through the blood access port once a day for five consecutive days. Lipotrust (Hokkaido System Science) was used for the delivery of siRNA. The number and areas of metastases were evaluated 14 days after implantation.
Statistical analysis
All data are presented as the mean ± SE. Significant differences were analyzed using Student's t‐test or the Kaplan–Meier method with Statview for Windows (SAS Institute Inc., Cary, NC, USA). A difference is considered statistically significant when P < 0.05.
Results
Nek2 expression in pancreatic cancer tissues and cell lines
Nek2 protein was highly expressed in eight pancreatic cell lines: KLM1, KP4, Panc1, PK45H, PK8, PK9 and MIA Paca2. However, Nek2 protein was not detected in the HFF human fibroblast cell line (Fig. 1a). Nek2 has a splice variant, which consists of Nek2A and Nek2B in mammals.11 Expression of Nek2A and Nek2B were verified in eight pancreatic cell lines. The expression of Nek2 mRNA was significantly higher in pancreatic cancer tissues (3.9 ± 5.2) than in normal tissues (1.0 ± 0.5) (Fig. 1b).
Figure 1.
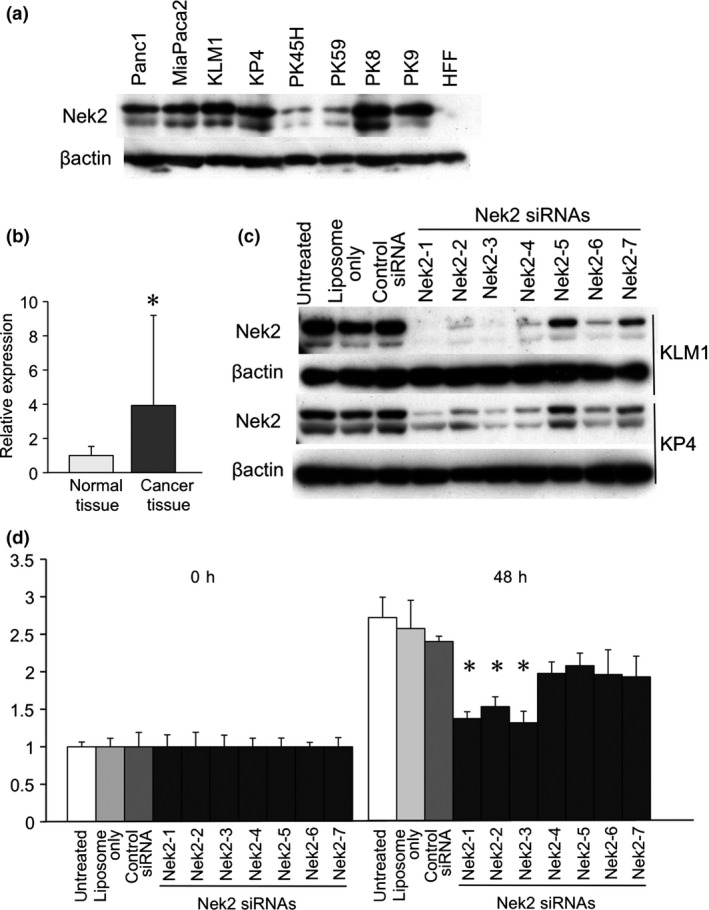
(a) Nek2 expression was examined in pancreatic cancer cell lines (Panc1, Mia Paca2, KLM1, KP4, PK45H, PK59, PK8 and PK9) and human foreskin fibroblast (HFF) cells by western blot analysis. β‐actin was used as an internal loading control. (b) Nek2 expression was analyzed in normal pancreatic tissues and cancer tissues by real time RT‐PCR. The expression levels in pancreatic cancer tissues are shown relative to the levels detected in normal tissues. *Statistically significant (P < 0.05 vs normal tissues by Student's t‐test). (c) Nek2 suppression by Nek2 siRNA (Nek2‐1 siRNA, Nek2‐2, Nek2‐3, Nek2‐4, Nek2‐5, Nek2‐6 and Nek2‐7) was analyzed in KLM‐1 cells (upper) and KP4 cells (lower) by western blot analysis. β‐actin was used as an internal loading control. (d) Cell proliferation was assessed in KLM‐1 cells using the WST‐1 cell proliferation assay. The cells were untreated, liposome only, transfected with control siRNA, or transfected with the Nek2 siRNA (Nek2‐1 siRNA, Nek2‐2, Nek2‐3, Nek2‐4, Nek2‐5, Nek2‐6 and Nek2‐7). The time points were 0 and 48 h after transfection. The data are shown relative to the zero time point. Each point represents the mean of six replicate wells. *Statistically significant (P < 0.05 vs control siRNA by Student's t‐test).
We ablated Nek2 protein using the seven types of Nek2 targeting siRNA in KLM1 and KP4 cells (Fig. 1c). Nek2 siRNA were able to suppress Nek2 protein in these cells. Interestingly, the degree of Nek2 suppression varied widely with each Nek2 siRNA.
The relative proliferation rate in KLM cells treated with Nek2 siRNA were lower than in untreated cells, cells treated with liposome only or cells treated with control siRNA. These data indicate that Nek2‐1 and Nek2‐3 siRNA are more effective in suppressing Nek2 in a pancreatic cell line. Therefore, in subsequent animal studies, we chose Nek2‐1 siRNA for Nek2 silencing (Fig. 1d).
Antitumor effects of Nek2 siRNA in xenograft nude mouse models
To examine the inhibitory effects of Nek2 siRNA on tumor growth in pancreatic cancer, Nek2 siRNA was administered to the tumor in a xenograft nude mouse. As shown, the mean tumor volumes of Nek2 siRNA‐treated mice were significantly lower than those in control siRNA‐treated mice (Fig. 2a).
Figure 2.
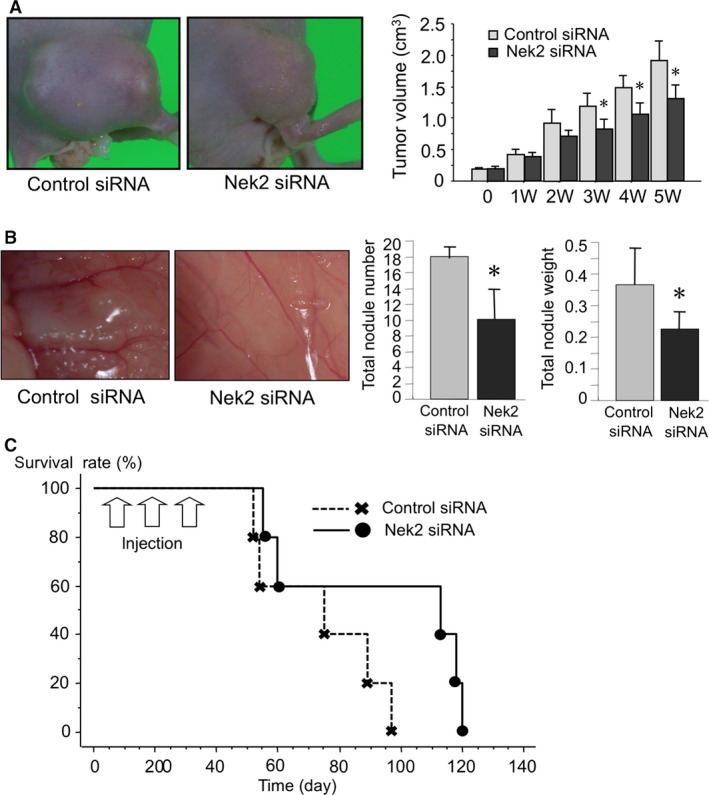
(a) Effect of Nek2 siRNA on tumor growth was examined in a subcutaneous xenograft mouse model. Photographs show the KLM1 xenograft tumor treated with Nek2‐1 siRNA and control siRNA at the end of the experiment. Graphs show the tumor volume of KLM xenograft tumors. Each bar in the graph represents the average tumor volume in each experimental group ± SE. *Statistically significant (P < 0.05 vs control siRNA by Student's t‐test). (b) Effect of intraperitoneal administration of Nek2 siRNA on an intraperitoneal xenograft mouse model. Photographs show peritoneal dissemination after treatment with Nek2‐1 siRNA and control siRNA. Total nodule number and weight were analyzed and compared between Nek2‐1 siRNA and control siRNA treated mice. Each bar in the graph represents the average tumor volume in each experimental group ± SE. *Statistically significant (P < 0.05 vs control siRNA by Student's t‐test). (c) Survival time was compared between Nek2‐1 siRNA and control siRNA treated mice.
Effectiveness of Nek2 siRNA for treating peritoneal dissemination in nude mice
We previously reported that Nek2 siRNA improved survival in nude mice with peritoneal dissemination. To look for a similar effect in peritoneal dissemination in pancreatic cancer, Nek2 siRNA was injected intraperitoneally into this model. The nodules of peritoneal dissemination were diffusely identified in control siRNA‐treated mice (Fig. 2b). A few nodules were identified in the peritoneal cavity of Nek2 siRNA‐treated mice. Total nodule number and weight in the Nek2 siRNA‐treated group was significantly lower than those in the control siRNA‐treated group. The survival time was significantly prolonged in Nek2 siRNA‐treated mice compared to control siRNA‐treated mice (Fig. 2c).
Nek2 siRNA inhibited cell motility and invasion
To determine the effect of Nek2 siRNA on metastasis in pancreatic cancer, a scratch wound healing assay and an invasion assay were analyzed in KP4 cells. In the scratch wound healing assay, Nek2 siRNA significantly decreased the motility of KP4 cells compared to control siRNA and untreated cells (Fig. 3a). In the invasion assay, Nek2 siRNA significantly inhibited the invasion of KP4 cells compared to control siRNA and untreated cells (Fig. 3b).
Figure 3.
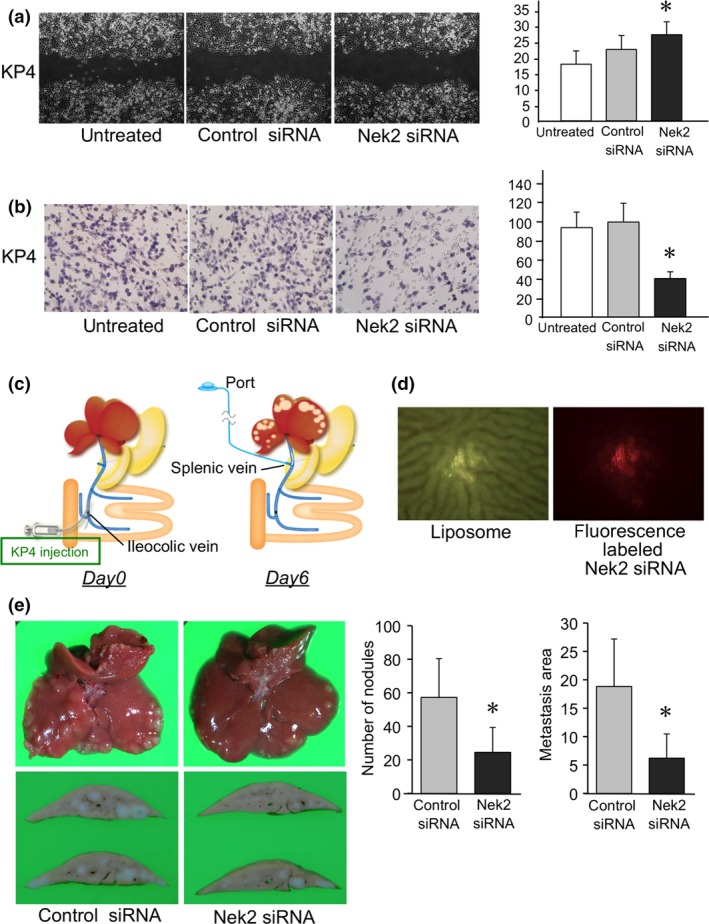
(a) Cell motility was assessed in KP4 cells 48 h after treatment with Nek2‐1 siRNA using a scratch wound healing assay. Graphs show the distance of the scratch at six points. Each bar in the graph represents the average distance in each experimental group ± SE. *Statistically significant (P < 0.05 vs control siRNA by Student's t‐test). (b) Cell invasion was assessed in KP4 cells 18 h after treatment with Nek2‐1 siRNA with an invasion assay using modified Boyden chambers. Invaded cells were stained with HE. Graphs show the area of invaded cells in five randomly selected fields. *Statistically significant (P < 0.05 vs control siRNA by Student's t‐test) (c) Schema demonstrating the procedure for the liver xenograft rat model and portal venous port–catheter system. (d) Photographs show that fluorescently labeled Nek2 siRNA and liposome was injected in the liver using the portal venous port–catheter system. (e) Photographs show liver metastasis after treatment with Nek2‐1 siRNA or control siRNA using the portal venous port–catheter system. Graphs show the number and area of liver metastasis in each group. Each bar in the graph represents the average number and area of liver metastasis in each experimental group ± SE. *Statistically significant (P < 0.05 vs control siRNA by Student's t‐test).
Inhibitory effect of Nek2 siRNA on liver metastasis using a portal venous port–catheter system
To examine the potential of Nek2 siRNA for preventing metastasis in pancreatic cancer, Nek2 siRNA was administered to the liver metastasis of a xenograft rat model using a portal venous port–catheter system (Fig. 3c). KP4 cells grew into visible metastatic tumors in rat livers 1 week after inoculation. Fluorescence‐labeled siRNA, which was injected using a portal venous port–catheter system, was identified in the liver (Fig. 3d). Nek2 siRNA or control siRNA was administered to these liver metastases five times for a week using this system. The photographs show the liver metastasis after Nek2 siRNA treatment (Fig. 3e). The number and the area of the liver metastasis decreased after Nek2 siRNA treatment compared with control siRNA treatment. In addition, lung metastasis and peritoneal dissemination were not identified in any of the rats at the end of the experiments.
Discussion
We previously reported the efficiency of Nek2 as a molecular therapeutic target in several cancers, such as cholangiocarcinoma, breast cancer and colon cancer. Nek2 expression in pancreatic cancer is similar to that in these cancers. In this paper, we studied the efficiency of Nek2 siRNA in xenograft mouse and rat models with pancreatic cancer. Nek2 silencing suppressed tumor growth and prolonged survival time in peritoneal dissemination. Furthermore, Nek2 siRNA decreased the number and the area of liver metastases.
Nek2 in mammals has two types of splice variant, Nek2A and Nek2B.12 Nek2A plays a role in centrosomal splitting and bipolar spindle formation during mitosis.13 Nek2B leads to a mitotic delay in the majority of cells.14 Our data did not clarify which variant, Nek2A or Nek2B, is important. Nek2 siRNA can suppress the expression of both Nek2A and Nek2B. Nek2 is considered to be an effective target for pancreatic cancer therapy.
siRNA is a beneficial tool to inhibit the expression of specific genes. However, developing a drug delivery system for siRNA is an important challenge for future clinical applications. We previously reported the efficiency of local administration using biocollagen as a drug delivery system for siRNA.8 Many pancreatic cancer patients die of peritoneal dissemination even after surgical resection.15 Early peritoneal dissemination of undetectable cancer cells is considered to be the main reason for the dismal outcome.16 The local administration of siRNA has a limitation in early peritoneal dissemination because it is impossible to treat undetectable and numerous tumor cells. Although several groups have reported using viral vectors, such as adenoviral and retroviral vectors as drug delivery systems for siRNA, these vectors may cause severe side effects in clinical use.17, 18 In contrast, liposome has often been used as a delivery carrier for nucleic acids, including siRNA.19 We applied liposome in peritoneal dissemination as a delivery carrier for Nek2 siRNA.8 Liposome had an efficient transfection rate in the peritoneal dissemination of pancreatic cancer. Liposome was considered to be an adequate delivery carrier in peritoneal dissemination because the intraperitoneal cavity is a confined space.
The high invasiveness and motility in pancreatic cancer has been associated with various genes.20, 21 In this study, we were unable to identify the novel genes, affect phenotype, including proliferation, migration and invasion in pancreas cancer.
We previously reported that Nek2 siRNA could affect invasion and motility in breast cancer cells.9 Nek2 siRNA also inhibited invasion and motility in pancreatic cancer cells. To examine the transfection rate of Nek2 siRNA to the liver, fluorescence‐labeled Nek2 siRNA was injected into the rat penile vein using liposome. However, the fluorescence‐labeled Nek2 siRNA was not identified in the liver after 1 h of administration. The delivery of siRNA in liver metastasis was more difficult than in peritoneal dissemination. Another method was required as a drug delivery system of siRNA to liver metastasis. We focused on using a venous port–catheter system as a drug delivery system for siRNA. The venous port–catheter system has already been applied in the clinic.22 Anticancer drugs are administered directly into the liver metastasis. The venous port–catheter system is less invasive than a surgical operation. The mixture of fluorescence‐labeled Nek2 siRNA and liposome was administered using the venous port–catheter system in the portal vein. In this procedure, fluorescence‐labeled Nek2 siRNA was identified in the liver 1 h after administration. Nek2 siRNA was able to prevent the progression of liver metastasis efficiently. There were no complications related to the portal venous port–catheter system. Our results showed that this procedure is effective in liver metastasis as a drug delivery system for siRNA.
Taken together, our data show that an adequate delivery system is important to treat advanced pancreatic cancer, including peritoneal dissemination and liver metastasis. Further investigation is required regarding the safety and side effects of the portal venous port–catheter system. Nek2 silencing was able to prevent the progression of peritoneal dissemination and liver metastasis in xenograft models. We hope that Nek2 siRNA will be a novel therapeutic strategy for pancreatic cancer with liver metastasis and peritoneal dissemination.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
We thank members and staff of the Hamaguchi Laboratory for excellent technical assistance and helpful suggestions regarding the experiments.
Cancer Sci 107 (2016) 1315–1320
Funding Information
No sources of funding were declared in this study.
References
- 1. Okusaka T, Kosuge T. Systemic chemotherapy for pancreatic cancer. Pancreas 2004; 28: 301–4. [DOI] [PubMed] [Google Scholar]
- 2. Beger HG, Rau B, Gansauge F, Poch B, Link KH. Treatment of pancreatic cancer: challenge of the facts. World J Surg 2003; 27: 1075–84. [DOI] [PubMed] [Google Scholar]
- 3. Schultz SJ, Fry AM, Sutterlin C, Ried T, Nigg EA. Cell cycle‐dependent expression of Nek2, a novel human protein kinase related to the NIMA mitotic regulator of Aspergillus nidulans. Cell Growth Differ 1994; 5: 625–35. [PubMed] [Google Scholar]
- 4. O'Connell MJ, Krien MJ, Hunter T. Never say never. The NIMA‐related protein kinases in mitotic control. Trends Cell Biol 2003; 13: 221–8. [DOI] [PubMed] [Google Scholar]
- 5. Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J 1998; 17: 470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayward DG, Clarke RB, Faragher AJ, Pillai MR, Hagan IM, Fry AM. The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res 2004; 64: 7370–6. [DOI] [PubMed] [Google Scholar]
- 7. Hayward DG, Fry AM. Nek2 kinase in chromosome instability and cancer. Cancer Lett 2006; 237: 155–66. [DOI] [PubMed] [Google Scholar]
- 8. Kokuryo T, Senga T, Yokoyama Y, Nagino M, Nimura Y, Hamaguchi M. Nek2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma. Cancer Res 2007; 67: 9637–42. [DOI] [PubMed] [Google Scholar]
- 9. Tsunoda N, Kokuryo T, Oda K et al Nek2 as a novel molecular target for the treatment of breast carcinoma. Cancer Sci 2009; 100: 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki K, Kokuryo T, Senga T, Yokoyama Y, Nagino M, Hamaguchi M. Novel combination treatment for colorectal cancer using Nek2 siRNA and cisplatin. Cancer Sci 2010; 101: 1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hames RS, Fry AM. Alternative splice variants of the human centrosome kinase Nek2 exhibit distinct patterns of expression in mitosis. Biochem J 2002; 361: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uto K, Nakajo N, Sagata N. Two structural variants of Nek2 kinase, termed Nek2A and Nek2B, are differentially expressed in Xenopus tissues and development. Dev Biol 1999; 208: 456–64. [DOI] [PubMed] [Google Scholar]
- 13. Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol Biol Cell 2003; 14: 2876–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fletcher L, Cerniglia GJ, Yen TJ, Muschel RJ. Live cell imaging reveals distinct roles in cell cycle regulation for Nek2A and Nek2B. Biochim Biophys Acta 2005; 1744: 89–92. [DOI] [PubMed] [Google Scholar]
- 15. Weden S, Klemp M, Gladhaug IP et al Long‐term follow‐up of patients with resected pancreatic cancer following vaccination against mutant K‐ras. Int J Cancer 2011; 128: 1120–8. [DOI] [PubMed] [Google Scholar]
- 16. Bogoevski D, Strate T, Yekebas EF, Izbicki JR. Pancreatic cancer: a generalized disease–prognostic impact of cancer cell dissemination. Langenbecks Arch Surg 2008; 393: 911–7. [DOI] [PubMed] [Google Scholar]
- 17. Giering JC, Grimm D, Storm TA, Kay MA. Expression of shRNA from a tissue‐specific pol II promoter is an effective and safe RNAi therapeutic. Mol Ther 2008; 16: 1630–6. [DOI] [PubMed] [Google Scholar]
- 18. Chen M, Payne WS, Dunn JR et al Retroviral delivery of RNA interference against Marek's disease virus in vivo . Poult Sci 2009; 88: 1373–80. [DOI] [PubMed] [Google Scholar]
- 19. Shim MS, Kwon YJ. Efficient and targeted delivery of siRNA in vivo . FEBS J 2010; 277: 4814–27. [DOI] [PubMed] [Google Scholar]
- 20. He X, Zheng Z, Li J et al DJ‐1 promotes invasion and metastasis of pancreatic cancer cells by activating SRC/ERK/uPA. Carcinogenesis 2012; 33: 555–62. [DOI] [PubMed] [Google Scholar]
- 21. Dumartin L, Whiteman HJ, Weeks ME et al AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res 2011; 71: 7091–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Darwish AA, Sokal E, Stephenne X, Najimi M, de Goyet Jde V, Reding R. Permanent access to the portal system for cellular transplantation using an implantable port device. Liver Transpl 2004; 10: 1213–5. [DOI] [PubMed] [Google Scholar]


