Abstract
Tumor budding has been defined as an isolated single cancer cell or a cluster composed of fewer than five cancer cells scattered in the stroma. It is a strong predictor for lymph node metastasis in T1 colorectal cancer. We introduced this concept to T1 non‐muscle invasive bladder cancer and evaluated whether tumor budding could have a prognostic impact on the clinical outcome. We identified 121 consecutive patients with newly diagnosed T1 bladder cancer between 1994 and 2014 at Keio University Hospital. All slides were re‐reviewed by a dedicated uropathologist. Budding foci were counted under ×200 magnification. When the number of budding foci was 10 or more, tumor budding was defined as positive. The relationship between tumor budding and clinical outcomes was assessed using a multivariate analysis. The median follow‐up was 52 months. Tumor budding was positive in 21 patients (17.4%). Tumor budding was significantly associated with T1 substaging, tumor architecture and lymphovascular invasion. The 5‐year progression‐free survival rate in T1 bladder cancer patients with tumor budding was 53.8%, which was significantly lower than that in patients without tumor budding (88.4%, P = 0.001). A multivariate Cox regression analysis revealed that tumor budding was independently associated with stage progression (P = 0.002, hazard ratio = 4.90). In a subgroup of patients treated with bacillus Calmette‐Guérin instillation (n = 88), tumor budding was also independently associated with stage progression (P = 0.003, hazard ratio = 5.65). Tumor budding may be a novel indicator for predicting stage progression in T1 bladder cancer, and would likely be easily introduced in clinical practice.
Keywords: Disease progression, pathology, recurrence, transitional cell, urinary bladder neoplasms
Tumor recurrence is common among patients with T1 non‐muscle invasive bladder cancer (NMIBC) and the risk of stage progression is also high.1 Despite adjuvant bacillus Calmette–Guérin (BCG) therapy following transurethral resection of bladder tumor (TURBT), recurrence and progression to muscle invasive tumors occurs in 45–62% and 17–23% of patients, respectively.2, 3 Immediate total cystectomy for T1 NMIBC is one of the therapeutic options available for these patients.4 Although the 10‐year disease‐specific survival rate of immediate cystectomy is favorable (approximately 80%),5, 6 cystectomy for all patients with T1 NMIBC is regarded as an overtreatment. Total cystectomy also reduces the quality of life of patients and is associated with a wide variety of complications.7 The most important factor in the management of T1 NMIBC is the identification of patients at very high risk of progression and cancer death. Several prognostic factors that predict poor outcomes in patients with NMIBC have been identified to date: sex, age, tumor diameter, multifocality, concomitant carcinoma in situ (CIS), CIS in the prostatic urethra, and lymphovascular invasion (LVI).8 However, these factors are not the only critical indicators. Therefore, we have focused on tumor budding, which is regarded as an important prognostic factor in patients with colorectal cancer.9, 10, 11, 12, 13, 14, 15, 16, 17 Tumor budding has been defined as an isolated single cancer cell or a cluster composed of fewer than five cancer cells scattered in the stroma at the invasive tumor margin (Fig. 1),16 reflects the detachment of tumor cells, and is associated with epithelial–mesenchymal transition (EMT).13, 18, 19 In 1993, Hase et al.9 reported that tumor budding, which was defined as small clusters of undifferentiated cancer cells ahead of the invasive front of a lesion, correlated with recurrence and survival rates. Many studies have since considered tumor budding to be a poor prognostic factor following surgical resection of colorectal cancer.9, 10, 11, 12, 13, 17 Furthermore, tumor budding and lymph node metastasis are closely related in T1 colorectal cancer.14, 15, 16 Therefore, current guidelines recommend surgical resection and lymphadenectomy following endoscopic mucosal resection when tumor budding is positive in T1 colorectal cancer.20 In the present study, we introduced this concept to T1 NMIBC and investigated whether tumor budding had a prognostic impact on T1 NMIBC clinical outcomes.
Figure 1.
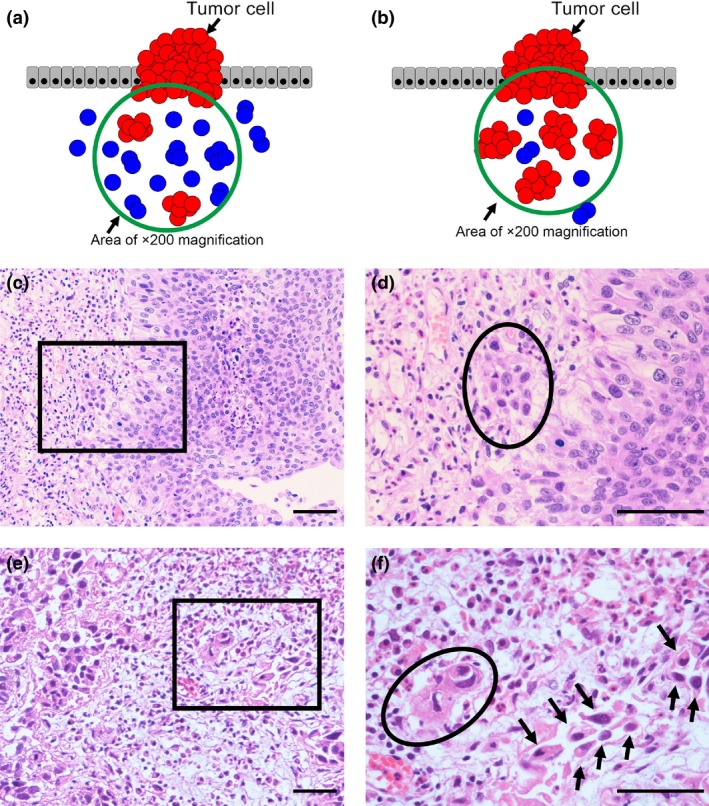
(a) Schema of positive tumor budding. Blue cells indicate budding foci that consist of an isolated single cancer cell or a cluster composed of fewer than five cancer cells. The green circle has 10 or more budding foci, indicating positive tumor budding. (b) Schema of negative tumor budding. Blue cells indicate budding foci that consist of an isolated single cancer cell or a cluster composed of fewer than five cancer cells. The green circle has fewer than 10 budding foci, indicating negative tumor budding. (c–f) H&E staining of tumor budding‐negative and ‐positive cases. Scale bar = 50 μm. (c) A tumor budding‐negative case (×200) and (d) a tumor budding‐negative case (the black square lesion in (c) was set to ×400). The black circle indicates a cluster of cancer cells inside the circle that was composed of more than five cells. (e) A tumor budding‐positive case (×200) and (f) a tumor budding‐positive case (the black square lesion in (e) was set to ×400). There were 10 or more budding foci, which were isolated single cancer cells (allows) or a cluster composed of fewer than five cancer cells (black circle).
Materials and Methods
Patient selection
A total of 691 patients newly diagnosed with NMIBC were treated between January 1994 and August 2014 at Keio University Hospital. During this period, 173 patients were newly diagnosed with T1 NMIBC. T staging was pathologically defined from the results of TURBT specimens. We excluded patients who had undergone TURBT at other institutions (15 patients) and patients with pure non‐urothelial carcinoma histology (e.g. squamous cell carcinoma or adenocarcinoma, four patients). Patients with a history of upper tract urothelial carcinoma and a follow‐up period of <6 months were also excluded (23 and five patients, respectively). We also excluded five patients who had been treated with total cystectomy without confirmed stage progression. Three cases were immediate total cystectomy, which meant that T1 NMIBC patients received total cystectomy soon after TURBT without further intravesical therapy. The other 2 cases were early total cystectomy, which meant that T1 NMIBC patients received total cystectomy after having tumor recurrence but before the occurrence of stage progression. Therefore, 121 cases were included in the analysis.
Specimen evaluation
Tumor budding was evaluated based on HE staining. Tumor budding has been defined as an isolated single cancer cell or a cluster composed of fewer than five cancer cells scattered in the stroma at the invasive tumor margin. After selecting one field in which tumor budding was the most intensive, budding foci were counted under ×200 magnification. When the number of budding foci was 10 or more, tumor budding was defined as positive (Fig. 1). All cases were re‐reviewed by a dedicated uropathologist who was unaware of the prognoses of the cases. Tumor budding, grade, T stage, concomitant CIS, and LVI status were evaluated. We also evaluated pT substaging (microinvasive vs extensive invasive).21
Treatments
We performed intravesical BCG therapy for intermediate or high‐risk NMIBC according to current clinical guidelines.8 However, due to side effects, the attending physicians and/or patients decided against BCG instillation in some cases. BCG instillation was initiated 4–5 weeks after TURBT and continued weekly for 6–8 weeks at a dose of 80 mg (Tokyo 172 strain) or 81 mg (Connaught strain). Patients were followed postoperatively with cystoscopy and urinary cytology every 3 months for 2 years, every 6 months for the next 3 years, and then annually thereafter. Excretory urograms and/or computed tomography were used to evaluate the upper urinary tract every year for 5 years after the treatment.
Statistical analysis
The variables of different groups were compared using the χ2‐test. The recurrence‐free survival rate and the progression‐free survival rate were estimated using the Kaplan–Meier method. Survival curves were compared using the log‐rank test. A multivariate analysis for tumor recurrence and stage progression was performed using the Cox proportional hazards model with stepwise forward regression. Independent variables included in the survival analysis were patient age (<70 vs ≥70 years), sex, tumor grade (low grade vs high grade), presence or absence of concomitant CIS, multifocality, whether intravesical BCG therapy was performed, whether intravesical chemotherapy was performed, the presence or absence of a history of Ta NMIBC, tumor size, T1 substaging, tumor architecture, tumor appearance, status of LVI, and tumor budding. Differences among groups were regarded as significant when P < 0.05. These analyses were performed with the SPSS v. 22.0 statistical software package (IBM, Somers, NY, USA).
Endpoints
We defined tumor recurrence as any evidence of disease on follow‐up evaluations. Stage progression was defined as muscle invasion or metastases. Recurrence‐free survival was calculated as the time between TURBT and the date of tumor recurrence. Progression‐free survival was determined from the date of TURBT to stage progression.
Clinical Trial Registeration Information
This study was conducted subject to the guidelines of the Declaration of Helsinki and approved by Keio university hospitals ethical committee. The reference number is 20130101.
Results
Clinicopathological characteristics in overall 121 patients
The median follow‐up period was 52 months (interquartile range, 29–85) and the median patient age was 71.8 years (interquartile range, 64–79). Tumor budding was positive in 21 out of 121 patients (17.4%). Table 1 shows the relationship between clinicopathological parameters and the tumor budding status in overall patients. Tumor budding was significantly associated with T1 substaging, tumor architecture and LVI status (P = 0.002, 0.023 and 0.001, respectively). Disease recurrence was noted in 50 patients (41.3%), while stage progression was detected in 16 patients (13.2%). One of the 16 patients with stage progression exhibited distant metastasis. Eight patients died of the disease.
Table 1.
Clinicopathological parameters in overall 121 patients according to the tumor budding status
| Characteristics | Total (%) | Tumor budding (%) | P‐value | |
|---|---|---|---|---|
| Positive | Negative | |||
| Number of patients | 121 | 21 | 100 | |
| Age | ||||
| <70 years | 55 (45.5) | 9 (42.9) | 46 (46) | 0.793 |
| ≥70 years | 66 (54.5) | 12 (57.1) | 54 (54) | |
| Sex | ||||
| Male | 102 (84.3) | 18 (85.7) | 84 (84) | 0.573 |
| Female | 19 (15.7) | 3 (14.3) | 16 (16) | |
| Tumor grade | ||||
| Low | 6 (5.0) | 0 (0) | 6 (6) | 0.379 |
| High | 115 (95.0) | 21 (100) | 94 (94) | |
| Concomitant CIS | ||||
| Positive | 28 (23.1) | 4 (19) | 24 (24) | 0.432 |
| Negative | 93 (76.9) | 17 (81) | 76 (76) | |
| Multifocality | ||||
| Multiple | 87 (71.9) | 12 (57.1) | 75 (75) | 0.098 |
| Solitary | 34 (28.1) | 9 (42.9) | 25 (25) | |
| BCG instillation | ||||
| Yes | 88 (72.7) | 16 (76.2) | 72 (72) | 0.695 |
| No | 33 (27.3) | 5 (23.8) | 28 (28) | |
| Intravesical chemotherapy | ||||
| Yes | 16 (13.2) | 2 (9.5) | 14 (14) | 0.446 |
| No | 105 (86.8) | 19 (90.5) | 86 (86) | |
| History of Ta NMIBC | ||||
| Yes | 7 (5.8) | 1 (4.8) | 6 (6) | 0.650 |
| No | 114 (94.2) | 20 (95.2) | 94 (94) | |
| Tumor size | ||||
| <30 mm | 91 (75.2) | 12 (57.1) | 79 (79) | 0.121 |
| ≥30 mm | 17 (14.0) | 5 (23.8) | 12 (12) | |
| Unknown | 13 (10.7) | 4 (19.0) | 9 (9) | |
| T1 substaging | ||||
| Microinvasive | 26 (21.5) | 0 (0) | 26 (26) | 0.002 |
| Extensive invasive | 86 (71.1) | 21 (100) | 65 (65) | |
| Unknown | 9 (7.4) | 0 (0) | 9 (9) | |
| Tumor architecture | ||||
| Papillary | 82 (67.8) | 11 (52.4) | 71 (71) | 0.023 |
| Nodular | 21 (17.4) | 8 (38.1) | 13 (13) | |
| Unclassified | 18 (14.9) | 2 (9.5) | 16 (16) | |
| Tumor appearance | ||||
| Pedunculated | 31 (25.6) | 3 (14.3) | 28 (28) | 0.132 |
| Broad base or flat | 72 (59.5) | 16 (76.2) | 56 (56) | |
| Unclassified | 18 (14.9) | 2 (9.5) | 16 (16) | |
| Lymphovascular invasion | ||||
| Positive | 30 (24.8) | 11 (52.4) | 19 (19) | 0.001 |
| Negative | 91 (75.2) | 10 (47.6) | 81 (81) | |
| Recurrence | 50 (41.3) | 12 (57.1) | 38 (38) | |
| Progression | 16 (13.2) | 7 (33.3) | 9 (9) | |
| Cancer death | 8 (6.6) | 5 (23.8) | 3 (3) | |
BCG, bacillus Calmette‐Guérin; CIS, carcinoma in situ; NMIBC, non‐muscle invasive bladder cancer.
Predictors for tumor recurrence and stage progression in overall patients
We performed univariate and multivariate Cox regression analyses to identify the predictors of subsequent tumor recurrence and stage progression (Table 2). The univariate analysis revealed that BCG instillation (P = 0.002), intravesical chemotherapy (P = 0.012) and tumor size (P = 0.015) correlated with tumor recurrence. The multivariate analysis showed that BCG instillation was an independent risk factor for subsequent disease recurrence (P = 0.002, hazard ratio (HR) =0.39).
Table 2.
Univariate and multivariate analyses for tumor recurrence and stage progression in overall cases
| Characteristics | Recurrence‐free survival | Progression‐free survival | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| P‐value | HR (95% CI) | P‐value | P‐value | HR (95% CI) | P‐value | |
| Age | ||||||
| <70 years | 0.157 | 0.845 | ||||
| ≥70 years | ||||||
| Sex | ||||||
| Male | 0.280 | 0.867 | ||||
| Female | ||||||
| Tumor grade | ||||||
| Low | 0.659 | 0.361 | ||||
| High | ||||||
| Concomitant CIS | ||||||
| Positive | 0.788 | 0.876 | ||||
| Negative | ||||||
| Multifocality | ||||||
| Single | 0.137 | 0.326 | ||||
| Multiple | ||||||
| BCG instillation | ||||||
| Yes | 0.002 | 0.39 (0.21–0.71) | 0.002 | 0.971 | ||
| No | ||||||
| Intravesical chemotherapy | ||||||
| Yes | 0.012 | 0.613 | ||||
| No | ||||||
| History of Ta NMIBC | ||||||
| Yes | 0.772 | 0.215 | ||||
| No | ||||||
| Tumor size | ||||||
| <30 mm | 0.015 | 0.850 | ||||
| ≥30 mm | ||||||
| T1 substaging | ||||||
| Microinvasive | 0.995 | 0.804 | ||||
| Extensive invasive | ||||||
| Tumor architecture | ||||||
| Papillary | 0.395 | 0.871 | ||||
| Nodular | ||||||
| Tumor appearance | ||||||
| Pedunculated | 0.295 | 0.818 | ||||
| Broad base or flat | ||||||
| Lymphovascular invasion | ||||||
| Positive | 0.155 | 0.003 | ||||
| Negative | ||||||
| Tumor budding | ||||||
| Positive | 0.068 | 0.001 | 4.90 (1.81–13.33) | 0.002 | ||
| Negative | ||||||
BCG, bacillus Calmette‐Guérin; CI, confidence interval; CIS, carcinoma in situ; HR, hazard ratio; NMIBC, non‐muscle invasive bladder cancer.
Furthermore, LVI and tumor budding were significant risk factors for stage progression in the univariate analysis (P = 0.003 and 0.001, respectively). Kaplan–Meier curves showed that the 5‐year progression‐free survival rate was 53.8% in tumor budding‐positive patients and 88.4% in tumor budding‐negative patients (Fig. 2a). The multivariate analysis identified tumor budding as the only significant risk factor for stage progression (P = 0.002, HR = 4.90).
Figure 2.
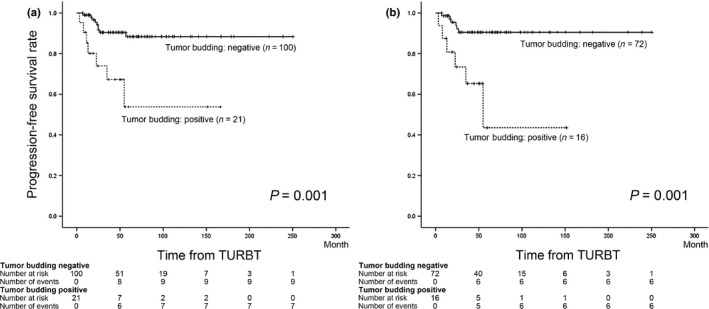
(a) Progression‐free survival rate according to the tumor budding status in all cases. (b) Progression‐free survival rate according to the tumor budding status in cases treated with bacillus Calmette–Guérin instillation.
Predictors of tumor recurrence and stage progression in patients treated with bacillus Calmette–Guérin instillation
A subgroup analysis of the 88 patients treated with BCG instillation was performed. We determined whether tumor budding had a prognostic impact. Sixteen cases (18.2%) were judged to be tumor budding positive. Tumor budding was significantly associated with T1 substaging, tumor architecture and LVI status (P = 0.017, 0.048 and 0.001, respectively). Disease recurrence was observed in 30 patients (34.1%), while stage progression was noted in 12 (13.6%). LVI was identified as an independent prognostic factor for tumor recurrence in the univariate analysis (P = 0.038) and multivariate analysis (P = 0.042, HR = 2.11, Table 3).
Table 3.
Univariate and multivariate analyses for tumor recurrence and stage progression in cases treated with BCG instillation
| Characteristics | Recurrence‐free survival | Progression‐free survival | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| P‐value | HR (95% CI) | P‐value | P‐value | HR (95% CI) | P‐value | |
| Age | ||||||
| <70 years | 0.086 | 0.598 | ||||
| ≥70 years | ||||||
| Sex | ||||||
| Male | 0.751 | 0.499 | ||||
| Female | ||||||
| Tumor grade | ||||||
| Low | 0.771 | 0.455 | ||||
| High | ||||||
| Concomitant CIS | ||||||
| Positive | 0.499 | 0.967 | ||||
| Negative | ||||||
| Multifocality | ||||||
| Single | 0.113 | 0.235 | ||||
| Multiple | ||||||
| History of Ta NMIBC | ||||||
| Yes | 0.695 | 0.066 | ||||
| No | ||||||
| Tumor size | ||||||
| <30 mm | 0.074 | 0.864 | ||||
| ≥30 mm | ||||||
| T1 substaging | ||||||
| Microinvasive | 0.804 | 0.913 | ||||
| Extensive invasive | ||||||
| Tumor architecture | ||||||
| Papillary | 0.224 | 0.783 | ||||
| Nodular | ||||||
| Tumor appearance | ||||||
| Pedunculated | 0.218 | 0.423 | ||||
| Broad base or flat | ||||||
| Lymphovascular invasion | ||||||
| Positive | 0.038 | 2.11 (1.03–4.35) | 0.042 | 0.014 | ||
| Negative | ||||||
| Tumor budding | ||||||
| Positive | 0.094 | 0.001 | 5.65 (1.80–17.54) | 0.003 | ||
| Negative | ||||||
BCG, bacillus Calmette‐Guérin; CI, confidence interval; CIS, carcinoma in situ; HR, hazard ratio; NMIBC, non‐muscle invasive bladder cancer.
The univariate analysis revealed that LVI and tumor budding were significant risk factors for stage progression (P = 0.014 and P = 0.001, respectively). Kaplan–Meier curves showed that the 5‐year progression‐free survival rate was 43.5% in tumor budding‐positive patients and 90.5% in the tumor budding‐negative patients (Fig. 2b). In the multivariate analysis, tumor budding was independently associated with stage progression in T1 NMIBC patients treated with BCG instillation (P = 0.003, HR = 5.65).
Of the 12 patients who developed stage progression, six underwent total cystectomy, 3 were treated with chemoradiation and 1 was treated with chemotherapy. The remaining two patients received no treatment as per their request. Six patients died of the disease, as did 4 out of 16 tumor budding‐positive patients (25%) and 2 out of 72 tumor budding‐negative patients (2.8%).
Discussion
Tumor budding has been identified as a predictor of a poor outcome in many carcinomas: colon,9, 10, 11, 12, 13, 14, 15, 16, 17 lung,22 breast,23 pancreatic24 and oral25 carcinomas. However, to the best of our knowledge, its prognostic impact on urothelial carcinoma has not yet been determined. Therefore, this is the first study to identify tumor budding as an independent predictor for stage progression in T1 NMIBC.
Tumor budding is strongly associated with EMT, which is characterized by losing cell‐to‐cell interaction and cell adhesion, resulting in acquired migratory capacity, resistance to apoptosis, and invasiveness as well as increased production of extracellular matrix components.26 E‐cadherin, which is one of the strong markers of epithelial cells,26 decreases through the EMT process. A previous study demonstrated that the E‐cadherin expression observed in the tumor center had disappeared in tumor budding sites.19 We also evaluated E‐cadherin expression in tumor centers and tumor budding sites separately in cases that were tumor budding positive. In the cases that were tumor budding positive (N = 21), E‐cadherin expression was observed in the tumor center in 18 (86%) cases and in the tumor budding site in 6 (29%) cases. These findings seem to indicate that tumor budding is associated with EMT in T1 bladder cancers.
In colorectal cancer, tumor budding has been associated with other factors, including advanced T stage,12 N stage,11, 12 tumor grade,10 LVI10, 11, 12, 17 and perineural invasion.11 In addition, tumor budding has been linked to disease‐free survival10, 11, 12 and cancer‐specific survival.10, 17 The prognosis of T1 colorectal cancer is excellent, with most patients being successfully managed with endoscopic therapy.27 However, lymph node metastasis has been reported in 11–14% of patients with T1 colorectal cancer, with this rate being significantly higher in cases of positive tumor budding.14, 15, 16 Based on these findings, current guidelines recommend surgical resection and lymphadenectomy for T1 colorectal cancer after endoscopic therapy when tumor budding is positive.20 Therefore, we speculated that tumor budding also had an important clinical impact on T1 NMIBC.
In the clinical treatment of T1 NMIBC, clinicians have yet to agree on whether aggressive therapy such as immediate total cystectomy is needed. Many clinical and biological parameters identify aggressive T1 tumors.8 However, clinicians lack a crucial parameter for detecting T1 NMIBC with high potential for stage progression. In the present study, we introduced the concept of tumor budding as a possible tool for identifying poor clinical outcomes in patients with T1 NMIBC, and found that tumor budding was an independent risk factor for stage progression in patients with T1 NMIBC as well as in a subgroup of patients treated with BCG instillation. The estimation of tumor budding may provide new practical information for selecting populations with a highly malignant potential and planning an appropriate management strategy for T1 NMIBC.
Urothelial carcinoma sometimes shows a variety of invasive patterns histologically. Tumor budding might not be a homogeneously distributed feature along the invasive front. Inter‐observer bias in evaluating tumor budding will be expected in such heterogeneous tumors. To reduce the bias, the critical point at the diagnosis of tumor budding is to select the area where the tumor budding is most intensive. We used the definition of tumor budding from a previous study by Ueno et al.,16, 28, 29 the counting method of which is widely used for colorectal cancer.10, 11, 12, 13, 14, 15, 16, 17 In a previous colorectal cancer study, an inter‐observer study showed a κ‐value of 0.69–0.84 by using this definition,10, 29 indicating good to excellent agreement and the effects of intra‐observer bias being very minimal. Therefore, a simple evaluation of tumor budding by HE staining may provide the prognostic information needed to predict a poorer outcome with strong agreement and without the need for expensive and time‐consuming immunohistochemical staining not only in patients with T1 colorectal cancer, but also in those with T1 NMIBC.
Our study has several limitations. Several treatments are available after TURBT: BCG instillation, intravesical chemotherapy and surveillance. Therefore, we performed a subgroup analysis of 88 patients treated with BCG instillation after diagnosis of T1 NMIBC and showed the prognostic impact of tumor budding in this population. Because we also included cases from 1994 onwards, not all patients were treated with second TURBT and, in our institution, maintenance BCG was not routinely performed. The reasons for this were that the optimal schedule of maintenance BCG has not yet been established and its effects are currently being debated30 and the continuation rate of the maintenance BCG was low due to side effects. In addition, direct damage to the mucosa and stroma surrounding the bladder cancer by the thermal degeneration of TURBT may affect strict evaluations of tumor budding in T1 NMIBC. However, at least in our study population, the dedicated uropathologist easily estimated tumor budding in all specimens.
Although further studies are needed in a larger series, we consider tumor budding to be an important and essential pathological indicator for predicting subsequent stage progression in T1 NMIBC. In addition, tumor budding would likely be easily introduced in clinical practice because of its strong agreement and no need for expensive and time‐consuming immunohistochemical staining.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
This work was supported by a Keio University Grant‐in‐Aid for Encouragement of Young Medical Scientists (Grant Number 02‐002‐0027) and the Japan Society for the Promotion of Science KAKENHI Grant Number 15K20107.
Cancer Sci 107 (2016) 1338–1344
Funding Information
Keio University Grant‐in‐Aid for Encouragement of Young Medical Scientists, (Grant/Award Number: ‘02‐002‐0027’) Japan Society for the Promotion of Science KAKENHI, (Grant/Award Number: ‘15K20107’).
Clinical Trial Registration Information: This study was conducted subject to the guidelines of the Declaration of Helsinki and approved by Keio university hospitals ethical committee. The reference number is 20130101.
References
- 1. Tanaka N, Kikuchi E, Matsumoto K, Miyajima A, Nakagawa K, Oya M. Frequency of tumor recurrence: a strong predictor of stage progression in initially diagnosed nonmuscle invasive bladder cancer. J Urol 2011; 185: 450–5. [DOI] [PubMed] [Google Scholar]
- 2. Hemdan T, Johansson R, Jahnson S et al 5‐Year outcome of a randomized prospective study comparing bacillus Calmette‐Guerin with epirubicin and interferon‐alpha2b in patients with T1 bladder cancer. J Urol 2014; 191: 1244–9. [DOI] [PubMed] [Google Scholar]
- 3. Palou J, Sylvester RJ, Faba OR et al Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease‐specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette‐Guerin. Eur Urol 2012; 62: 118–25. [DOI] [PubMed] [Google Scholar]
- 4. Lebret T, Neuzillet Y. Indication and timing of cystectomy in high‐risk bladder cancer. Curr Opin Urol 2012; 22: 427–31. [DOI] [PubMed] [Google Scholar]
- 5. Denzinger S, Fritsche HM, Otto W, Blana A, Wieland WF, Burger M. Early versus deferred cystectomy for initial high‐risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder‐sparing approach? Eur Urol 2008; 53: 146–52. [DOI] [PubMed] [Google Scholar]
- 6. Hautmann RE, Volkmer BG, Gust K. Quantification of the survival benefit of early versus deferred cystectomy in high‐risk non‐muscle invasive bladder cancer (T1 G3). World J Urol 2009; 27: 347–51. [DOI] [PubMed] [Google Scholar]
- 7. Roghmann F, Trinh QD, Braun K et al Standardized assessment of complications in a contemporary series of European patients undergoing radical cystectomy. Int J Urol 2014; 21: 143–9. [DOI] [PubMed] [Google Scholar]
- 8. Babjuk M, Burger M, Zigeuner R et al EAU guidelines on non‐muscle‐invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013; 64: 639–53. [DOI] [PubMed] [Google Scholar]
- 9. Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum 1993; 36: 627–35. [DOI] [PubMed] [Google Scholar]
- 10. Betge J, Kornprat P, Pollheimer MJ et al Tumor budding is an independent predictor of outcome in AJCC/UICC stage II colorectal cancer. Ann Surg Oncol 2012; 19: 3706–12. [DOI] [PubMed] [Google Scholar]
- 11. Choi HJ, Park KJ, Shin JS, Roh MS, Kwon HC, Lee HS. Tumor budding as a prognostic marker in stage‐III rectal carcinoma. Int J Colorectal Dis 2007; 22: 863–8. [DOI] [PubMed] [Google Scholar]
- 12. Park KJ, Choi HJ, Roh MS, Kwon HC, Kim C. Intensity of tumor budding and its prognostic implications in invasive colon carcinoma. Dis Colon Rectum 2005; 48: 1597–602. [DOI] [PubMed] [Google Scholar]
- 13. Kevans D, Wang LM, Sheahan K et al Epithelial–mesenchymal transition (EMT) protein expression in a cohort of stage II colorectal cancer patients with characterized tumor budding and mismatch repair protein status. Int J Surg Pathol 2011; 19: 751–60. [DOI] [PubMed] [Google Scholar]
- 14. Nakadoi K, Oka S, Tanaka S et al Condition of muscularis mucosae is a risk factor for lymph node metastasis in T1 colorectal carcinoma. Surg Endosc 2014; 28: 1269–76. [DOI] [PubMed] [Google Scholar]
- 15. Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol 2010; 23: 1068–72. [DOI] [PubMed] [Google Scholar]
- 16. Ueno H, Mochizuki H, Hashiguchi Y et al Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004; 127: 385–94. [DOI] [PubMed] [Google Scholar]
- 17. Wang LM, Kevans D, Mulcahy H et al Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol 2009; 33: 134–41. [DOI] [PubMed] [Google Scholar]
- 18. Dawson H, Lugli A. Molecular and pathogenetic aspects of tumor budding in colorectal cancer. Front Med 2015; 2: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 2010; 1: 651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watanabe T, Itabashi M, Shimada Y et al Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20: 207–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Rhijn BW, van der Kwast TH, Alkhateeb SS et al A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol 2012; 61: 378–84. [DOI] [PubMed] [Google Scholar]
- 22. Kadota K, Yeh YC, Villena‐Vargas J et al Tumor budding correlates with protumor immune microenvironment and is an independent prognostic factor for recurrence of stage I lung adenocarcinoma. Chest 2015; 148: 711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salhia B, Trippel M, Pfaltz K et al High tumor budding stratifies breast cancer with metastatic properties. Breast Cancer Res Treat 2015; 150: 363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Connor K, Li‐Chang HH, Kalloger SE et al Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma. Am J Surg Pathol 2015; 39: 472–8. [DOI] [PubMed] [Google Scholar]
- 25. Angadi PV, Patil PV, Hallikeri K, Mallapur MD, Hallikerimath S, Kale AD. Tumor budding is an independent prognostic factor for prediction of lymph node metastasis in oral squamous cell carcinoma. Int J Surg Pathol 2015; 23: 102–10. [DOI] [PubMed] [Google Scholar]
- 26. Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Invest 2009; 119: 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoda Y, Ikematsu H, Matsuda T et al A large‐scale multicenter study of long‐term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy 2013; 45: 718–24. [DOI] [PubMed] [Google Scholar]
- 28. Ueno H, Mochizuki H, Shinto E, Hashiguchi Y, Hase K, Talbot IC. Histologic indices in biopsy specimens for estimating the probability of extended local spread in patients with rectal carcinoma. Cancer 2002; 94: 2882–91. [DOI] [PubMed] [Google Scholar]
- 29. Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002; 40: 127–32. [DOI] [PubMed] [Google Scholar]
- 30. Ehdaie B, Sylvester R, Herr HW. Maintenance bacillus Calmette‐Guerin treatment of non‐muscle‐invasive bladder cancer: a critical evaluation of the evidence. Eur Urol 2013; 64: 579–85. [DOI] [PubMed] [Google Scholar]


