Abstract
Immunotherapy has recently become widely used in lung cancer. Many oncologists are focused on cytotoxic T lymphocyte antigen‐4 (CTLA‐4), programmed cell death ligand‐1 (PD‐L1) and programmed cell death‐1 (PD‐1). Immunotherapy targeting the PD‐1/PD‐L1 checkpoints has shown promising efficacy in non‐small cell lung cancer (NSCLC), but questions remain to be answered. Among them is whether the simultaneous inhibition of other checkpoints could improve outcomes. Lymphocyte‐activation gene‐3 (LAG‐3) is another vital checkpoint that may have a synergistic interaction with PD‐1/PD‐L1. Here we review the LAG‐3 function in cancer, clinical trials with agents targeting LAG‐3 and the correlation of LAG‐3 with other checkpoints.
Keywords: Cancer checkpoints, clinical trial, immunotherapy, lymphocyte‐activation gene‐3, soluble LAG‐3
In 2013, American Science ranked cancer immunotherapy as one of the most important scientific breakthroughs.1 Cancer immunotherapy can reverse tumor immune escape by suppressing immune checkpoint pathways. It is possible that the inhibition of pathway checkpoints, including cytotoxic T lymphocyte antigen‐4 (CTLA‐4), programmed cell death‐1 (PD‐1) and programmed cell death ligand‐1 (PD‐L1), could activate T cells to attack and eliminate cancer.2, 3, 4, 5, 6, 7
In recent years, several trials of agents that block CTLA‐4, PD‐L1 and PD‐1 have demonstrated durable efficacy against melanoma, renal, lung and other cancers.1, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 In March 2015, immunotherapy reached another milestone when the FDA approved nivolumab as a second line treatment for metastatic lung squamous carcinoma. Previously, nivolumab had been approved for use in patients with melanoma that is either not resectable or had not responded to other therapies.6, 7, 8, 10 For reasons that remain unclear, not all PD‐1 or PD‐L1 positive patients have good outcomes with the treatment of anti‐PD‐1 or PD‐L1 monoclonal antibody. It remains to be analyzed whether PD‐1 or PD‐L1 have synergistic effects with other checkpoints in a clinical setting (Fig. 1).
Figure 1.
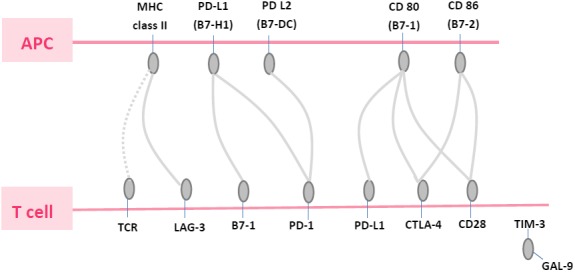
Some checkpoint pathways in cancer. The lymphocyte‐activation gene‐3 (LAG‐3) protein binds a nonholomorphic region of MHC class II.
Preliminary data indicates that another important checkpoint, lymphocyte‐activation gene‐3 (LAG‐3) (CD 223) may have a synergistic effect with PD‐1/PD‐L1.18, 19, 20
Lymphocyte‐activation gene‐3 and soluble lymphocyte‐activation gene‐3
LAG‐3 (CD223) is encoded by the LAG‐3 gene. LAG‐3 is a member of the immunoglobulin superfamily (IgSF) and exerts a wide variety of biologic impacts on T cell function.21 LAG‐3 is expressed on cell membranes of natural killer cells (NK),21 B cells,22 tumor‐infiltrating lymphocytes (TIL), a subset of T cells,23 and dendritic cells (DC)24, 25. The LAG‐3 gene encompasses 8 exons, and the cDNA encodes a 498 amino acid membrane protein. Human LAG‐3 is highly homologous with both murine (70%) and pig (78%) LAG‐3.21, 26
LAG‐3 is closely related to CD 4.27 LAG‐3 is located on the human chromosome 12 (12p13.32) adjacent to the CD 4 gene, and its sequence is approximately 20% identical to CD 4.21 The LAG‐3 protein binds a nonholomorphic region of major histocompatibility complex 2 (MHC class II) with greater affinity than CD 4.28, 29, 30, 31, 32, 33, 34, 35 LAG‐3 is one of the various immune‐checkpoint receptors that are coordinately upregulated on both regulatory T cells (Tregs) and anergic T cells, and the simultaneous blockade of these receptors can result in an enhanced reversal of this anergic state relative to the blockade of one receptor alone.18 The LAG‐3/MHC class II molecule interaction leads to the downregulation of CD4+ Ag‐specific T cell clone proliferation and cytokine secretion. T cell MHC class II molecules downregulate T cell proliferation following LAG‐3 binding and suggest a role for LAG‐3 in control of the CD4+ T cell response.31 LAG‐3 can negatively regulated T cell proliferation, activation and homeostasis.
LAG‐3 plays a complicated role in the immune pathway. Soluble lymphocyte‐activation gene‐3 (sLAG‐3) likely performs different functions from LAG‐3. sLAG‐3 is a Th1 activity marker in serum that can be detected by ELISA.36, 37 sLAG‐3 causes DCs to mature 38, 39, 40, 41, 42, 43, 44 and attack tumor cells.43, 44 Studies of the mechanisms that underlie monocyte and DC activation38, 40 by sLAG‐3 indicate that sLAG‐3 induces protein phosphorylation in immature DC that triggers the functional maturation.38, 39 This process requires sLAG‐3 binding with MHC class II.28
Lymphocyte‐activation gene‐3 in disease
Beyond the role it plays in a variety of autoimmune diseases, LAG‐3 can also reduce the body's ability to resist infection and promote chronic infection. LAG‐3 prevents autoimmune disorders in the eye by inducing anterior chamber‐associated immune deviation.45 LAG‐3 may regulate the functions of CD4+ and CD8+ T cells during autoimmune diabetes, and limit autoimmunity in disease‐prone environments.46 In bone marrow transplant (BMT) patients, LAG‐3 can regulate CD8+ cells involved in alloreactivity, T cell proliferation and activation after BMT.47, 48 In patients with chronic viral infection, the blockade of both PD‐1 and LAG‐3 could synergistically activate T cell responses and control the virus.49 LAG‐3 negatively regulates CD8+ T cells in chronic hepatitis C patients.50 In tuberculosis, sLAG‐3 is elevated both in healthy people who have been exposed to the bacteria and in tuberculosis patients with good prognoses,51 indicating that sLAG‐3 could modulate an anti‐bacterial immune response in mycobacterium tuberculosis.52 In acquired immune deficiency, high expression of LAG‐3 was correlated with impaired invariant natural killer T cell cytokine production for the duration of chronic human immunodeficiency virus (HIV)‐1 infection and treatment.53, 54 Targeting the LAG‐3 pathway has an immune regulatory effect and can enhance immune reconstitution in HIV‐infected patients.55
Lymphocyte‐activation gene‐3 in cancer
LAG‐3 expression was also observed in various cancer types. Vital preclinical studies have demonstrated that LAG‐3 antibodies have potential for cancer immunotherapy (Table 1).
Table 1.
LAG‐3 and cancer
| Year | Disease | Finding | References |
|---|---|---|---|
| 1999 | Cancer | sLAG‐3 could be a vaccine since it could active antigen presenting cells (APC). | 41 |
| 2001 | Cancer | sLAG‐3 could improve interactions between in situ T cells and DC, and potentiate Th1‐type response to target tumors, and, thus, could be a cancer vaccine. | 40 |
| 2003 | Breast cancer | Therapy involving LAG‐3 relative could block the progression of mammary carcinogenesis in an animal model. | 42 |
| 2005 | Cancer | LAG‐3 related anti‐cancer therapy was effective and shared a similar mechanism with IL‐12 | 27 |
| 2006 | Cancer (melanoma or colorectal cancer) | Human LAG‐3 Ig induced specific CD8+ T‐cell activity. The activation of this protein is a potential adjuvant treatment for cancer vaccines | 56 |
| 2006 | Cancer | sLAG‐3, used as a cancer vaccine, bound MHC class II+ APC, induced DC maturation and was well tolerated | 57 |
| 2006 | Hodgkin's lymphoma (HL) | LAG‐3 played important roles in the suppression of EBV immunity in HL | 58 |
| 2010 | Ovarian cancer | Inhibiting both LAG‐3 and PD‐1 pathways could efficiently improve T‐cell function | 59 |
| 2010 | Chronic lymphocytic leukemia (CLL) | High LAG‐3 expression indicated poor treatment outcomes in CLL | 60 |
| 2010 | Cancer | LAG‐3 defined Tregs were more numerous in tumor sites | 61 |
| 2010 | Multiple myeloma | LAG‐3 gene single nucleotide polymorphism (SNP) increased susceptibility to multiple myeloma | 62 |
| 2011 | Melanoma | LAG‐3/MHC class II interaction in MHC class II‐positive melanoma tumors might serve as a bidirectional immune escape pathway shared by tumor cells and immune cells and renews the interest in MHC class II phenotyping for more efficient therapeutic strategies | 63 |
| 2012 | Cancer | LAG‐3/Pdcd1 mice lived markedly longer than wild type and could eliminate multiple transplantable tumors | 20 |
| 2012 | Hepatocellular carcinoma (HCC) | Increased LAG‐3 expression was observed in TIL in HCC | 64 |
| 2014 | Melanoma | LAG‐3 activated pDC were found in tumor areas, which could suppress the immune environment | 65 |
| 2015 | Gastric cancer | In gastric cancer, expression of PD‐1 and LAG‐3 on CD4+ and CD8+ T cells was elevated and might impair cell‐mediated immunity after surgery | 66 |
DC, dendritic cells; LAG‐3, lymphocyte‐activation gene‐3; sLAG‐3, soluble LAG‐3; TIL, tumor‐infiltrating lymphocytes.
Lymphocyte‐activation gene‐3 and treatment
LAG‐3 may be an even more promising target in cancer immunotherapy, because anti‐LAG‐3 antibodies can activate T effector cells and affect Tregs function.67 Many companies are now focusing on the LAG‐3 immune checkpoint in their search for novel approaches to treat malignant tumors and autoimmune disorders, many of which are now in clinical development (Table 2).
Table 2.
Clinical trials with LAG‐3
| Year | Drug | Phase | Company | Type | Objective | Clinical trial.gov identifier |
|---|---|---|---|---|---|---|
| 2006 | IMP321 | I | Immutep S.A. | sLAG‐3 | IMP321 given alone or with a reference flu antigen | NCT00354263 |
| 2006 | IMP321 | I | Immutep S.A. | sLAG‐3 | IMP321 combined with a hepatitis B antigen | NCT00354861 |
| 2006 | IMP321 | I | Immutep S.A. | sLAG‐3 | IMP321 metastatic breast carcinoma receiving first line paclitaxel | NCT00349934 |
| 2006 | IMP321 | I | Immutep S.A. | sLAG‐3 | IMP321 in metastatic renal cell carcinoma | NCT00351949 |
| 2008 | IMP321 | I | Immutep S.A. | sLAG‐3 | IMP321 and gemcitabine in advanced pancreatic cancer | NCT00732082 |
| 2015 | IMP321 | II | Immutep S.A. | sLAG‐3 | Adjunctive IMP321 to paclitaxel in metastatic breast carcinoma | NCT02614833 |
| 2013 | BMS‐986016 | I | BMS | Anti‐LAG‐3 | The safety of anti‐LAG‐3 alone or with anti‐PD‐1 in solid tumors | NCT01968109 |
| 2014 | BMS‐986016 | I | BMS | Anti‐LAG‐3 | The safety of anti‐LAG‐3 in hematological malignant tumors | NCT02061761 |
| 2016 | BMS‐986016 | I | BMS | Anti‐LAG‐3 | Anti‐LAG‐3 or urelumab alone or with nivolumab in recurrent glioblastoma | NCT02658981 |
| 2014 | GSK2831781 | I | GSK | Anti‐LAG‐3 | GSK2831781 in healthy people and patients with plaque psoriasis | NCT02195349 |
Correlation of lymphocyte‐activation gene‐3 and other checkpoints
LAG‐3 and CTLA‐4 function similarly.19, 68, 69 CTLA‐4 inhibits T cell activation, suppresses T cell receptor signaling, and promotes cell cycle arrest.70 Activated LAG‐3−/− T cells extend cell cycle progression and increase T cell death. The similarity of function between LAG‐3 and CTLA‐4 may be related to some intersection in their signal transduction pathways. Tetravalent CTLA‐4‐Ig and LAG‐3‐Ig could have a synergistic effect in preventing acute graft‐versus‐host disease (GVHD). The combination therapy could more effectively inhibit T cell proliferation and reduce GVHD lethality.71
LAG‐3 has synergistic action with PD‐1/PD‐L1.20, 48, 72 LAG‐3 and PD‐1 are critical for the prevention of autoimmunity. Their synergistic function reverses autoimmune disease.19 A deficiency of LAG‐3 and PD‐1 caused lethal myocarditis in a mouse model. The respective ligand receptor interactions between PD‐L1 and LAG‐3, together with the molecules themselves, synergistically inhibit T cell responses during persistent plasmodium. Blockade of PD‐L1 and LAG‐3 activated CD 4+ T cells, increased helper T cells and B cells, enhanced protective antibodies and rapidly cleared blood‐stage malaria in mice.73 In chronic viral infection, LAG‐3 and PD‐1 maintain CD8+ T cell exhaustion.18, 49 In vivo research has shown that the blockade of PD‐1 and LAG‐3 pathways can activate T cells to achieve better viral control compared to either blockade alone.49 Co‐expression of LAG‐3 and PD‐1 can induce greater T cell exhaustion and more severe infection.23 PD‐1 and LAG‐3 signaling pathways can inhibit CD 8 by antigen and cytokine signaling.18 In ovarian cancer, CD8+ TIL could be negatively regulated by LAG‐3 and PD‐1. CD8+LAG‐3+PD‐1+ T cells significantly reduced IFN‐γ/TNF‐α. Blockade of both LAG‐3 and PD‐1 could increase specific CD8+ T cells producing cytokine.59 It was also reported that LAG‐3 and PD‐1 synergistically regulate T‐cell function, blunting the anti‐tumor immune response. Lag‐3−/−Pdcd‐1−/− mice developed an early onset, lethal autoimmune condition, but not a single knockout or wild‐type mice. Cytokine analysis revealed high levels of IFN‐γ, TNF‐α and MCP‐1 in the serum of Lag‐3−/−Pdcd‐1−/− recipients but not a single knockout or wild‐type control recipient. Although CTLA‐4, PD‐1 and LAG‐3 are all negative regulators expressed during T‐cell activation, high level, dual LAG‐3/PD‐1 expression is largely restricted to infiltrating TIL. Thus, LAG‐3/PD‐1 combinatorial immunotherapy may promote the tumor‐specific responses relative to nonspecific or self‐antigen‐specific immune responses and, thus, may be less toxic than the CTLA‐4 blockade.20 Dual anti‐LAG‐3 and anti‐PD‐1 antibody therapy has a better prognosis than single antibody therapy. Dual knockout mice survive longer than single knockout mice. The strong synergy between the PD‐1 and LAG‐3 inhibitory pathways could be the foundation for novel cancer treatments (Fig. 2).20
Figure 2.
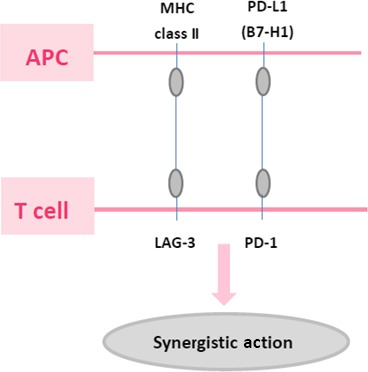
Lymphocyte‐activation gene‐3 (LAG‐3)/MHC class II and PD‐1/PD‐L1 pathways. LAG‐3 has synergistic action with PD‐1/PD‐L1.
Summary
Immune checkpoints play vital roles in tumor immune escape. However, the mechanisms of the synergy between various immune checkpoints remain unknown. Cancer treatments related to CTLA‐4 and PD‐1/PD‐L1 have achieved remarkable results. Another important immune checkpoint, LAG‐3, which is closely related to CD4, can regulate T cell proliferation, activation and homeostasis. LAG‐3 plays an important role in a variety of autoimmune diseases and promotes chronic infection and cancer. LAG‐3's synergistic function with PD‐1 and PD‐L1 warrants further exploration.
Disclosure Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
This project was supported by the IASLC Young Investigator Award, the Pia and Fred R. Hirsch Endowed Chair at the University of Colorado, and the Young Program of Shanghai Health Development Planning Commission.
Cancer Sci 107 (2016) 1193–1197
Funding Information
IASLC Young Investigator Award; Pia and Fred R. Hirsch Endowed Chair at the University of Colorado; Young Program of Shanghai Health Development Planning Commission.
Contributor Information
Caicun Zhou, Email: caicunzhoudr@163.com.
Fred R. Hirsch, Email: Fred.hirsch@ucdenver.edu
References
- 1. Couzin‐Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013; 342: 1432–3. [DOI] [PubMed] [Google Scholar]
- 2. Dong H, Zhu G, Tamada K, Chen L. B7‐H1, a third member of the B7 family, co‐stimulates T‐cell proliferation and interleukin‐10 secretion. Nat Med 1999; 5: 1365–9. [DOI] [PubMed] [Google Scholar]
- 3. Dong H, Strome SE, Salomao DR et al Tumor‐associated B7‐H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800. [DOI] [PubMed] [Google Scholar]
- 4. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007; 8: 239–45. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer JR, Drake CG, Wollner I et al Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer JR, Tykodi SS, Chow LQ et al Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamid O, Robert C, Daud A et al Safety and tumor responses with lambrolizumab (anti‐PD‐1) in melanoma. N Engl J Med 2013; 369: 134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbst RS, Soria JC, Kowanetz M et al Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Powles T, Eder JP, Fine GD et al MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–62. [DOI] [PubMed] [Google Scholar]
- 11. Tumeh PC, Harview CL, Yearley JH et al PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asaoka Y, Ijichi H, Koike K. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015; 373: 1979. [DOI] [PubMed] [Google Scholar]
- 13. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diaz LA Jr, Le DT. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015; 373: 1979. [DOI] [PubMed] [Google Scholar]
- 16. Garon EB, Rizvi NA, Hui R et al Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 17. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 18. Grosso JF, Goldberg MV, Getnet D et al Functionally distinct LAG‐3 and PD‐1 subsets on activated and chronically stimulated CD8 T cells. J Immunol 2009; 182: 6659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okazaki T, Okazaki IM, Wang J et al PD‐1 and LAG‐3 inhibitory co‐receptors act synergistically to prevent autoimmunity in mice. J Exp Med 2011; 208: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woo SR, Turnis ME, Goldberg MV et al Immune inhibitory molecules LAG‐3 and PD‐1 synergistically regulate T‐cell function to promote tumoral immune escape. Cancer Res 2012; 72: 917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Triebel F, Jitsukawa S, Baixeras E et al LAG‐3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 1990; 171: 1393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kisielow M, Kisielow J, Capoferri‐Sollami G. Expression of lymphocyte activation gene 3 (LAG‐3) on B cells is induced by T cells. Eur J Immunol 2005; 35: 2081–8. [DOI] [PubMed] [Google Scholar]
- 23. Grosso JF, Kelleher CC, Harris TJ et al LAG‐3 regulates CD8+ T cell accumulation and effector function in murine self‐ and tumor‐tolerance systems. J Clin Invest 2007; 117: 3383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Workman CJ, Wang Y, El Kasmi KC et al LAG‐3 regulates plasmacytoid dendritic cell homeostasis. J Immunol 2009; 182: 1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andreae S, Piras F, Burdin N, Triebel F. Maturation and activation of dendritic cells induced by lymphocyte activation gene‐3 (CD223). J Immunol 2002; 168: 3874–80. [DOI] [PubMed] [Google Scholar]
- 26. Kim SS, Kim SH, Kang HS et al Molecular cloning and expression analysis of pig lymphocyte activation gene‐3 (LAG‐3; CD223). Vet Immunol Immunopathol 2010; 133: 72–9. [DOI] [PubMed] [Google Scholar]
- 27. Di Carlo E, Cappello P, Sorrentino C et al Immunological mechanisms elicited at the tumour site by lymphocyte activation gene‐3 (LAG‐3) versus IL‐12: sharing a common Th1 anti‐tumour immune pathway. J Pathol 2005; 205: 82–91. [DOI] [PubMed] [Google Scholar]
- 28. Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4‐ and lymphocyte activation gene‐3 (LAG‐3)‐Ig fusion proteins. Eur J Immunol 1995; 25: 2718–21. [DOI] [PubMed] [Google Scholar]
- 29. Bruniquel D, Borie N, Triebel F. Genomic organization of the human LAG‐3/CD4 locus. Immunogenetics 1997; 47: 96–8. [DOI] [PubMed] [Google Scholar]
- 30. Baixeras E, Huard B, Miossec C et al Characterization of the lymphocyte activation gene 3‐encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med 1992; 176: 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huard B, Prigent P, Pages F, Bruniquel D, Triebel F. T cell major histocompatibility complex class II molecules down‐regulate CD4+ T cell clone responses following LAG‐3 binding. Eur J Immunol 1996; 26: 1180–6. [DOI] [PubMed] [Google Scholar]
- 32. Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex‐associated lymphocyte activation gene‐3 molecules inhibit CD3/TCR signaling. J Immunol 1998; 161: 4058–65. [PubMed] [Google Scholar]
- 33. Hannier S, Triebel F. The MHC class II ligand lymphocyte activation gene‐3 is co‐distributed with CD8 and CD3‐TCR molecules after their engagement by mAb or peptide‐MHC class I complexes. Int Immunol 1999; 11: 1745–52. [DOI] [PubMed] [Google Scholar]
- 34. Sakihama T, Smolyar A, Reinherz EL. Oligomerization of CD4 is required for stable binding to class II major histocompatibility complex proteins but not for interaction with human immunodeficiency virus gp120. Proc Natl Acad Sci USA 1995; 92: 6444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li S, Satoh T, Korngold R, Huang Z. CD4 dimerization and oligomerization: implications for T‐cell function and structure‐based drug design. Immunol Today 1998; 19: 455–62. [DOI] [PubMed] [Google Scholar]
- 36. Annunziato F, Manetti R, Tomasevic I et al Expression and release of LAG‐3‐encoded protein by human CD4+ T cells are associated with IFN‐gamma production. FASEB J 1996; 10: 769–76. [DOI] [PubMed] [Google Scholar]
- 37. Triebel F. LAG‐3: a regulator of T‐cell and DC responses and its use in therapeutic vaccination. Trends Immunol 2003; 24: 619–22. [DOI] [PubMed] [Google Scholar]
- 38. Avice MN, Sarfati M, Triebel F, Delespesse G, Demeure CE. Lymphocyte activation gene‐3, a MHC class II ligand expressed on activated T cells, stimulates TNF‐alpha and IL‐12 production by monocytes and dendritic cells. J Immunol 1999; 162: 2748–53. [PubMed] [Google Scholar]
- 39. Andreae S, Buisson S, Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG‐3 protein (CD223). Blood 2003; 102: 2130–7. [DOI] [PubMed] [Google Scholar]
- 40. Demeure CE, Wolfers J, Martin‐Garcia N, Gaulard P, Triebel F. T lymphocytes infiltrating various tumour types express the MHC class II ligand lymphocyte activation gene‐3 (LAG‐3): role of LAG‐3/MHC class II interactions in cell–cell contacts. Eur J Cancer 2001; 37: 1709–18. [DOI] [PubMed] [Google Scholar]
- 41. Prigent P, El Mir S, Dreano M, Triebel F. Lymphocyte activation gene‐3 induces tumor regression and antitumor immune responses. Eur J Immunol 1999; 29: 3867–76. [DOI] [PubMed] [Google Scholar]
- 42. Cappello P, Triebel F, Iezzi M et al LAG‐3 enables DNA vaccination to persistently prevent mammary carcinogenesis in HER‐2/neu transgenic BALB/c mice. Cancer Res 2003; 63: 2518–25. [PubMed] [Google Scholar]
- 43. El Mir S, Triebel F. A soluble lymphocyte activation gene‐3 molecule used as a vaccine adjuvant elicits greater humoral and cellular immune responses to both particulate and soluble antigens. J Immunol 2000; 164: 5583–9. [DOI] [PubMed] [Google Scholar]
- 44. Buisson S, Triebel F. MHC class II engagement by its ligand LAG‐3 (CD223) leads to a distinct pattern of chemokine and chemokine receptor expression by human dendritic cells. Vaccine 2003; 21: 862–8. [DOI] [PubMed] [Google Scholar]
- 45. Zhu X, Yang P, Zhou H et al CD4+CD25+Tregs express an increased LAG‐3 and CTLA‐4 in anterior chamber‐associated immune deviation. Graefes Arch Clin Exp Ophthalmol 2007; 245: 1549–57. [DOI] [PubMed] [Google Scholar]
- 46. Bettini M, Szymczak‐Workman AL, Forbes K et al Cutting edge: accelerated autoimmune diabetes in the absence of LAG‐3. J Immunol 2011; 187: 3493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sega EI, Leveson‐Gower DB, Florek M, Schneidawind D, Luong RH, Negrin RS. Role of lymphocyte activation gene‐3 (Lag‐3) in conventional and regulatory T cell function in allogeneic transplantation. PLoS ONE 2014; 9: e86551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lucas CL, Workman CJ, Beyaz S et al LAG‐3, TGF‐beta, and cell‐intrinsic PD‐1 inhibitory pathways contribute to CD8 but not CD4 T‐cell tolerance induced by allogeneic BMT with anti‐CD40L. Blood 2011; 117: 5532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blackburn SD, Shin H, Haining WN et al Coregulation of CD8+ cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen N, Liu Y, Guo Y, Chen Y, Liu X, Liu M. LAG‐3 negatively regulates the function of intrahepatic HCV‐specific CD8 T cells. J Gastroenterol Hepatol 2015; 30: 1788–95. [DOI] [PubMed] [Google Scholar]
- 51. Lienhardt C, Azzurri A, Amedei A et al Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol 2002; 32: 1605–13. [DOI] [PubMed] [Google Scholar]
- 52. Phillips BL, Mehra S, Ahsan MH, Selman M, Khader SA, Kaushal D. LAG3 expression in active Mycobacterium tuberculosis infections. Am J Pathol 2015; 185: 820–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pena J, Jones NG, Bousheri S, Bangsberg DR, Cao H. Lymphocyte activation gene‐3 expression defines a discrete subset of HIV‐specific CD8+ T cells that is associated with lower viral load. AIDS Res Hum Retroviruses 2014; 30: 535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Juno JA, Stalker AT, Waruk JL et al Elevated expression of LAG‐3, but not PD‐1, is associated with impaired iNKT cytokine production during chronic HIV‐1 infection and treatment. Retrovirology 2015; 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tian X, Zhang A, Qiu C et al The upregulation of LAG‐3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV‐infected subjects. J Immunol 2015; 194: 3873–82. [DOI] [PubMed] [Google Scholar]
- 56. Casati C, Camisaschi C, Rini F et al Soluble human LAG‐3 molecule amplifies the in vitro generation of type 1 tumor‐specific immunity. Cancer Res 2006; 66: 4450–60. [DOI] [PubMed] [Google Scholar]
- 57. Fougeray S, Brignone C, Triebel F. A soluble LAG‐3 protein as an immunopotentiator for therapeutic vaccines: preclinical evaluation of IMP321. Vaccine 2006; 24: 5426–33. [DOI] [PubMed] [Google Scholar]
- 58. Gandhi MK, Lambley E, Duraiswamy J et al Expression of LAG‐3 by tumor‐infiltrating lymphocytes is coincident with the suppression of latent membrane antigen‐specific CD8+ T‐cell function in Hodgkin lymphoma patients. Blood 2006; 108: 2280–9. [DOI] [PubMed] [Google Scholar]
- 59. Matsuzaki J, Gnjatic S, Mhawech‐Fauceglia P et al Tumor‐infiltrating NY‐ESO‐1‐specific CD8+ T cells are negatively regulated by LAG‐3 and PD‐1 in human ovarian cancer. Proc Natl Acad Sci USA 2010; 107: 7875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kotaskova J, Tichy B, Trbusek M et al High expression of lymphocyte‐activation gene 3 (LAG3) in chronic lymphocytic leukemia cells is associated with unmutated immunoglobulin variable heavy chain region (IGHV) gene and reduced treatment‐free survival. J Mol Diagn 2010; 12: 328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Camisaschi C, Casati C, Rini F et al LAG‐3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J Immunol 2010; 184: 6545–51. [DOI] [PubMed] [Google Scholar]
- 62. Lee KM, Baris D, Zhang Y et al Common single nucleotide polymorphisms in immunoregulatory genes and multiple myeloma risk among women in Connecticut. Am J Hematol 2010; 85: 560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hemon P, Jean‐Louis F, Ramgolam K et al MHC class II engagement by its ligand LAG‐3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol 2011; 186: 5173–83. [DOI] [PubMed] [Google Scholar]
- 64. Li FJ, Zhang Y, Jin GX, Yao L, Wu DQ. Expression of LAG‐3 is coincident with the impaired effector function of HBV‐specific CD8(+) T cell in HCC patients. Immunol Lett 2013; 150: 116–22. [DOI] [PubMed] [Google Scholar]
- 65. Camisaschi C, De Filippo A, Beretta V et al Alternative activation of human plasmacytoid DCs in vitro and in melanoma lesions: involvement of LAG‐3. J Invest Dermatol 2014; 134: 1893–902. [DOI] [PubMed] [Google Scholar]
- 66. Takaya S, Saito H, Ikeguchi M. Upregulation of immune checkpoint molecules, PD‐1 and LAG‐3, on CD4+ and CD8+ T cells after gastric cancer surgery. Yonago Acta Med 2015; 58: 39–44. [PMC free article] [PubMed] [Google Scholar]
- 67. Gagliani N, Magnani CF, Huber S et al Coexpression of CD49b and LAG‐3 identifies human and mouse T regulatory type 1 cells. Nat Med 2013; 19: 739–46. [DOI] [PubMed] [Google Scholar]
- 68. Workman CJ, Vignali DA. The CD4‐related molecule, LAG‐3 (CD223), regulates the expansion of activated T cells. Eur J Immunol 2003; 33: 970–9. [DOI] [PubMed] [Google Scholar]
- 69. Workman CJ, Cauley LS, Kim IJ. Lymphocyte activation gene‐3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol 2004; 172: 5450–5. [DOI] [PubMed] [Google Scholar]
- 70. Kamma H, Yazawa T, Ogata T, Horiguchi H, Iijima T. Expression of MHC class II antigens in human lung cancer cells. Virchows Arch B Cell Pathol Incl Mol Pathol 1991; 60: 407–12. [DOI] [PubMed] [Google Scholar]
- 71. Cho H, Chung YH. Construction, and in vitro and in vivo analyses of tetravalent immunoadhesins. J Microbiol Biotechnol 2012; 22: 1066–76. [DOI] [PubMed] [Google Scholar]
- 72. Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG‐3 in acute and chronic LCMV infection. Int Immunol 2010; 22: 13–23. [DOI] [PubMed] [Google Scholar]
- 73. Butler NS, Moebius J, Pewe LL et al Therapeutic blockade of PD‐L1 and LAG‐3 rapidly clears established blood‐stage Plasmodium infection. Nat Immunol 2012; 13: 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


