Abstract
B‐cell activating factor (BAFF) promotes the survival and adhesion of multiple myeloma (MM) cells. Tabalumab (LY2127399) is an anti‐BAFF monoclonal antibody. This phase 1, multicenter, open‐label, nonrandomized, dose‐escalation study evaluated the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of tabalumab in combination with bortezomib and dexamethasone in Japanese patients with relapsed or refractory MM (RRMM). Sixteen patients received intravenous i.v. tabalumab 100 mg (Cohort 1, n = 4) or i.v. tabalumab 300 mg (Cohort 2, n = 12) in combination with oral dexamethasone 20 mg/day and i.v. or s.c. bortezomib 1.3 mg/m2. All patients had treatment‐emergent adverse events (TEAE) possibly related to study treatment; the most common TEAE were thrombocytopenia (81.3%), lymphopenia (43.8%) and increased alanine aminotransferase (43.8%). Two (20.0%) dose‐limiting toxicities were observed, both in Cohort 2 (tabalumab 300 mg), which was below the predefined cutoff for tolerability (<33%). The pharmacokinetics of tabalumab were similar when bortezomib was coadministered i.v. versus s.c. The overall response rate was 56.3%, suggesting that the combined treatment was effective. In conclusion, combined treatment with these three agents was well tolerated in this population of Japanese patients with RRMM. The study was registered at www.clinicaltrials.gov (NCT01556438).
Keywords: Anti‐B‐cell activating factor monoclonal antibody, LY2127399, multiple myeloma, phase 1 study, tabalumab
Multiple myeloma (MM), a clonal B‐cell malignancy, accounts for 1% of all malignancies worldwide.1 The age‐standardized incidence rate (per 100 000 individuals) in 2005 was estimated as 1.5 for men and 1.2 for women worldwide and 2.3 for men and 1.7 for women in Japan.2 Treatment of MM includes high‐dose chemotherapy with autologous stem cell transplantation and more recently approved therapies such as thalidomide, bortezomib and lenalidomide.1 Currently, the recommended treatment for patients with relapsed or refractory MM (RRMM) is dexamethasone combined with bortezomib or lenalidomide.1 However, most patients eventually develop resistant or refractory disease and therapies targeting several molecular pathways need to be developed and refined to further improve disease control.3
B‐cell activating factor (BAFF), a member of the tumor necrosis factor (TNF) superfamily, is critical for B‐cell development.3, 4 BAFF is elevated in serum and bone marrow mononuclear cells from patients with MM and is inversely correlated with cell survival.4 In nonclinical studies, BAFF protects B‐cells against apoptosis5, 6, 7 and is stimulated by and increases MM cell adhesion to bone marrow stromal cells.8 Thus, BAFF promotes the survival and adhesion of MM cells and is a potential therapeutic target for MM treatment.3
Tabalumab (LY2127399) is a potent, selective, fully human immunoglobulin G subclass 4 (IgG4) monoclonal antibody that neutralizes soluble and membrane‐bound BAFF.9 Tabalumab prevents free BAFF from binding its receptor but does not interfere with bound BAFF and, thus, does not directly interact with B‐cells. In a model using an interleukin‐6 (IL‐6)‐dependent MM cell line grafted onto a human fetal bone chip in severe combined immunodeficiency mice, tabalumab significantly reduced tumor burden (measured by soluble IL‐6 receptor levels) and increased survival compared with controls.10 Regarding tabalumab monotherapy, there are three phase 3 studies for rheumatoid arthritis (RA) and SLE.11, 12, 13 Results from these studies suggested partial efficacy for both indications, but could not show robust enough efficacy data to meet new drug application criteria. Anticipated adverse events (AE) were infusion reaction or infection, which could be induced by durable blockage of B‐cell function. However, no unexpected safety signals, including infection or infusion reaction, were detected in these studies. In a phase 1 clinical study conducted in the US in patients with previously treated RRMM, the observed safety profile of tabalumab at doses up to 300 mg in combination with bortezomib (with or without dexamethasone) was similar to that of bortezomib alone, and the overall response rate was 46% (partial response or better).14 In another global phase 1 study, the tabalumab dose was tested up to 300 mg i.v. every 21 days in combination with biweekly 1.3 mg/m2 bortezomib i.v. No dose‐limiting toxicities (DLT) were observed in the study. PK/PD results revealed a plateau in the LY2127399 peak/through fluctuation at over 100 mg, suggesting target saturation.15 Based on these data, we selected two doses (100 mg or 300 mg) for evaluating the Japanese population, because over 100 mg could be considered to block BAFF signal completely without DLT. The 300‐mg dose was chosen for inhibiting potential outrageous high BAFF level in myeloma patients. Based on these results, tabalumab 100 mg and 300 mg were used for clinical development.
The primary objective of this study was to evaluate the safety and tolerability of tabalumab 100 mg and 300 mg in combination with bortezomib and dexamethasone in Japanese patients with RRMM. Secondary objectives included assessment of pharmacokinetics, pharmacodynamics, and efficacy of tabalumab in combination with bortezomib and dexamethasone.
The rationale for choosing the combination of tabalumab with bortezomib and dexamethasone (BD) was: (i) BD combination is one of the standard treatments for relapsed myeloma, and there are no potential overlapping toxicities between tabalumab and bortezomib; (ii) preclinical data showed that tabalumab inhibited osteoclastogenesis in an in vivo model;10 and (iii) dexamethasone induces apoptosis in myeloma cells,6 and tabalumab was shown to inhibit cytoprotection from dexamethasone‐induced apoptosis of myeloma cells by BAFF/APRIL. Bortezomib is also known to function on bone marrow microenvironment and to activate osteogenesis,16 so the combination would be expected to improve bone disease associated with myeloma.
Materials and Methods
Study design
This phase 1, multicenter, open‐label, nonrandomized dose‐escalation study evaluated the safety and efficacy of tabalumab in combination with bortezomib and dexamethasone in patients with RRMM who were eligible for bortezomib therapy. The study was conducted from December 2011 to February 2015 at five sites in Japan.
This study was conducted in accordance with consensus ethics principles derived from international ethics guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, International Conference on Harmonisation Good Clinical Practices Guideline, and applicable laws and regulations. The study protocol was approved by the institutional review board at each site and all patients provided written informed consent before undergoing any study procedure. Patients who continued study treatment after the first treatment cycle signed a second informed consent form before starting the second treatment cycle. The study was registered at www.clinicaltrials.gov (NCT01556438).
Study population
Patients aged ≥20 years who had RRMM and had been treated with at least one prior regimen were eligible for inclusion; prior therapy with bortezomib was allowed if there was previously at least a minimal response. Patients had to have measurable disease defined by one or more of the following criteria: serum M‐protein concentration ≥1 g/dL (≥10 g/L); urine monoclonal light chain concentration ≥200 mg/24 h; involved serum free light chain (SFLC) concentration ≥10 mg/dL (≥100 mg/L); and an abnormal SFLC ratio. Patients were to have adequate organ function and an Eastern Cooperative Oncology Group performance status ≤2. Patients were excluded if they had more than one serious pre‐existing medical condition or a medical history that would preclude study participation: uncontrolled infection; pregnant or breastfeeding; known positive test results for human immunodeficiency virus, hepatitis B or hepatitis C; ≥Grade 2 peripheral neuropathy or any grade with neuralgic pain; a significant allergy to human monoclonal antibodies; previous tabalumab treatment; an allogenic hematopoietic stem cell transplant or an experimental agent targeting BAFF; corrected QT interval ˃470 ms; interstitial pneumonitis or pulmonary fibrosis; or any other active malignancy within the past five years.
Study treatments
All patients who met the eligibility criteria were assigned to a treatment cohort by the Sponsor. All patients were to receive i.v. tabalumab 100 or 300 mg, in combination with i.v. or s.c. bortezomib 1.3 mg/m2 and oral dexamethasone 20 mg/day, according to the schedule shown in Table 1.
Table 1.
Study treatment regimen
| Cycles 1–8 | Cycles ≥9 | |
|---|---|---|
| Cycle length | 21 days | 35 days |
| Tabalumab (Cohort 1: 100 mg; Cohort 2: 300 mg) | Day 1 | Day 1 |
| Bortezomib (1.3 mg/m2)†,‡ | Days 1, 4, 8, 11 | Days 1, 8, 15, 22 |
| Dexamethasone (20 mg/day) | Days 1, 2, 4, 5, 8, 9, 11, 12 | Days 1, 2, 8, 9, 15, 16, 22, 23 |
†Patients in Cohort 1 and Cohort 2‐IV received bortezomib i.v.; patients in Cohort 2‐SC received bortezomib s.c. ‡When tabalumab and bortezomib were administered on the same day, bortezomib was administered immediately after tabalumab.
Patients in Cohort 1 were administered tabalumab 100 mg, dexamethasone and i.v. bortezomib. Patients in Cohort 2 were administered tabalumab 300 mg, dexamethasone and bortezomib i.v. (Cohort 2‐IV) or s.c. (Cohort 2‐SC); both cohorts were treated concomitantly. The separate i.v. and s.c. cohorts were designed to assess tabalumab safety using the two marketed bortezomib dose formulations. No other chemotherapy, radiotherapy, immunotherapy, cancer‐related hormone therapy, corticosteroids (except low‐dose chronic corticosteroid therapy for conditions other than myeloma) or experimental medications were permitted during the study. However, local palliative radiation was permitted from Cycle 2 onwards.
Dose escalation
The study used a conventional 3 + 3 dose‐escalation design where three patients were initially enrolled per cohort. If one of the initial three patients experienced a DLT during Cycle 1, the cohort was to be expanded to six patients.
In Cohort 1 (tabalumab 100 mg), dose escalation to tabalumab 300 mg proceeded if <33% of patients experienced a DLT during Cycle 1. In Cohort 2 (tabalumab 300 mg), the dose was considered tolerable if <33% patients experienced a DLT during Cycle 1.
Dose intensities
Dose adjustments of tabalumab were not permitted, but the schedule was delayed to allow concomitant use with Day 1 bortezomib therapy. The bortezomib dose and/or schedule was modified in response to signs of toxicity. Patients who had bortezomib‐related neuropathy Grade ≤1 or Grade 2 without pain had their bortezomib dose or schedule modified to reduce toxicity. Patients who had bortezomib‐related neuropathy Grade 2 with pain or ≥Grade 3 had their treatment with bortezomib and dexamethasone discontinued, and were considered for single‐agent tabalumab. Dexamethasone was only permitted on the day of and day after bortezomib treatment. Dexamethasone was withheld in the event of Grade ≥3 AE (except hematological toxicity) related to dexamethasone; after AE had resolved to Grade ≤1, dexamethasone could be reinitiated at a 50% dose reduction. If a patient could not receive the standard doses of bortezomib or dexamethasone in Cycle 1 for reasons other than DLT, they were replaced with a new patient for DLT evaluation. From Cycle 1 and beyond, switching between bortezomib i.v. and bortezomib s.c. inter/intracycle was allowed.
Safety
Safety evaluation included the type, severity and incidence of treatment‐emergent AE (TEAE), laboratory variables, physical examination and vital signs. A DLT was defined as an AE during Cycle 1 that was possibly related to the study medication(s) and fulfilled any one of the following criteria according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03: Grade 4 neutropenia ˃5 days and/or resulting in neutropenic fever (>38.3°C); thrombocytopenia with a platelet count of <10 000/mm3 on ≥2 occasions; a Grade ≥3 non‐hematological toxicity (except for nausea and vomiting); Grade 3 electrolyte abnormalities; tumor lysis syndrome; increased alkaline phosphatase or lactate dehydrogenase; or a TEAE that caused Day 1 of Cycle 2 to be delayed by ≥14 days due to toxicity. Lymphopenia, a recognized toxicity of bortezomib and tabalumab, was not considered a DLT in this study.
Peripheral neuropathy was assessed using the “Additional Concerns” subscale of the patient‐rated Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity questionnaire17 on Day 1 of each cycle and at the 30‐day follow‐up visit.
Pharmacokinetics
Blood samples for tabalumab pharmacokinetics were collected as follows: Cycle 1, Day 1 (before, 2 h after and 6 ± 0.25 h after bortezomib), Day 4 (before bortezomib), Day 8 (any time) and Day 11 (any time); Cycle 2, Day 1 (before tabalumab and immediately after bortezomib); Cycle 3, Day 1 (immediately after bortezomib); Cycle 6, Day 1 (before tabalumab); Cycle 7, Day 1 (immediately after bortezomib and 2 h after tabalumab), Day 4 (72 ± 1 h after tabalumab), Day 8 (any time) and Day 11 (any time); Cycle 8: Day 11 (any time); Cycles ≥9: Day 1 (immediately after tabalumab); and the 30‐day follow‐up visit. Blood samples for bortezomib pharmacokinetics were collected on Day 1 (immediately after and at 0.5, 1, 2, 4 and 6 ± 0.25 h after bortezomib) and Day 2 (at least 24 ± 1 h after bortezomib) of Cycle 1. Tabalumab and bortezomib concentrations were analyzed using a validated ELISA.
Tumor response
Tumor response was assessed using the International Myeloma Working Group's International Uniform Response Criteria for MM during every cycle from Cycle 2 onwards. A repeat assessment (at any time) was required to confirm a response or progressive disease. The tumor response rate was defined as the proportion of patients who experienced a complete or partial response.18 Immunoglobulins IgA, IgG and IgM were measured at a central laboratory using standard methods. Baseline BAFF levels were measured using a validated ELISA method.
Pharmacodynamics
Blood samples for disease‐related biomarkers were collected ≤28 days before the first dose of study therapy; before the first tabalumab dose of Cycles 1–8; immediately after the tabalumab dose for Cycles ≥9; and at the 30‐day follow‐up visit. The B‐cell mature naïve CD19+, IgD+, CD27− subset was determined by flow cytometry.
Statistical analysis
This study used a conventional 3 + 3 dose escalation design. The plan was to enroll up to six patients per cohort (9–18 patients). All patients who received at least one dose of any study drug were evaluated for safety and toxicity (full analysis set, FAS). All patients who completed Cycle 1 or who discontinued study treatment due to a DLT were included in the DLT‐related safety analysis. DLT were summarized by DLT criteria for each dose level and cohort. Analyses of pharmacokinetic and pharmacodynamic parameters were conducted on all patients in the FAS who had pharmacokinetic or pharmacodynamic samples collected. Descriptive statistics were used to summarize safety, tumor response and pharmacokinetic parameters. Pharmacokinetic parameter estimates for tabalumab were calculated by standard noncompartmental methods of analysis. Imputation for missing values was not performed for the outcome variables. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient disposition
Of 21 patients screened, 16 were enrolled and received at least one dose of tabalumab (Fig. 1).
Figure 1.
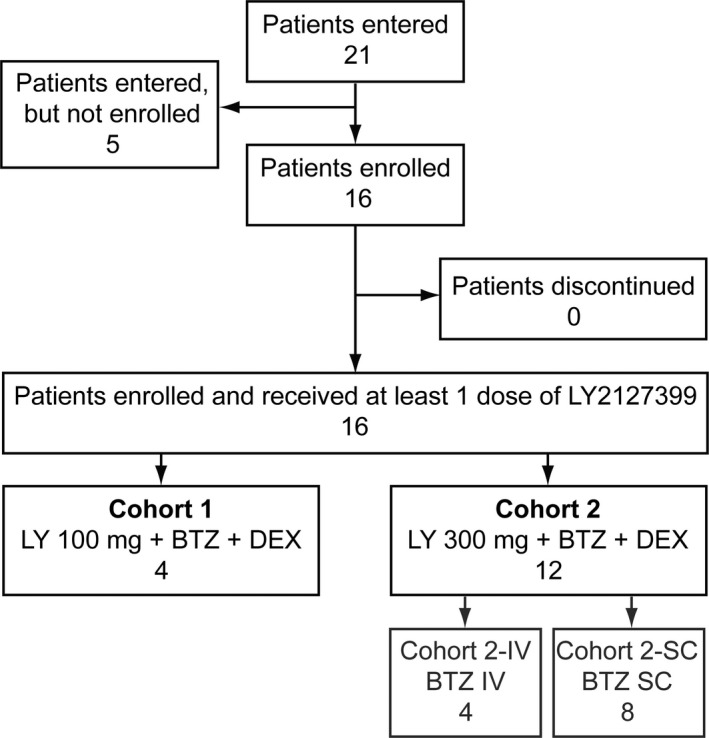
Patient disposition. BTZ, bortezomib 1.3 mg/m2; DEX, dexamethasone 20 mg/day; IV, intravenous; LY, LY2127399 (tabalumab); SC, subcutaneous.
In Cohort 1 (tabalumab 100 mg + bortezomib + dexamethasone), one of three patients originally enrolled reported ileus and did not receive the scheduled full dose of bortezomib during Cycle 1. This meant that DLT could not be determined in Cycle 1 for this patient, and an additional patient was added to the cohort. All four patients were evaluated for DLT, safety, pharmacokinetics and pharmacodynamics.
In Cohort 2 (tabalumab 300 mg + bortezomib + dexamethasone), 12 patients were enrolled and received at least one dose of tabalumab and one patient continued the study at data cut‐off. In Cohort 2‐SC, one of the initial three patients had a DLT in Cycle 1 and the cohort was expanded to six patients. However, two patients did not meet the criteria for DLT evaluation (both discontinued before the end of Cycle 1 because another antitumor therapy was needed) and two more patients were added to the cohort (total = 8). In Cohort 2‐IV, three patients were enrolled initially, but one patient had a DLT in Cycle 1 and so an extra patient was included in the cohort (total = 4). The i.v. cohort was not expanded to six patients as future trials planned to use bortezomib s.c. All 12 patients were included in the safety, pharmacokinetics, pharmacodynamics and efficacy analyses, and 10 patients were included in the DLT analysis.
Demographic and baseline clinical characteristics
All patients in Cohort 1 and half of the patients in Cohort 2 had relapsed/progressive MM; the remaining patients in Cohort 2 had relapsed/refractory MM (Table 2). All patients had received one or more systemic therapy, half the patients had received an autologous stem cell transplant, and none had prior surgery. Of the 12 patients in Cohort 2, two had received radiation.
Table 2.
Patient characteristics
| Characteristic | Cohort 1 LY 100 mg + BTZ + DEX (N = 4) | Cohort 2 LY 300 mg + BTZ + DEX (N = 12) |
|---|---|---|
| Sex, n (%) | ||
| Female | 2 (50.0) | 8 (66.7) |
| Male | 2 (50.0) | 4 (33.3) |
| Age, median (range) years | 68.1 (66.4–80.2) | 75.0 (52.0–82.1) |
| ≤65 years, n (%) | 0 | 3 (25.0) |
| ˃65 years, n (%) | 4 (100.0) | 9 (75.0) |
| ECOG performance status, n (%) | ||
| 0 | 3 (75.0) | 5 (41.7) |
| 1 | 1 (25.0) | 5 (41.7) |
| 2 | 0 | 2 (16.7) |
| Disease response status, n (%) | ||
| Relapsed/progressive MM | 4 (100.0) | 6 (50.0) |
| Relapsed/refractory MM | 0 | 6 (50.0) |
| Prior therapies | ||
| Surgery | 0 | 0 |
| Radiotherapy | 0 | 2 (16.7) |
| Systemic therapies | 4 (100.0) | 12 (100.0) |
| Bortezomib | 3 (75.0) | 10 (83.3) |
| Melphalan | 3 (75.0) | 11 (91.7) |
| Lenalidomide | 2 (50.0) | 5 (41.7) |
| Thalidomide | 1 (25.0) | 3 (25.0) |
| 1 regimen | 1 (25.0) | 5 (41.7) |
| 2 regimens | 3 (75.0) | 5 (41.7) |
| ≥3 regimens | 0 | 2 (16.7) |
| Stem cell transplant | 2 (50.0) | 6 (50.0) |
BTZ, bortezomib 1.3 mg/m2; DEX, dexamethasone 20 mg/day; ECOG, Eastern Cooperative Oncology Group; LY, LY2127399 (tabalumab); MM, multiple myeloma; N, number of patients; relapsed/progressive, the relapsed patients who once achieved response (PR, VGPR, CR, sCR) in the prior therapy; relapsed/refractory, the refractory patient who did not respond to prior therapy or the relapsed patient from SD after prior therapy.
Extent of drug exposure and dose modifications
The median number (range) of treatment cycles was 3 (2–11) in Cohort 1 and 4.5 (1–15) in Cohort 2. Three (75%) patients in Cohort 1 and 9 (75%) patients in Cohort 2 received 3 or more treatment cycles. After Cycle 1, 2 (50%) patients in Cohort 1 had tabalumab dose delays and bortezomib dose reductions, and 1 (25%) of these patients also had a bortezomib dose delay; no patients had dexamethasone dose adjustments. In Cohort 2, 8 (66.7%) patients had tabalumab dose delays; 3 (25%) of these patients also had bortezomib and dexamethasone dose reductions, 1 (8.3%) patient also had a bortezomib dose delay, one patient (8.3%) also had a dexamethasone dose increase, and 1 (8.3%) patient also had dexamethasone dose reduction.
Safety and tolerability
All 16 patients who received study medication experienced at least one TEAE and 13 (81.3%) patients had a Grade ≥3 TEAE (Table 3). Ten (62.5%) patients had ≥1 serious adverse event (SAE; Table 3); 6 (37.5%) of these patients had SAE that were possibly related to study treatment (one patient had peripheral sensory neuropathy and syncope, one patient had febrile neutropenia and tumor lysis syndrome, one patient had an embolism and infection, one patient had ileus, one patient had gastroenteritis and one patient had bronchopulmonary aspergillosis). Six patients discontinued due to an AE, five of which were considered possibly related to study treatment. One patient died within 30 days of the last dose of study the drug due to a subarachnoid hemorrhage, but this was not considered related to treatment by the investigator. No acute toxicities relevant to tabalumab were observed, and no topical infusion reactions or allergic reactions were seen after tabalumab administration.
Table 3.
Summary of all adverse events
| Cohort 1 LY 100 mg + BTZ + DEX (N = 4) | Cohort 2 LY 300 mg + BTZ + DEX (N = 12) | Total (N = 16) | |
|---|---|---|---|
| Patients with ≥1 AE | 4 (100.0) | 12 (100.0) | 16 (100.0) |
| Patients with ≥1 TEAE | 4 (100.0) | 12 (100.0) | 16 (100.0) |
| Patients with ≥1 Grade ≥3 TEAE | 3 (75.0) | 10 (83.3) | 13 (81.3) |
| Patients with ≥1 SAE | 2 (50.0) | 8 (66.7) | 10 (62.5) |
| Patients who discontinued due to AE | 4 (100.0) | 2 (16.7) | 6 (37.5) |
| Patients who died during the study | 0 | 1 (8.3) | 1 (6.3) |
| Patients who died within 30 days of last dose of study medication | 0 | 1 (8.3) | 1 (6.3) |
AE, adverse event; BTZ, bortezomib 1.3 mg/m2; DEX, dexamethasone 20 mg/day; LY, LY2127399 (tabalumab); SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
The most common TEAE possibly related to study treatment were thrombocytopenia, lymphopenia and increased alanine aminotransferase (Table 4). Other common TEAE were fatigue, constipation, peripheral sensory neuropathy and anemia (Table 4). TEAE of CTCAE Grade ≤3 were mostly hematologic, including 7 (43.8%) patients with lymphopenia and 7 (43.8%) patients with anemia. Febrile neutropenia was observed in one patient (6.3%) and no cases of interstitial lung disease were observed. Most of the clinically significant AE were considered to be related to the underlying condition and concomitant medications. There were no clear differences in TEAE between Cohort 1 and Cohort 2 (tabalumab 100 vs 300 mg), or between Cohort 2‐IV and Cohort 2‐SC (bortezomib i.v. vs s.c.).
Table 4.
Treatment‐emergent adverse events related to study drugs
| System order class† Preferred term‡ | Cohort 1 LY 100 mg + BTZ + DEX (N = 4) | Cohort 2‐IV LY 300 mg + BTZ IV + DEX (N = 4) | Cohort 2‐SC LY 300 mg + BTZ SC + DEX (N = 8) | Total (N = 16) |
|---|---|---|---|---|
| Patients with ≥1 possibly related TEAE | 4 (100.0) | 4 (100.0) | 8 (100.0) | 16 (100.0) |
| Blood and lymphatic system disorders | 4 (100.0) | 4 (100.0) | 5 (62.5) | 13 (81.3) |
| Thrombocytopenia | 4 (100.0) | 4 (100.0) | 5 (62.5) | 13 (81.3) |
| Lymphopenia | 0 | 3 (75.0) | 4 (50.0) | 7 (43.8) |
| Anemia | 1 (0.25) | 3 (75.0) | 1 (12.5) | 5 (31.3) |
| Neutropenia | 1 (0.25) | 2 (50.0) | 1 (12.5) | 4 (25.0) |
| Febrile neutropenia | 0 | 1 (25.0) | 0 | 1 (6.3) |
| General disorders and administration site conditions | 2 (50.0) | 4 (100) | 7 (87.5) | 13 (81.3) |
| Fatigue | 2 (50.0) | 2 (50.0) | 2 (25.0) | 6 (37.5) |
| Edema | 0 | 0 | 3 (37.5) | 3 (18.8) |
| Injection site reaction | 0 | 1 (25.0) | 1 (12.5) | 2 (12.5) |
| Malaise | 0 | 2 (50.0) | 0 | 2 (12.5) |
| Peripheral edema | 0 | 1 (25.0) | 1 (12.5) | 2 (12.5) |
| Pyrexia | 0 | 0 | 2 (25.0) | 2 (12.5) |
| Investigations | 4 (100.0) | 3 (75.0) | 4 (50.0) | 11 (68.8) |
| Increased ALT | 4 (100.0) | 1 (25.0) | 2 (25.0) | 7 (43.8) |
| Decreased WBC count | 1 (25.0) | 2 (50.0) | 1 (12.5) | 4 (25.0) |
| Increased AST | 1 (25.0) | 0 | 2 (25.0) | 3 (18.8) |
| Increased blood creatinine | 1 (25.0) | 1 (25.0) | 0 | 2 (12.5) |
| Gastrointestinal disorders | 3 (75.0) | 4 (100.0) | 3 (37.5) | 10 (62.5) |
| Constipation | 3 (75.0) | 1 (25.0) | 2 (25.0) | 6 (37.5) |
| Nausea | 1 (25.0) | 2 (50.0) | 0 | 3 (18.8) |
| Abdominal distension | 0 | 1 (25.0) | 1 (12.5) | 2 (12.5) |
| Diarrhea | 0 | 0 | 2 (25.0) | 2 (12.5) |
| Stomatitis | 1 (25.0) | 1 (25.0) | 0 | 2 (12.5) |
| Metabolism and nutrition disorders | 3 (75.0) | 3 (75.0) | 4 (50.0) | 10 (62.5) |
| Decreased appetite | 2 (50.0) | 1 (25.0) | 2 (25.0) | 5 (31.3) |
| Hypophosphatemia | 2 (50.0) | 1 (25.0) | 2 (25.0) | 5 (31.3) |
| Hypoalbuminemia | 0 | 0 | 3 (37.5) | 3 (18.8) |
| Hyponatremia | 0 | 1 (25.0) | 1 (12.5) | 2 (12.5) |
| Nervous system disorders | 3 (75.0) | 3 (75.0) | 3 (37.5) | 9 (56.3) |
| Peripheral sensory neuropathy | 2 (50.0) | 1 (25.0) | 3 (37.5) | 6 (37.5) |
| Dysgeusia | 1 (25.0) | 1 (25.0) | 1 (12.5) | 3 (18.8) |
| Vagus nerve disorder | 1 (25.0) | 2 (50.0) | 0 | 3 (18.8) |
| Dizziness | 0 | 1 (25.0) | 1 (12.5) | 2 (12.5) |
| Neuralgia | 1 (25.0) | 0 | 1 (12.5) | 2 (12.5) |
| Infections and infestations | 2 (50.0) | 1 (25.0) | 4 (50.0) | 7 (43.8) |
| Nasopharyngitis | 1 (25.0) | 1 (25.0) | 1 (12.5) | 3 (18.8) |
| Skin and subcutaneous tissue disorders | 2 (50.0) | 0 | 3 (37.5) | 5 (31.3) |
| Psychiatric disorders | 1 (25.0) | 1 (25.0) | 1 (12.5) | 3 (18.8) |
| Insomnia | 1 (25.0) | 1 (25.0) | 1 (12.5) | 3 (18.8) |
| Respiratory, thoracic and mediastinal disorders | 1 (25.0) | 0 | 2 (25.0) | 3 (18.8) |
| Vascular disorders | 0 | 2 (50.0) | 1 (25.0) | 3 (18.8) |
| Eye disorders | 1 (25.0) | 1 (25.0) | 0 | 2 (12.5) |
| Ear and labyrinth disorders | 0 | 0 | 1 (12.5) | 1 (6.3) |
†Patients were only counted once for each preferred term but could be counted in more than one preferred term within each system organ class; events were coded using MedDRA Version 17.1. ‡Preferred terms are only shown for TEAE that occurred in ˃ 1 patient (total). System order classes are shown for all TEAE. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BTZ, bortezomib 1.3 mg/m2; DEX, dexamethasone 20 mg/day; IV, intravenous; LY, LY2127399 (tabalumab); MedDRA, Medical Dictionary for Regulatory Activities; N, number of patients; SC, subcutaneous; TEAE, treatment‐emergent adverse event; WBC, white blood cell.
Two DLT occurred during Cycle 1. One patient in Cohort 2‐IV had febrile neutropenia and peripheral sensory neuropathy and one patient in Cohort 2‐SC had syncope and peripheral sensory neuropathy (all CTCAE Grade 3). No other DLT were observed in subsequent cycles.
Discontinuation from the study
A total of 4 (100%) patients in Cohort 1 and 11 (91.7%) patients in Cohort 2 discontinued the study prematurely (Table 5).
Table 5.
Summary of reasons for discontinuation of study treatment
| Cohort 1 LY 100 mg + BTZ + DEX (N = 4) | Cohort 2 LY 300 mg + BTZ + DEX (N = 12) | Total (N = 16) | |
|---|---|---|---|
| Patients entered | 4 (100.0) | 12 (100.0) | 16 (100.0) |
| Patients who received at least one study medication | 4 (100.0) | 12 (100.0) | 16 (100.0) |
| Patients discontinued from study | 4 (100.0) | 11a (91.7) | 15a (93.8) |
| Reasons for discontinuation | |||
| Adverse events | 4 (100.0) | 2 (16.7) | 6 (37.5) |
| Colon cancer | 0 | 1 (8.3) | 1 (6.3) |
| Constipation | 2 (50.0) | 0 (0.0) | 2 (12.5) |
| Peripheral sensory neuropathy | 2 (50.0) | 1 (8.3) | 3 (18.8) |
| Death | 0 (0.0) | 1 (8.3) | 1 (6.3) |
| Sponsor decision | 0 (0.0) | 2 (16.7) | 2 (12.5) |
| Progressive disease | 0 (0.0) | 6 (50.0) | 6 (37.5) |
One patient was still continuing the study at the data cut‐off. BTZ, bortezomib 1.3 mg/m2; DEX, dexamethasone 20 mg/day; LY, LY2127399 (tabalumab).
Pharmacokinetics
Tabalumab
Serum tabalumab concentrations decreased biexponentially over time with a terminal half‐life of 18–25 days following the first dose (Table 6 and Fig. 2). The pharmacokinetics of tabalumab were similar when bortezomib was coadministered i.v. versus s.c. (Fig. 2).
Table 6.
Summary of tabalumab pharmacokinetic parameters
| Parameter | Cycle 1, Day 1 | Cycle 7, Day 1 | ||||
|---|---|---|---|---|---|---|
| Cohort 1 LY 100 mg + BTZ + DEX (N = 4) | Cohort 2‐IV LY 300 mg + BTZ IV + DEX (N = 4) | Cohort 2‐SC LY 300 mg + BTZ SC + DEX (N = 8) | Cohort 1 LY 100 mg + BTZ + DEX (N = 1) | Cohort 2‐IV LY 300 mg + BTZ IV + DEX (N = 1) | Cohort 2‐SC LY 300 mg + BTZ SC + DEX (N = 3) | |
| C max,† μg/mL | 38.5 (12) | 154 (21) | 139 (14) | 70.5 | 179 | 220 (25) |
| t max,‡ h | 2.58 (0.68–6.37) | 0.65 (0.60–2.52) | 1.56 (0.55–6.75) | 0.60 | 0.55 | 0.68 (0.58–2.50) |
| t 1/2,§ h | 434 (343–528) | 602 (363–920) | 433 (145–916) | NC | 443 | 379¶ |
| AUC(0–t last),† μg·h/mL | 8100 (15) | 34 600 (23) | 26 900 (48) | 9400 | 27 300 | 22 900 (71) |
†Geometric mean (CV%). ‡Median (range). §Geometric mean (range). AUC, area under the concentration–time curve; AUC(0–t last), area under the concentration–time curve from 0 h to the last measurable concentration; BTZ, bortezomib 1.3 mg/m2; C max, maximum concentration; CV, coefficient of variation; DEX, dexamethasone 20 mg/day; LY, LY2127399 (tabalumab); NC, not calculated; t 1/2, half‐life; t max, time at which maximum concentration is reached.¶n=1
Figure 2.
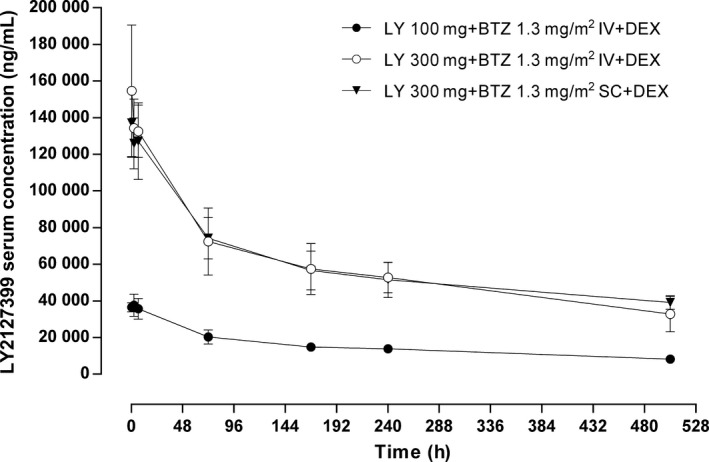
Mean ± SD serum LY2127399 (tabalumab) concentration–time profiles (linear) following intravenous infusion of LY2127399 (tabalumab) 100 or 300 mg in combination with i.v. or s.c. bortezomib 1.3 mg/m2 and oral dexamethasone 20 mg during Day 1 of Cycle 1 and Cycle 7. BTZ, bortezomib 1.3 mg/m2; DEX, dexamethasone 20 mg; IV, intravenous; LY, LY2127399 (tabalumab); SC, subcutaneous.
Bortezomib
Plasma bortezomib concentrations decreased exponentially over time (Table 7 and Fig. 3). No differences in bortezomib pharmacokinetics were observed when bortezomib was administered i.v. versus s.c. (Fig. 3). The ratio of the area under the curve from time 0 to infinity (AUC(0–∞)) following s.c. administration relative to i.v. administration was 74.5%.
Table 7.
Summary of bortezomib pharmacokinetic parameters
| Parameter | Cycle 1, Day 1 | ||
|---|---|---|---|
| Cohort 1 LY 100 mg + BTZ + DEX (N = 4) | Cohort 2‐IV LY 300 mg + BTZ IV + DEX (N = 4) | Cohort 2‐SC LY 300 mg + BTZ SC + DEX (N = 8) | |
| C max,† ng/mL | 103 (650) | 185 (42) | 15.9 (23) |
| t max,‡ h | 0.05 (0.02–0.50) | 0.06 (0–0.08) | 0.50 (0.48–1.00) |
| t 1/2,§ h | 16.8 (10.0–25.4) | 16.2 (12.1–23.6) | 14.8 (9.05–31.8) |
| AUC(0–∞),† ng·h/mL | 69.4 (52) | 77.7 (41) | 57.9 (36) |
†Geometric mean (CV%). ‡Median (range). §Geometric mean (range). AUC, area under the concentration–time curve; AUC(0–∞), area under the concentration–time curve from 0 h to infinity; BTZ, bortezomib 1.3 mg/m2; C max, maximum concentration; CV, coefficient of variation; DEX, dexamethasone 20 mg/day; t 1/2, half‐life; t max, time at which maximum concentration is reached.
Figure 3.
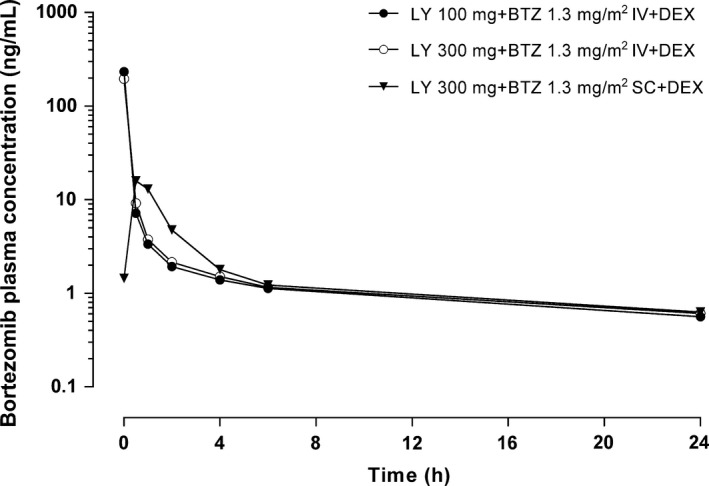
Mean plasma bortezomib concentration–time profiles (semi‐logarithmic) following intravenous infusion of LY2127399 (tabalumab) 100 or 300 mg in combination with i.v. or s.c. bortezomib 1.3 mg/m2 and oral dexamethasone 20 mg during Day 1 of Cycle 1. BTZ, bortezomib 1.3 mg/m2; DEX, dexamethasone 20 mg; IV, intravenous; LY, LY2127399 (tabalumab); SC, subcutaneous.
Overall tumor response
All four patients in Cohort 1 and five of 12 (41.7%) patients in Cohort 2 responded to treatment; the overall response rate was 56.3% (Table 8). Of the three patients in Cohort 2 with progressive disease, two progressed in Cycle 1 after one dose of tabalumab and one progressed in Cycle 2 after two doses of tabalumab. There was no clear relationship between the baseline BAFF level and tumor response (Fig. 4). For most patients, the immunoglobulins IgA, IgG and Ig M either remained stable or decreased during treatment (Fig. 5).
Table 8.
Best overall tumor response
| Response to treatment | Cohort 1 LY 100 mg + BTZ + DEX (N = 4) | Cohort 2 LY 300 mg + BTZ + DEX (N = 12) | Total (N = 16) |
|---|---|---|---|
| Best overall response, n (%) | |||
| CR | 0 (0.0) | 0 (0.0) | 0 |
| VGPR | 2 (50.0) | 1 (8.3) | 3 (18.8) |
| PR | 2 (50.0) | 4 (33.3) | 6 (37.5) |
| SD | 0 (0.0) | 2 (16.7) | 2 (1.3) |
| PD | 0 (0.0) | 3a (25.0) | 3 (18.8) |
| Unknown | 0 (0.0) | 2 (16.7) | 2 (12.5) |
| Overall response rate, % | 100.0 | 41.7 | 56.3 |
Two patients had PD in Cycle 1 after one dose of tabalumab; one patient had PD in Cycle 2 after two doses of tabalumab. BTZ, bortezomib 1.3 mg/m2; DEX, dexamethasone 20 mg/day; CR, complete response; LY, LY2127399 (tabalumab); PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response.
Figure 4.
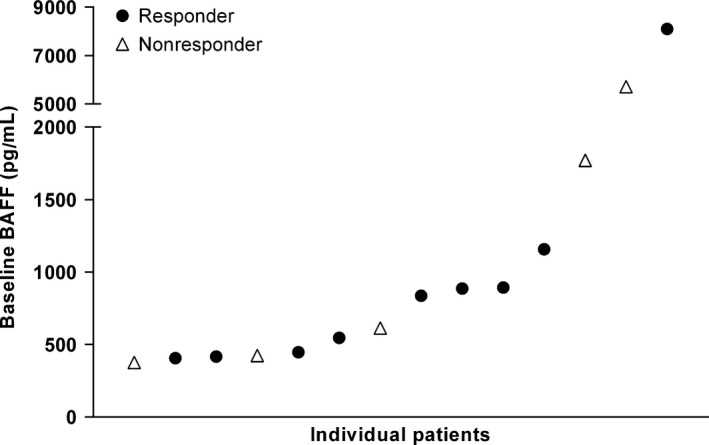
Relationship between baseline B‐cell activating factor (BAFF) levels and tumor response. Each data point represents an individual patient for responders (partial response or better: ●) and nonresponders (stable or progressive disease: Δ). For two patients, the response status was unknown.
Figure 5.
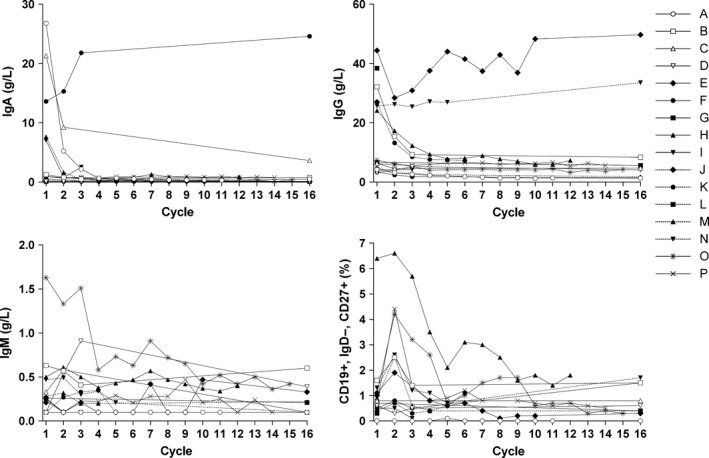
Change in the immunoglobulins (Ig) A, G and M, and CD19+, IgD−, CD27+ (mature naïve B‐cell subset) over time for each patient in Cohort 1 (……..) and Cohort 2 (____). BTZ, bortezomib 1.3 mg/m2; DEX, dexamethasone 20 mg; IV, intravenous; LY, LY2127399 (tabalumab); SC, subcutaneous.
Pharmacodynamics
The mature naïve B‐cell subset also largely remained stable or decreased during treatment in most patients (Fig. 5). Patient H, who started at the highest CD19+, IgD−, CD27+ value and had the largest decrease over time, had a very good partial response to treatment and continued for 12 cycles.
Discussion
This is the first study to evaluate the safety and efficacy of tabalumab 100 or 300 mg in combination with bortezomib and dexamethasone in Japanese patients with RRMM. The safety profile of the combined treatment was consistent with the known safety profile of the individual treatments and 2 (20.0%) DLT were observed at the 300‐mg dose of tabalumab, below the predetermined 33% cutoff for tolerability. The overall response rate was 56.3%, suggesting that the combined treatment was effective even in aggressively pretreated patients. We revaluated the relationship between tumor response and clinical profile in each patient. In Cohort 2, refractory patients occupied 50% (0% in Cohort 1), and 16.7% of patients were treated with more than three regimens. Therefore, the lower response rate in Cohort 2 was likely due to the more heavily treated or refractory population compared with patients in Cohort 1.
All patients experienced at least one TEAE possibly related to the study drug. However, the most commonly observed TEAE (thrombocytopenia, lymphopenia, anemia, neutropenia, fatigue and constipation) have been associated with bortezomib.19, 20 Mild lymphopenia and neutropenia were reported but there was no indication that long‐term treated patients were immunocompromised. The number and type of TEAE and the pharmacokinetics of Cohorts 2‐IV and 2‐SC were similar, thus no tabalumab dose adjustments were needed when switching between bortezomib i.v. and s.c.
Although the patient numbers were small, the tumor response findings in the present phase 1 study suggested that tabalumab in combination with bortezomib and dexamethasone was effective in some patients with RRMM. The combined overall response rate for both cohorts was 56.3%, similar to an earlier phase 1 study in previously treated RRMM patients in the USA (46%),11 and there were no safety concerns. This suggests that tabalumab may be a promising treatment for patients with RRMM. However, a phase 2 study of tabalumab 100 or 300 mg versus placebo in combination with bortezomib s.c. and dexamethasone in some patients with RRMM failed to demonstrate a difference in progression‐free survival, the primary efficacy endpoint (JDCG; N = 220; ClinicalTrial.gov: NCT01602224).
Informal assessment of the combined results of the current phase 1 study and the phase 2 JDCG study suggest that very few RRMM patients who have baseline BAFF concentrations above 1500 pg/mL respond to tabalumab (unpublished data). It is possible that at these concentrations, BAFF cannot be fully neutralized by tabalumab 300 mg. Although full BAFF neutralization may be achieved with higher tabalumab doses, the risk was considered to outweigh any potential benefits and clinical development of tabalumab in RRMM was discontinued. It is also possible that a high serum BAFF concentration is a surrogate biomarker in terms of the disease activity in patients with RRMM.3
The lack of efficacy in the phase 2 JDCG study may also be due to the activity of a proliferation‐inducing ligand (APRIL), another member of the TNF family, which induces proliferation independently of BAFF. Both BAFF and APRIL bind to BCMA (B‐cell maturation antigen) and TACI (transmembrane activation and calcium modulator and cyclophilin ligand interaction), resulting in the activation of the nuclear factor‐kappa B, mitogen‐activation protein kinase, and phosphatidylinositol‐3 kinase to Akt pathways in MM cells.10 Thus, tabalumab combined with anti‐APRIL antibody or TACI‐Fc fusion protein, a potent inhibitor of both BAFF and APRIL, could augment clinical efficacy in RRMM.21
This is the first report of a clinical test of anti‐BAFF treatment for multiple myeloma patients, so we believe the data will be valuable when considering the clinical significance of BAFF‐APRIL pathway involved in myeloma pathogenesis. In this study we evaluated tabalumab in combination with bortezomib, the standard regimen for MM. We believe that the data of this study could be a valuable future reference for these potential combination therapies, even though the result was negative. Investigating a future combination therapy with anti‐APRIL antibody or TACI‐Fc fusion protein which might enhance blockages of the pathway would be very interesting.
In conclusion, i.v. tabalumab 100 or 300 mg administered in combination with i.v. or s.c. bortezomib and dexamethasone was well tolerated in this population of Japanese patients with RRMM.
Disclosure Statement
HA, KM, KU, SN, TI and IC are employees of Eli Lilly and Company. SI has received honoraria from Janssen, Celgene, Ono, Takeda and Bristol‐Myers Squibb and research funding from Ono, Eli Lilly, Celgene, Janssen, Chugai, Kyowa Hakko Kirin and Takeda. MT has received research funding from Chugai, Bristol‐Myers Squibb, and Kyowa Hakko Kirin. KT has received research funding from Chugai, Kyowa Hakko Kirin, Ono, Celgene, Janssen, GlaxoSmithKline, Eisai, Mundipharma, Takeda, Servier and Abbvie. DO and YA have no conflicts of interest to declare.
Acknowledgments
The authors would like to thank all study participants. This study was sponsored by Eli Lilly and Company. Medical writing assistance was provided by Michelle Anderson, PhD, and Rebecca Lew, PhD, CMPP of ProScribe – Envision Pharma Group, and was funded by Eli Lilly Japan K.K.
Cancer Sci 107 (2016) 1281–1289
Funding Information
Eli Lilly and Company.
References
- 1. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364: 1046–60. [DOI] [PubMed] [Google Scholar]
- 2. Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T. Cancer incidence and incidence rates in Japan in 2005: based on data from 12 population‐based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2011; 41: 139–47. [DOI] [PubMed] [Google Scholar]
- 3. Hengeveld PJ, Kersten MJ. B‐cell activating factor in the pathophysiology of multiple myeloma: a target for therapy? Blood Cancer J 2015; 5: e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009; 9: 491–502. [DOI] [PubMed] [Google Scholar]
- 5. He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol 2004; 172: 3268–79. [DOI] [PubMed] [Google Scholar]
- 6. Moreaux J, Legouffe E, Jourdan E et al BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004; 103: 3148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haiat S, Billard C, Quiney C, Ajchenbaum‐Cymbalista F, Kolb JP. Role of BAFF and APRIL in human B‐cell chronic lymphocytic leukaemia. Immunology 2006; 118: 281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tai YT, Li XF, Breitkreutz I et al Role of B‐cell‐activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res 2006; 66: 6675–82. [DOI] [PubMed] [Google Scholar]
- 9. Manetta J, Bina H, Ryan P, Fox N, Witcher DR, Kikly K. Generation and characterization of tabalumab, a human monoclonal antibody that neutralizes both soluble and membrane‐bound B‐cell activating factor. J Inflamm Res 2014; 7: 121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neri P, Kumar S, Fulciniti MT et al Neutralizing B‐cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res 2007; 13: 5903–9. [DOI] [PubMed] [Google Scholar]
- 11. Merrill JT, van Vollenhoven RF, Buyon JP et al Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B‐cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE‐2, a 52‐week, phase III, multicentre, randomised, double‐blind, placebo‐controlled study. Ann Rheum Dis 2016; 75: 332–40. [DOI] [PubMed] [Google Scholar]
- 12. Smolen JS, Weinblatt ME, van der Heijde D et al Efficacy and safety of tabalumab, an anti‐B‐cell‐activating factor monoclonal antibody, in patients with rheumatoid arthritis who had an inadequate response to methotrexate therapy: results from a phase III multicentre, randomised, double‐blind study. Ann Rheum Dis 2015; 74: 1567–70. [DOI] [PubMed] [Google Scholar]
- 13. Isenberg AD, Petri M, Kalunian K et al Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE‐1, a 52‐week, phase III, multicentre, randomised, double‐blind, placebo‐controlled study. Ann Rheum Dis 2016; 75: 323–31. [DOI] [PubMed] [Google Scholar]
- 14. Ocio EM, Richardson PG, Rajkumar SV et al New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia 2014; 28: 525–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raje NS, Hohl RJ, Faber EA et al Phase I study of LY2127399, a human anti‐BAFF antibody, and bortezomib in patients with previously treated multiple myeloma. J Clin Oncol 2011; 29: (suppl; abstr 8012). [Google Scholar]
- 16. Li X, Pennisi A, Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood 2008; 112: 159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calhoun EA, Welshman EE, Chang CH et al Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity (Fact/GOG‐Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 2003; 13: 741–8. [DOI] [PubMed] [Google Scholar]
- 18. Durie BG, Harousseau JL, Miguel JS et al International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–73. [DOI] [PubMed] [Google Scholar]
- 19. Millennium Pharmaceuticals . VELCADE (Bortezomib): Highlights of Prescribing Information. Cambridge, MA: Millennium Pharmaceuticals, 2014. [Google Scholar]
- 20. Moreau P, Pylypenko H, Grosicki S et al Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non‐inferiority study. Lancet Oncol 2011; 12: 431–40. [DOI] [PubMed] [Google Scholar]
- 21. Abe M, Kido S, Hiasa M et al BAFF and APRIL as osteoclast‐derived survival factors for myeloma cells: a rationale for TACI‐Fc treatment in patients with multiple myeloma. Leukemia 2006; 20: 1313–5. [DOI] [PubMed] [Google Scholar]


