Abstract
BACKGROUND
IDH1mutated glioblastoma (GB) has a better prognosis than IDH1wildtype GB. However, it remains unknown whether patients (pts)with IDH1mutated GB ha ve a higher 6-month progression free survival (PFS6) or radiographic response (RR) rate on clinical trials for recurrence.
DESIGN/METHODS
Retrospective review of GB pts at MDACC between 2006–2012 identified 330 pts in recurrent GB trials. 93 pts (28%) had either PFS6 or a complete/partial RR per RANO criteria. 49/93 (53%) pts with PFS6 or a complete/partial RR had tumor tissue for IDH1testing. A matched cohort of 49 pts on recurrent GB clinical trials without PFS6 or RR (also with tissue for IDH1 testing)was identified for comparison.
RESULTS
IDH1status was obtained in 92/98 (94%) pts of which 17 (18%) had an IDH1mutation. PFS6 was seen in 26/49 (53%) pts. IDH status was unknown in 2 of these pts. 5/24 (21%) were IDH1 mutated compared to 5/24 (21%) of their matched cohort without PFS6. RR was found in 47/49 (94%) pts. IDH status was unknown in 4 of these pts. IDH1mutation was present in 7/43 (16%) pts with RR compared to 10/43 (23%) in the matched cohort without RR (p=0.48). Median OS for trials at 1strecurrence was 9.8 months for IDH1wildtype GB vs. 19.32 months for IDH1 mutated GB (p=0.14).
CONCLUSIONS
IDH1mutation status was notpredictive of PFS6 or RR in recurrent GB trials for this data set . However, further examination in larger randomized prospective studies is needed.
Keywords: IDH1mutation, recurrent glioblastoma, clinical trials
INTRODUCTION
Glioblastoma (GB) is the most common malignant primary brain tumor in adults.1 Overall median survival for patients with GB treated with maximal resection, 6 weeks of concurrent chemoradiation with daily temozolomide followed by 6 to 12 cycles of adjuvant temozolomide is approximately 16 months.2 Although bevacizumab is approved for recurrent disease, treatment with clinical trials is recommended.
GB historically has been classified as primary GB if it develops de novo or as secondary GB if it progresses clinically from a low-grade or anaplastic astrocytoma.3 The histopathologic findings for primary and secondary GBs are indistinguishable (consisting of microvascular proliferation or necrosis). Secondary GB occurs less frequently (~5% of GBs) and typically in younger patients (median age ~45 years vs. ~60 years for primary GB).4 However, the prognosis of primary and secondary GB does not appear to be different after adjustment for age5 and recurrent GBs clinical trials currently do not stratify patients based upon this distinction.
Recently, molecular profiling is being utilized to separate diffuse gliomas including GB into prognostic groups.6,7 A mutation affecting codon 132 of the isocitrate dehydrogenase 1 (IDH1) gene was found to occur in up to 12% of glioblastomas.8,9 A review of sequencing studies on large glioma patient cohorts have found IDH1 mutations present in 6% (range 3–16) of clinically defined primary glioblastoma and 76% (range 73–88) of clinically defined secondary glioblastoma.10
However, IDH1 mutation is now considered the definitive diagnostic molecular marker of secondary glioblastomas and is considered more reliable and objective than clinical criteria.11 Long term outcome for patients with high-grade glioma including GB directly correlates with IDH1 mutation status. IDH1 mutated tumors have been associated with an improved outcome comparable to IDH1 wild-type tumors.12,13 IDH1 mutation has remained an independent favorable prognostic marker even after adjustment for age, grade, MGMT status, genomic profile, and treatment in multivariate analysis.14
Despite IDH1mutated tumors being associated with an improved outcome, clinical trials for recurrent GB currently do not stratify patients based upon IDH status. Historically, the PFS6 rate for recurrent glioblastoma trials is approximately 9–12% which could be similar to the estimated percentage of patients on clinical trials expected by chance to have IDH1 mutated tumors. It remains unknown if patients with IDH1mutated GB on clinical trials are more likely to have a higher 6-month progression free survival (PFS6) or radiographic response (RR) rate.
The goal of this study was to examine if patients on recurrent GB trials with PFS6 and/or RR were more likely to have an IDH1 mutation and thus require future recurrent GB clinical trials to stratify or exclude patients with IDH1mutation .
METHODS
Patients
After obtaininginstitutional board review approval (protocol PA12 –0938), we retrospectively identified 330 GB patients treated at MD Anderson on clinical trials for recurrent disease from 2006–2012 in our institutional database. All of the patients had a pathologically-confirmed diagnosis of glioblastoma using World Health Organization (WHO) criteria.
Data was then collected on all patients including date of birth, date of GB diagnosis, extent of resection at GB diagnosis as determined by imaging or operative reports if imaging was not available, Karnofsky performance status score (KPS) at GB diagnosis and recurrent GB trial, date of recurrent GB trial, type of chemotherapies and/or targeted therapies used on recurrent GB trial, number of chemotherapy regimens tried prior to start of recurrent GB trial, PFS on recurrent GB trial, imaging response on recurrent GB clinical trial per RANO criteria15, date of imaging response, date of death and/or last follow up.
For patients with a previous diagnosis of a lower grade tumor (low grade or anaplastic glioma), additional data was collected including date of initial tumor diagnosis, histopathologic grade of initial tumor, extent of resection at initial tumor diagnosis, and KPS at initial tumor diagnosis.
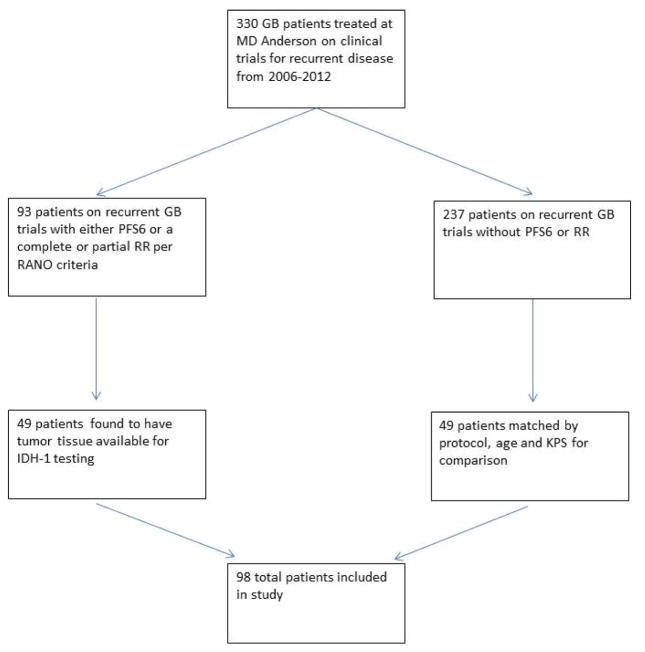
93 patients (28%) on recurrent GB trials were found to have had either a PFS6 or a complete or partial RR per RANO criteria. Of these 93 patients, 49 (53%) were found to have tumor tissue available for IDH1testing. These 49 patients were then matched by protocol, age and KPS to 49 of the 237 patients on recurrent GB trials that did not have a PFS6 or RRfor a total study cohort of 98 patients. (Figure 1)
Figure 1.
Study diagram
Retrospectively identified 330 GB patients treated at MD Anderson on clinical trials for recurrent disease from 2006–2012. 93 patients on recurrent GB trials were found to have had either a PFS6 or a complete or partial RR per RANO criteria. Of these 93 patients, 49 were found to have tumor tissue available for IDH1 testing. These 49 patients were then matched by protocol, age and KPS to 49 of the 237 patients on recurrent GB trials that did not have a PFS6 or RR but also had tumor tissue available for IDH1 testing for a total study cohort of 98 patients.
IDH1mutation testing was performed by immunohistochemistry. Immunohistochemistry was manually performed with anti-IDH1-R132H mouse anticlonal antibody (Dianova, Hamburg, Germany, clone H09, dilution 1:200). All controls were appropriate. Two experienced neuropathologists (K.A. and G.F) were blinded from previously obtained clinical data and independently analyzed the tissue samples.
Statistical Analysis
Patient demographic and clinic-pathologicalcharacteristics such as age, performance status (KPS), extent of surgical resections, grades of tumors were summarized as a whole and by IDH groups, using standard descriptive statistics and frequency tabulation. Chi-squared test or Fisher’s exact test were used to test the any difference of categorical variables between IDH groups. Progression free survival and overall survival from initial diagnosis, GB diagnosis and start of recurrent GB trial were estimated using the Kaplan-Meier method and the comparisons between IDH groups were assessed using log-rank test. Multicovariate Cox proportional hazard models were applied to assess the effect of covariates of interest (age, grade, extent of surgical resections, KPS at initial tumor diagnosis and/or GBMdi agnosis, and IDH1status) on progression free survival and overall survival. All computations were carried out in SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and Splus 8.2 (TIBCO Software Inc, Palo Alto, CA) or R 2.15.1.
RESULTS
Median age at GB diagnosis was 48.2 years (range 19.3–78.6). Median time from GB diagnosis to recurrent GB clinical trial was 9.7 months. KPS prior to enrollment on recurrent GBtrial was equal to or greater than 90 in 34.7% of patients. (Table 1)
Table 1.
Clinical characteristics of patients on recurrent GB trials
| IDH | |||||
|---|---|---|---|---|---|
| covariate | levels | ALL | wildtype | mutated | p_value |
| Initial tumor grade | 2 | 13(13.3%) | 5(6.7%) | 7(41.2%) | <.0001 |
| 3 | 10(10.2%) | 5(6.7%) | 4(23.5%) | ||
| 4 | 75(76.5%) | 65(86.7%) | 6(35.3%) | ||
| Imaging response | 0 | 49(50%) | 37(49.3%) | 10(58.8%) | .4797 |
| 1 | 49(50%) | 38(50.7%) | 7(41.2%) | . | |
| Number of recurrences prior to trial | 1 | 61(62.2%) | 54(72%) | 5(29.4%) | .0039 |
| 2 | 25(25.5%) | 12(16%) | 9(52.9%) | ||
| 3 | 8(8.2%) | 6(8%) | 2(11.8%) | ||
| 4 | 4(4.1%) | 3(4%) | 1(5.9%) | ||
| Extent of prior GB resection | Gross total | 23(23.5%) | 12(16%) | 8(47.1%) | .0052 |
| Near total | 18(18.4%) | 12(16%) | 5(29.4%) | ||
| Subtotal | 10(10.2%) | 8(10.7%) | 0(0%) | ||
| Biopsy | 47(48%) | 43(57.3%) | 4(23.5%) | ||
| KPS at initial tumor diagnosis | Unknown | 3 | |||
| <=80 | 21(22.1%) | 19(25.7%) | 1(6.7%) | .2744 | |
| 90 | 32(33.7%) | 25(33.8%) | 6(40%) | ||
| 100 | 42(44.2%) | 30(40.5%) | 8(53.3%) | ||
| KPS at GB diagnosis | <=80 | 21(21.4%) | 17(22.7%) | 3(17.6%) | .9417 |
| 90 | 36(36.7%) | 27(36%) | 7(41.2%) | ||
| 100 | 41(41.8%) | 31(41.3%) | 7(41.2%) | ||
| KPS at recurrent GB trial | <=80 | 64(65.3%) | 49(65.3%) | 9(52.9%) | .4661 |
| 90 | 16(16.3%) | 13(17.3%) | 3(17.6%) | ||
| 100 | 18(18.4%) | 13(17.3%) | 5(29.4%) | ||
| PFS6 | No | 72(73.5) | 56(74.7) | 12(70.6%) | .7635 |
| Yes | 26(26.5%) | 19(25.3%) | 5(29.4) | ||
Recurrent GB trial was at 1st-4th tumor recurrence from GB diagnosis with 62% of trials at the 1strecurrence, 26% at 2nd, 8% at 3rd and 4% at 4th recurrence. Median OS for patients on trial for 1strecurrence was 11.5 months, 2 ndrecurrence 8.11 months, 3 rdrecurrence 6.44 months, and 4threcurrence 5.67 months. (p=0.17) Recurrent GB trial was at 1st recurrence in 30 patients (61%) with PFS6 and/or RR compared to 31 patients (63%) in the matched cohort without PFS6 or RR. Median time from GB diagnosis to recurrent GB trial for thecohort of patients with PFS6 and/or RR was 9.79 months and 8.44 months for the cohort of patients without PFS6 or RR (p= 0.60)
The most common recurrent trial consisted of a bevacizumab containing regimen (38%), followed by XL-184 (cabozantinib, Exelixis;22%), temozolomide containing regimen (12%), carboplatin containing regimen (10%), lapatinib and pazopanib (8%), other (6%) and VEGF trap (4%). (Supplementary Table 1)
Of the 49 patients in the cohort with PFS6 and/or RR, RR consisted ofa partial respo nse in 45 patients and complete response in 2 patients(the other 2 patients had PFS6 without RR but had stable imaging). IDH1mutation status was in determinate in 4 patientswith RR . IDH1 mutation was present in 7/43 (16%) pts with RR compared to 10/43 (23%) patients of their matched cohort without RR (p=0.48).
PFS6 was seen in 26/49 (53%) patients. IDH1mutation status was incomplete in 2 patients. 5/24 (21%) pts with PFS6 had IDH1 mutations compared to 5/24 (21%) pts of their matched cohort without PFS6 (p=1.0).
Prior diagnosis of low grade or anaplastic glioma
Prior diagnosis of low grade or anaplastic glioma was found in 23% (23/98) of patients of which 13 (57%) were initially diagnosed as low grade and 10 as anaplastic.
Prior diagnosisof low grade or anaplastic glioma was seen in 18% (9/49) of patients with PFS6 and/or RR compared to 29% of patients (14/49) in the matched cohort without PFS6 or RR.
Initial tumor diagnosis in the PFS6 and/or RR cohort included 3 anaplastic astrocytomas, an infiltrating diffuse glioma, a low grade oligodendroglioma, a low grade oligoastrocytoma, and 3 low grade astrocytomas.
Initial tumor diagnosis in the matched cohort without PFS6 or RR included 6 anaplastic astrocytomas, an anaplastic oligodendroglioma, a low grade oligoastrocytoma, a low grade oligodendroglioma, and 5 low grade astrocytomas.
IDH1Mutation status
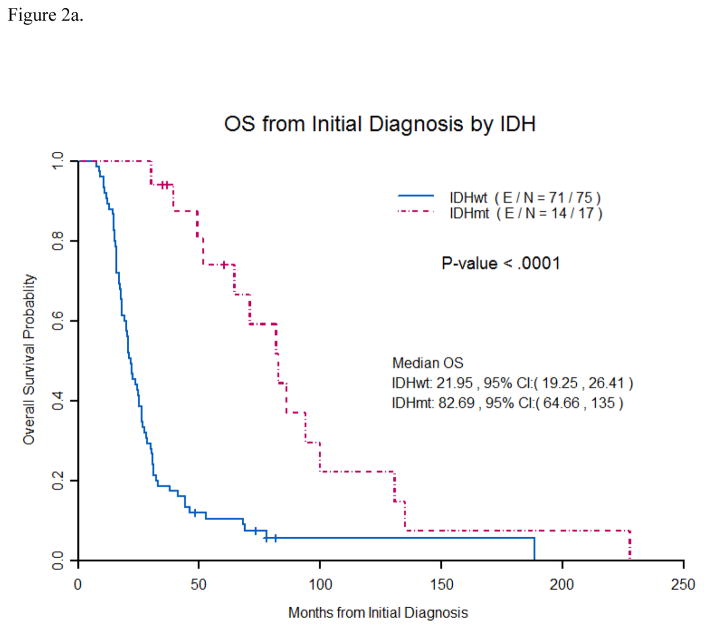
IDH1status was obtained in 92 (94%) patients of which 17 (18%) had an IDH1R132H mutation. IDH1mutation was seen in 8% (6/71) of patients with initial diagnosis ofGB (4 unknown) and 52% (11/21) ofGB patients with a prior diagnosis of low grade or anaplastic glioma(2 unknown). Median age at initial tumor diagnosis for IDH1mutated tumors was 34.9 years (range 23.3–56.3) and 52.7 years (range 19.3–77.4) for IDH1wil dtype tumors(p=.0001) . Median overall survival(OS) from initial tumor diagnosis was 22 months for IDH1 wildtype tumors vs. 83 months for IDH1 mutated tumors (p<0.0001). (Figure 2a) IDH1mutation status as a prognostic marker remained significant in multivariate analysis (p=0.0005). (Table 2)
Figure 2.
Figure 2a. Overall survival from initial tumor diagnosis by IDH1 mutation status
Patients with an IDH1 mutation were found to have a significantly longer median OS than patients with an IDH1 wildtype tumor (83 months vs. 22 months) from initial tumor diagnosis. ( E/N- E stands for events and N is total number of patients).
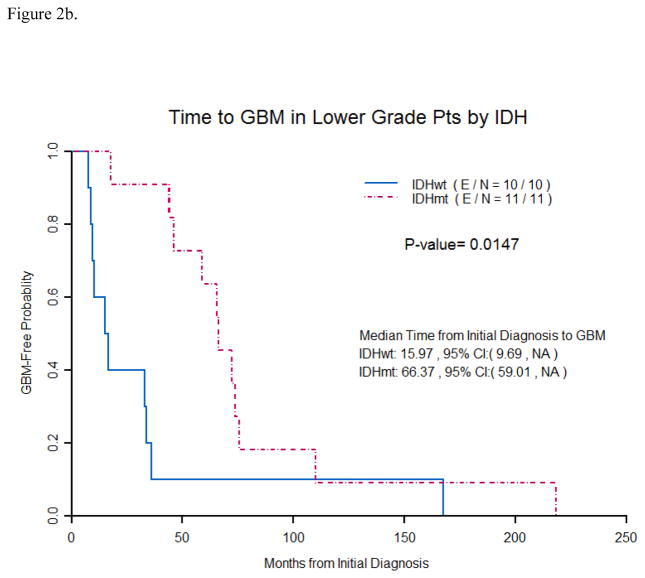
Figure 2b. Time from initial tumor diagnosis to GB diagnosis by IDH Mutation status
Patients initially diagnosed with a low grade or anaplastic glioma with an IDH1 mutation were found to have a significantly longer time (66 months vs. 16 months) from initial tumor diagnosis to GB diagnosis than patients initially diagnosed with a low grade or anaplastic glioma with an IDH1 wildtype tumor. ( E/N- E stands for events and N is total number of patients).
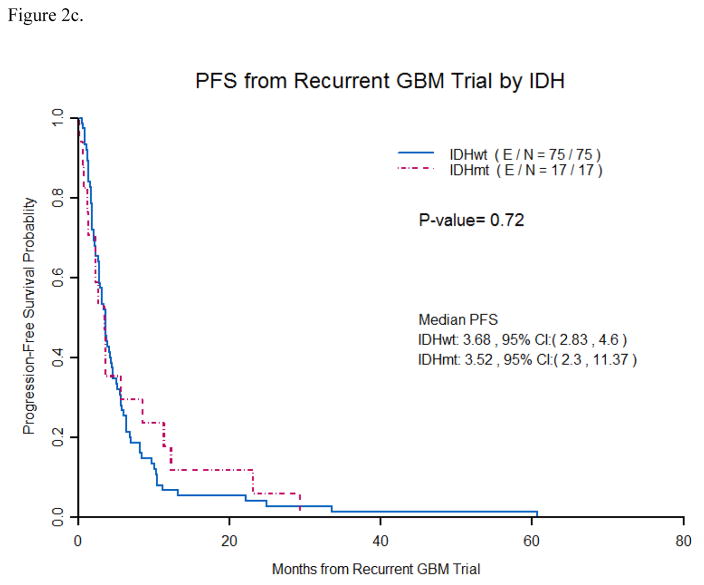
Figure 2c. Progression free survival from recurrent GB trial by IDH Mutation status
Patients with IDH1 mutations were not found to have a significantly longer median PFS (3.5 months vs. 3.6 months) than patients with IDH1 wildtype tumors from the start of recurrent GB trials. ( E/N- E stands for events and N is total number of patients).
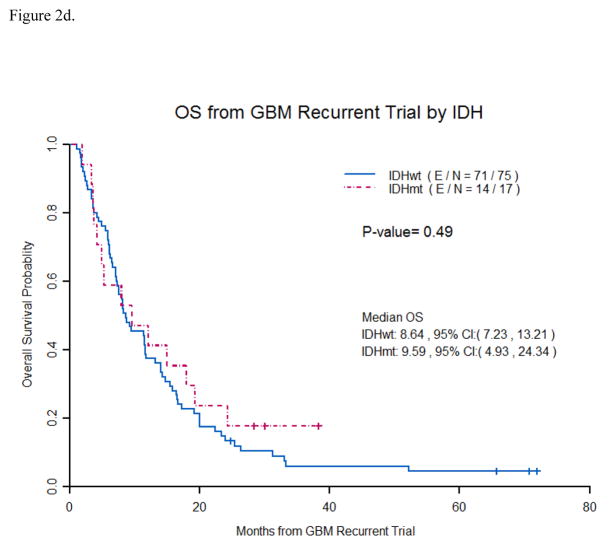
Figure 2d. Overall survival from recurrent GB trial by IDH Mutation status
Patients with IDH1 mutations were not found to have a significantly longer median OS (9.6 months vs.8.6 months) than patients with IDH1 wildtype tumors from the start of recurrent GB trials. ( E/N- E stands for events and N is total number of patients).
Table 2.
Multicovariate Cox Model for OS from Original Diagnosis
| Parameter | Parameter Estimate | Standard Error | p-value | Hazard Ratio | 95% HR CI | ||
|---|---|---|---|---|---|---|---|
| Grade | 2 vs 4 | −0.68 | 0.37 | 0.06 | 0.504 | 0.246 | 1.035 |
| 3 vs 4 | −0.81 | 0.42 | 0.06 | 0.444 | 0.193 | 1.021 | |
| IDH | 1 vs 0 | −1.20 | 0.35 | 0.0005 | 0.301 | 0.152 | 0.594 |
Of the lower grade tumors, 7/12 (58%) were IDH1mutated (1 unknown) and of the anaplastic glioma 4/9 (44%) were IDH1mutated (1 unknown). Median OS from initial tumor diagnosis for low grade or anaplastic glioma was 36.3 months for IDH1wildtype tumors vs. 82.7 months for IDH1mutated tumors (p=0.0327).
Median time from low grade or anaplastic glioma diagnosis to GB diagnosis was 35.9 months (range 6.7–218.4). Median time from low grade or anaplastic glioma diagnosis to GB diagnosis for IDH1mutated tumors was 66.4 months (range 17.7 –218.4) and 16 months (range 7.6–167.4) or IDH1wildtype tumors (p= 0.01 ). (Figure 2b)
KPS at GB diagnosis was greater than 80 in 78.6 % of patients (77/98) with no difference in KPS between IDH1mutated and IDH1wildtype tumors (p=0.94 ). Median overall survival from GB diagnosis was 20.6 months for IDH1 wildtype tumors vs. 25.0 months for IDH1mutated tumors (p=0.097). Median overall survival from GB diagnosis fortumors with initial diagnosis of GB was 20.8 months for IDH1wildtype tumors vs. 94 months for IDH1mutated tumors (p=0.0025).
For patients without an IDH1mutation, median time from GB diagnosis to recurrent GB clinical trial in the cohort of patients with 6-PFS or RR was 8.48 months and 8.44 months for the cohort of patients without 6-PFS or RR(p=0.60) . For patients with an IDH1mutation, median time from GB diagnosis to recurrent GB clinical trial in the cohort of patients with 6-PFS and/or or RR was 19.84 months and 6.46 months for the cohort of patients without 6-PFS or RR (p= 0.06).
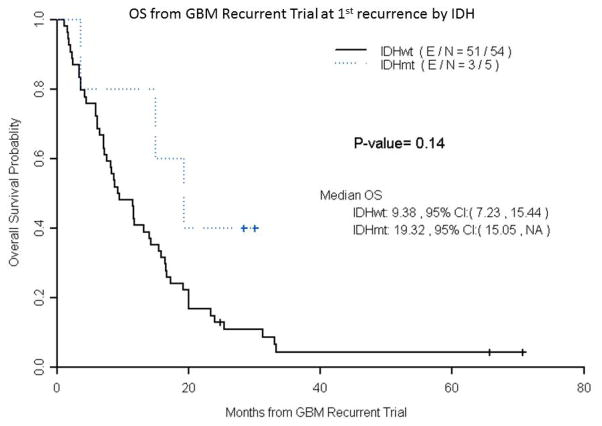
Median progression free survival(PFS) on a recurrent trial was 3.68 months for IDH1 wildtype GB vs. 3.52 months for IDH1mutated GB (p =0.72). (Figure 2c) Median OS on recurrent trial was 8.64 months for IDH1wildtype GB vs. 9.59 months for IDH1mutated GB (p =0.49). (Figure 2d) Median PFS for only 1strecurrence patients was 3.3 months for IDH1wildtype GB vs. 3.65 months for IDH1mutated GB (p =0.31). Median OS for only 1strecurrenc e patients was 9.38 months for IDH1wildtype GB vs. 19.32 months for IDH1mutated GB (p =0.14). (Figure 3)
Figure 3.
Overall survival from recurrent GB trial at 1st recurrence by IDH Mutation status
Patients placed on GB trials at first recurrence with IDH1 mutations were found to have a trend to longer median OS (19.3 months vs.9.4 months) than patients with IDH1 wildtype tumors from the start of recurrent GB trials. However, this analysis is limited by the small number of patients (n=5) on GB trials at first recurrence with IDH1 mutations. ( E/N- E stands for events and N is total number of patients).
DISCUSSION
Our study found that patients on clinical trials for recurrent GB with PFS-6 and/or RR were not more likely to be IDH1 mutated compared to a cohort of patients without PFS-6 or RRmatched by clinical trial, age, and KPS. Additionally, IDH1mutated tumors did not have a prolonged progression free survival or overall survival on recurrent GB trials compared to IDH1wildtype tumors. These results would seem to indicate that stratifying or excluding patients with IDH1 mutations may be unnecessaryif including patients at various recurrences. However, patients on clinical trials at first recurrence had a trend for improved OS (19.32 months for IDH-mutated tumors versus 9.8 months for IDH wildtype tumors). Although this finding is quite limited due to the very small number (n=5) on GB trials at first recurrence with IDH1 mutations, it is consistent with the results of the BELOB study which limited patients to 1st recurrence and found a median overall survival of 9 monthsin IDH wild -type tumors vs. 20 months in IDH mutant tumors.16 These results suggest that stratification of patients based on IDH mutation status could be necessary for recurrent GB clinical trials that limit patient enrollment to 1st recurrence. However, further validation is required in larger randomized prospective trials.
Prior studies have suggested that IDH1mutations are very early events in gliomagenesis mak ing theman appea ling potential therapeutic target.17 Additionally, an inhibitor of mutant IDH1has demonstrated ability to delay growth and promote differentiation of glioma cellsin murine models.18 While our study confirmed IDH1mutation status as strong prognostic mark erof survivalat initial tumor diagnosis ( 22 months for IDH1wildtype tumors vs. 83 months for IDH1 mutated tumors; p<0.0001), this benefit did not seem to persist at the time of recurrent GB trials (median OS on recurrent GB trial was 8.64 months for IDH1 wildtype GB vs. 9.59 months for IDH1mutated GB ;p=0.49). This suggests thatthe biology of IDH1mutated and wild type tumors mayconverge to the extent that IDH1is no longer a driver mutation at the time of recurrent GB trials. From this it is possible to speculate that IDH1inhibitors may be less likely to show efficacy in future clinical trials if used in recurrent GB trials compared to being started earlier in the course of the disease.
IDH1mutations were present in 18% of GB patients in our study which is slightly higher than seen in previous literature.8,9 This is presumed to be due to the high incidence (23%) of secondary GBs included in our study. We suspect that the higher rate of secondary GBs is likely related to referral bias to MD AndersonCancer Center as a specialized tertiary care facility. IDH1mutations were seen in 8% of patients with primary GB and 52% of secondary GB in our study. This is consistent with a review of prior studies that reported an IDH1mutation rate of 3 –16% for primary GB and 73–88% for secondary glioblastomas.10 Other smaller studies have found a lower rate of IDH1mutation in secondary glioblastoma of 50% –67%.19,20 Additionally, MD Anderson may have a higher incidence of low grade IDH1wildtype tumors as these tum ors typically behave more aggressively than low grade IDH1mutated tumors. The malignant nature of lower grade IDH1wildtype tumors may make these patients more likely to be referred to a dedicated brain tumor center. Similar to previous studies, patientswith IDH1mutated tumors were found to be diagnosed at a younger age and had an improved overall survival compared to IDH1wildtype tumors of the same histopathologic grade.
One possible explanation that patients on clinical trials for recurrent GB with PFS-6 and/or RR were not more likely to have an IDH1mutation compared to a matched cohort of patients without PFS-6 or RR is that treatment response in recurrent GB is likely dependent on more than just one genetic mutation. GB in particular is known to be a very heterogeneous tumor. A recent study has suggested that a combined analysis of mutations affecting the promoter region of the telomerase reverse transcriptase (TERT) gene, epidermal growth factor receptor (EGFR) amplification, and IDH status improves prognostic classes in GB.21 Additionally, another study has suggested that the presence of an IDH1mutation may not always indicate a favorable prognosis, as IDH1mutated GBs also with a TERT mutation were found to have a poor prognosis.22 It remainsa possibility that if we examined more genomic abnormalities in addition to the IDH1mutation that a combination of biomarkers could potentially predict response to treatment on recurrent trials.
Furthermore, lead time bias may have also been a factor in ourstudy due to our inclusion of both GBs with an initial diagnosis of low grade or anaplastic tumors and GBs with initial diagnosis of GB. We decided to allow GBs with an initial diagnosis of low grade or anaplastic tumorsin our study because they are currently included in recurrent GB trials. Patients with an initial diagnosis of low grade or anaplastic tumors occasionally received heterogenous treatments including radiation and/or a variety of chemotherapies prior to the diagnosis of GB as chosen by the treating physician. It is unclear the effect those earlier treatments may have had on the patients likelihood for response on later recurrent GB trials. However, overall survival from initial tumor diagnosis was similar for IDH1mutated tumors regardless of whether they were in the cohort of patients with PFS-6 and/or RR or the matched cohort of patients without PFS-6 or RR (83.07 months vs. 86.07 months, p <0.22).
Another potential limitation to our study was that only immunohistochemistry was used to determine IDH1status. Although the concordance rate between immunohistochemistry and sequencing is reported to range from 88% to 99%, a few studies have shown that the number of mutations detected by Sanger sequencing to be greater than those detected by immunohistochemistry.23,24,25,26 This discrepancy is thought to be due to immunohistochemistry failing to detect other types of IDH1mutations including R132C, R132L, R132S, R132G, and IDH-2 mutations.27 Additionally, by allowing tumors that had been biopsied only in our study, it isconceivable that the available tissue sample did not fully capture diagnostic material. There is also the possibility that specimens in which only a few scattered cells are R132H IDH1 immunopositive may not have the same properties as those with many more positive cells.
Our study was further limited due to its retrospective nature. Although we attemptedto match recurrent GB patients by clinical trial protocol, age and KPS, it is impossible to say if any slight differences in these factors may have influenced our results.
In conclusion, despite patients with IDH1mutated gliomas having improved overall survival from initial tumor diagnosis, IDH1mutations were not more common in patients with PFS -6 and/or RR on recurrent GB trialsfor this data set . This would seem to indicate that stratifying future recurrent GB trials based on IDH1mutation status or excluding patients with IDH1 mutated tumors may be unnecessaryif including patients at various recurrences . However, our findingssuggest that stratification of patients based on IDH mutation status may potentially be necessary for recurrent GB clinical trials that limit patient enrollment to 1st recurrence. Due to the limitations in this retrospective review, further examination regarding the role of IDH1 mutation and response on recurrent GB clinical trialsis needed in larger randomized prospective studies.
Supplementary Material
Acknowledgments
Funding: No funding was obtained for this project.
Footnotes
Conflict of Interest:No conflict of interests to report
Contributor Information
Jacob J. Mandel, Baylor College of Medicine, Department of Neurology, One Baylor Plaza, MS NB302, Houston, TX 77030
David Cachia, Medical College of South Carolina, Department of Neurosurgery, 96 Jonathan Lucas St, Charleston SC 29425.
Diane Liu, The University of Texas MDAnderson Cancer Center, Quantitative Sciences - Unit 1409, P. O. Box 301402, Houston, TX 77230-1402.
Charmaine Wilson, The University of Texas MDAnderson Cancer, Center Division of Neuro-Oncology, 1515 Holcombe Blvd Unit 431, Houston, Texas 77030-4009.
Ken Aldape, University Health Network, Department of Pathology, Toronto Medical Discovery Tower, 101 College St., Rm 14-601, Toronto, Ontario M5G 1L7.
Greg Fuller, The University of Texas MDAnderson Cancer, Center Division of Neuro-Oncology, 1515 Holcombe Blvd Unit 431, Houston, Texas 77030-4009.
John F. de Groot, The University of Texas MDAnderson Cancer, Center Division of Neuro-Oncology, 1515 Holcombe Blvd Unit 431, Houston, Texas 77030-4009
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. The New England Journal of Medicine. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 2005 Mar 10;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Scherer H. Cerebral astrocytomas and their derivatives. Am J Cancer. 1940;40:159. [Google Scholar]
- 4.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol. 1999 Jan;1(1):44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004 Oct 1;64(19):6892–9. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 6.Theeler BJ, Yung WK, Fuller GN, De Groot JF. Moving toward molecular classification of diffuse gliomas in adults. Neurology. 2012 Oct 30;79(18):1917–26. doi: 10.1212/WNL.0b013e318271f7cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis DN, Perry A, Burger P International Society Of Neuropathology. Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429. doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan H, Parsons DW, Jin G, et al. ID H1 and IDH2 Mutations in Gliomas. N Engl J Med. 2009 Feb 19;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons DW, Jones S, Zhang X, et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science. 2008 Sep 26;321(5897):1807. doi: 10.1126/science.1164382. Published online 2008 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labussiere M, Sanson M, Idbaih A, et al. IDH1 Gene Mutations: A New Para digm in Glioma Prognosis and Therapy? Oncologist. 2010 Feb;15(2):196–199. doi: 10.1634/theoncologist.2009-0218. Published online 2010 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013 Feb 15;19(4):764–72. doi: 10.1158/1078-0432.CCR-12-3002. Epub 2012 Dec 3. [DOI] [PubMed] [Google Scholar]
- 12.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009 Oct 1;15(19):6002–7. doi: 10.1158/1078-0432.CCR-09-0715. Epub 2009 Sep 15. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wildtype anaplastic astrocytomasexhibit worse prognosis than IDH1 -mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010 Dec;120(6):707–18. doi: 10.1007/s00401-010-0781-z. Epub 2010 Nov 19. [DOI] [PubMed] [Google Scholar]
- 14.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009 Sep 1;27(25):4150–4. doi: 10.1200/JCO.2009.21.9832. Epub 2009 Jul 27. [DOI] [PubMed] [Google Scholar]
- 15.Wen P, Macdonald D, Reardon D, et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010 Apr 10;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 16.Taal W, Oosterkamp HM, Walenkamp AM, et al. Sing le-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014 Aug;15(9):943–53. doi: 10.1016/S1470-2045(14)70314-6. Epub 2014 Jul. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1Mutations Are Early Events in the Development of Astrocytomas and Oligodendrogliomas. Am J Pathol. 2009 Apr 1;74(4):1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohle D1, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013 May 3;340(6132):626–30. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009 Aug;11(4):341–7. doi: 10.1215/15228517-2009-025. Epub 2009 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonoda Y1, Kumabe T, Nakamura T, et al. Analysis of IDH1 and IDH2 mutations in Japanese glioma patients. Cancer Sci. 2009 Oct;100(10):1996–8. doi: 10.1111/j.1349-7006.2009.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labussiere M, Boisselier B, Mokhtari K, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83:1200–1206. doi: 10.1212/WNL.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 22.Eckel-Passow JE1, Lachance DH, Molinaro AM. GliomaGroups Based on 1p/19q, IDH, and TERTPromoter Mutations in Tumors. N Engl J Med. 2015 Jun 25;372(26):2499–508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou Y, Xiao Bai H, Wang Z, et al. Comparison of immunohistochemistry and DNA sequencing for the detection of IDH1 mutations in gliomas. Neuro Oncol. 2015;17(3):477–478. doi: 10.1093/neuonc/nou351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellai M, Piazzi A, Caldera V, et al. IDH1 and IDH2 mutations, immunohistochemistry and associations in a series of brain tumors. J Neurooncol. 2011;105(2):345–357. doi: 10.1007/s11060-011-0596-3. [DOI] [PubMed] [Google Scholar]
- 25.Catteau A, Girardi H, Monville F, et al. A new sensitive PCR assay for one-step detection of 12 IDH1/2 mutations in glioma. Acta Neuropathol Commun. 2014;2:58. doi: 10.1186/2051-5960-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loussouarn D, Le Loupp AG, Frenel JS, et al. Comparison of immunohistochemistry, DNA sequencing and allele-specific PCR for the detection of IDH1 mutations in gliomas. Int J Oncol. 2012;40(6):2058–2062. doi: 10.3892/ijo.2012.1404. [DOI] [PubMed] [Google Scholar]
- 27.Preusser M, Capper D, Hartmann C Euro CNS Rsearch Committe. IDH testing in diagnostic neuropathology: review and practical guideline article invited by the Euro-CNS research committee. Clin Neuropathol. 2011;30(5):217–230. doi: 10.5414/np300422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.