Abstract
Rationale: Patient factors associated with development of abnormal lung function in children with sickle cell anemia (SCA) have not been fully characterized.
Objectives: To characterize lung function abnormalities among children with SCA and to determine whether these steady-state lung function results were associated with morbidity before or after testing among children with SCA.
Methods: This study was part of the prospective National Institutes of Health–funded Sleep and Asthma Cohort Study. Children with HbSS or Hb Sβo (SCA) were enrolled without regard for sickle cell–related comorbidities or diagnosis of asthma. Lung function was measured by spirometry and plethysmography on the same day, when free of acute disease. Standardized asthma symptom questionnaires and review of the medical records were also performed.
Measurements and Main Results: A total of 149 children aged 6 to 19 years completed lung function testing, of whom 139 participants had retrospective morbidity data from birth to the test date, and 136 participants were followed prospectively for a median of 4.3 years from the test date. At baseline, percentages with normal, obstructive, restrictive, nonspecific, and mixed lung function patterns were 70, 16, 7, 6, and 1, respectively. Neither retrospective rates of pain nor acute chest syndrome was associated with lung function patterns. Furthermore, baseline lung function pattern was not predictive of future pain or acute chest syndrome episodes.
Conclusions: The majority of children with SCA have lung function that is within the normal range. Abnormal lung function patterns were not associated with prior vasoocclusive pain or acute chest syndrome episodes, and baseline lung function patterns did not predict future vasoocclusive pain or chest syndrome episodes.
Keywords: sickle cell anemia, pulmonary function tests, respiratory physiological phenomena, child, risk factors
The lung is often affected in sickle cell disease (SCD), acutely with acute chest syndrome (ACS) (1) and chronically with dyspnea and reduced exercise capacity (2, 3), loss of lung function (4, 5), and pulmonary hypertension (6). Studies have sought to define pulmonary function abnormalities in children and adolescents with SCD (4, 7–13) using pulmonary function evaluations conducted when children and adolescents were at their baseline status, with tests most often obtained in clinical laboratories during routine care.
In his review of lung function in SCD, Koumbourlis (14) concluded that there has been “over-estimation of the prevalence of the restrictive pattern” and “under-estimation of the obstructive pattern,” which he attributed to inconsistencies across studies regarding criteria for defining those categories. In addition to discrepancies about the prevalence of lung function abnormalities, data are inconsistent about whether prior morbidity—either ACS or vasoocclusive pain—is associated with abnormal lung function in children with SCD (7, 9, 11, 13, 15–17).
In this analysis of pulmonary function test results in children with sickle cell anemia (SCA) studied at their baseline state of health, we hypothesized that (1) prior morbidity would be associated with baseline lung function pattern, and (2) abnormal lung function pattern at baseline would be associated with future rates of pain and ACS.
Methods
Patients and Methods
The current study uses data collected from the prospective, observational Sleep and Asthma Cohort study. Children with SCA (who had either hemoglobin SS [HbSS] or sickle beta zero thalassemia [HbSβ°] phenotypes) were enrolled at three clinical centers from 2006 to 2008 and followed prospectively until the end of the study in 2013 or until they were lost to follow up. Children were enrolled without regard to past morbidity or diagnosis of asthma, but those on chronic transfusions or participating in a clinical trial evaluating hydroxyurea (HU) therapy were excluded. Participants included in the current analysis completed spirometry and plethysmography on the same date when they were at their baseline of health and had been followed at their clinical center since birth. Participants’ medical records were available for determination of numbers of hospital admissions for pain crises and ACS episodes from birth to the dates of lung function testing and after lung function testing.
Institutional approval was obtained from participating sites: Washington University School of Medicine in St. Louis, Missouri; Case Western Reserve University School of Medicine in Cleveland, Ohio; University College London in London, UK (who recruited from three London hospitals); and the Coordinating Center at Vanderbilt School of Medicine in Nashville, Tennessee. Informed written parental consent was obtained, and children were consented or assented according to institutional policies of each institution.
Questionnaires
Caregivers and participants completed the following questionnaires at the time of enrollment: (1) the American Thoracic Society (ATS) Division of Lung Disease questionnaire (18); (2) a medical history questionnaire eliciting information on demographics, participant and family history of physician-diagnosed asthma and atopy, and what medications the participant was currently using; and (3) ascertainment of exposure to environmental tobacco smoke (ETS). Start and stop dates for prescribed medications were confirmed via review of the medical record.
Definitions
Asthma was defined as having both a parent-reported physician diagnosis of asthma and current prescription of an asthma medication (controller and/or rescue medication). A vasoocclusive pain episode (“pain”) was defined as bone pain in the chest, extremities, or other areas (not headaches only) directly associated with SCA that required hospitalization for opioid treatment. ACS was defined as an episode of acute respiratory distress requiring a new radiodensity on chest roentgenogram, temperature greater than 38°C, and increased respiratory effort with a decrease in oxygen saturation or increased respiratory rate documented in the medical record. To ensure a uniform definition of pain and ACS, all pain and ACS episodes requiring hospitalization were reviewed by a single investigator at each site, with over-reading by the principal investigator (M.R.D.).
Pulmonary Function Testing
Pulmonary function tests (PFTs) were obtained when participants were at baseline of health, (i.e., without current pain, respiratory symptoms, or recent illness) and at least 4 weeks after discharge from hospital for SCD complications. Height and weight measurements were performed using standard methods.
Spirometry was performed by study-certified pulmonary function technicians using a pneumotachograph-type spirometer interfaced with a personal computer system (Jaeger MasterScope; VIASYS, Hoechberg, Germany) as described in detail by Field and colleagues (19). Spirometry was performed at least 4 hours after the use of a short-acting bronchodilator and 12 hours after use of a long-action bronchodilator (20) according to ATS standards adapted for children (20, 21). Spirometry results from all three centers were over-read by a senior technician to ensure that results were valid.
Static lung volume measurements were performed on the same day as spirometry measurements using a Jaeger MasterScreen (Cleveland and London) or Sensor Medics VMAX (St. Louis) plethysmograph per ATS/European Respiratory Society (ERS) standards (22). Functional residual capacity (FRC) was the mean of the technically satisfactory FRC measurements, residual volume (RV) was FRC minus the mean of the technically acceptable expiratory reserve volume measurements, and total lung capacity (TLC) was value for RV plus the largest of the technically acceptable inspiratory vital capacity measurements. Plethysmography results from all three centers were over-read by a senior technician to ensure that results were valid.
Markers of Abnormal Lung Function
Predicted values were determined for each subject based on their age, sex, height, and race for FEV1, FVC, FEV1/FVC ratio, and forced expiratory flow midexpiratory phase (FEF25–75%) using the Global Lung Function 2012 Equations (23). Abnormal results for FEV1, FVC, FEV1/FVC ratio, and FEF25–75% were determined by comparison to their lower limits of normal (LLN) (23). Reference equations for TLC derived by Rosenthal and colleagues (24) were used with an African American race adjustment of 12% (25); LLN was defined as 80% predicted per ATS/ERS recommendations (26). Percent predicted values of FEV1, FEV1/FVC, FVC, and TLC were used to categorize lung function using the algorithm published by Pellegrino and colleagues (26) and modified by addition of the nonspecific pattern described by Hyatt and colleagues (27) and Iyer and colleagues (28), as shown in Figure 1.
Figure 1.
Algorithm used to assess lung function (Refs. 26–28). LLN = lower limit of normal; TLC = total lung capacity; VC = vital capacity.
Bronchodilator Reactivity
To measure bronchodilator (BD) response, technicians administered four inhalations of albuterol from a metered dose inhaler (90 μg/puff at U.S. sites, 100 μg/puff at UK sites) using an AeroChamber (Forest Pharmaceuticals, New York, NY). Spirometry was repeated 15 minutes post-albuterol. Baseline and post-BD FEV1 were compared, with percentage response to albuterol defined as [(post-BD FEV1 − pre-BD FEV1)/pre-BD FEV1] × 100. An increase of 12% or more in FEV1 was considered a positive BD response (26).
Laboratory Testing
Serum IgE was obtained from a peripheral blood draw on study entry. Complete blood count at steady state was obtained from the medical record.
Statistical Analysis
Demographic and clinical factors were compared between lung function categories with a chi-square test for categorical variables and an analysis of variance or Kruskal–Wallis test for continuous variables, depending on the distribution. Rates of ACS and pain episodes were separated into retrospective and prospective based on the date of the PFT session.
Logistic regression models to predict lung function pattern were constructed with the primary predictors of interest being retrospective rates of pain and ACS. In addition to unadjusted models, multivariate logistic regression models were constructed to adjust for factors potentially associated with lung function pattern among patients with SCD, including demographic, SCD-related, and respiratory-related factors including early life ETS exposure (from birth through age 2 years) (29–32), with a model for each factor grouping. Models were developed comparing each abnormal pattern to the normal lung function group. Our model-building approach was chosen because small numbers in the restriction and nonspecific groups made it statistically appropriate to build separate smaller models rather than one large screening model that included all potential confounders. Given the high degree of association between having asthma and having a history of wheezing leading to shortness of breath, models for obstruction replaced “asthma” with “history of wheezing leading to shortness of breath” as a potential covariate.
Associations between having an abnormal lung function pattern and prospective rates of pain and ACS were tested with negative binomial regression models. Analyses for prospective rates of ACS and pain were conducted with participants who had a minimum of 3 months of follow up after the lung function testing; we then repeated our analyses with participants who had 12 months or more of prospective follow up.
Multivariable models were built in two steps. Potential covariates were included in a screening model. In addition to lung function pattern (obstruction, restriction, or nonspecific versus normal), covariates we considered to be potentially associated with prospective rates of ACS included sex, prior history of ACS, white blood cell count, reticulocyte count, asthma, history of wheezing leading to shortness of breath, BD responsiveness, and use of inhaled corticosteroids. Covariates initially included in a screening model of the association between lung function pattern and prospective rate of pain episodes were age, sex, prior history of pain, hemoglobin, reticulocyte count, white blood cell count, BD responsiveness, wheezing leading to shortness of breath, early life ETS, and ln(IgE). All covariates with P < 0.20 in screening models were included in the final pain and ACS models. Preliminary analyses indicated that the children on HU at the time of testing had the highest rates of ACS and pain, suggesting confounding by indication of severe disease. Initially, HU was not added as a covariate in the models; models that include HU are presented in the online supplement.
Given recent publications examining the contribution of FEF25–75% in lung function classification and clinical decision making (33, 34), we conducted additional analyses using both the FEV1/FVC ratio and FEF25–75% less than LLN as an alternative criterion for having obstruction.
Analyses were conducted using Stata statistical software (Version 12; StataCorp LP, College Station, TX) and IBM SPSS Statistics (Version 22; IBM, Chicago, IL).
Results
Baseline Characteristics
One hundred forty-nine children completed valid spirometry and plethysmography testing at a median age of 11.4 years (range, 6.2–19.0 yr); 95% had the HbSS phenotype. All had SCD morbidity data available from birth to date of the PFT session. One hundred thirty-six were followed prospectively for a median of 4.3 years (range, 3 mo–6.7 yr) after their PFT session, and 121 participants had at least 12 months of prospective follow up. Twenty-nine percent had a physician diagnosis of asthma (see Table 1 for a complete description of the study cohort).
Table 1.
Baseline characteristics of 147 study participants
| Patient Characteristics | All (N = 147) | Normal (n = 104) | Obstruction (n = 24) | Restriction (n = 10) | Nonspecific (n = 9) | P Value* |
|---|---|---|---|---|---|---|
| Age at lung function test, median (range), yr | 11.4 (6.2–19.0) | 10.8 (6.3–19.0) | 11.8 (7.1–17.4) | 13.4 (8.3–18.4) | 12.1 (6.2–18.1) | 0.55† |
| Prospective follow up, median (range), yr | 4.3 (0.3–6.7) | 4.2 (0.3–6.7) | 4.4 (0.6–6.4) | 4.9 (1.0–6.3) | 4.5 (1.7–6.3) | 0.42† |
| Sex, % male | 55.8 | 56.7 | 54.2 | 50.0 | 55.6 | 0.97 |
| BMI z-score, mean ± SD | −0.06 ± 1.15 | 0.04 ± 1.12 | −0.17 ± 1.31 | −1.08 ± 0.71 | 0.13 ± 1.07 | 0.03‡ |
| Hb phenotype, HbSS, % | 94.6 | 94.2 | 95.8 | 90.0 | 100.0 | 0.70 |
| Hemoglobin, mean ± SD, g/dL | 8.2 ± 1.2 | 8.2 ± 1.2 | 7.9 ± 1.5 | 8.2 ± 0.8 | 8.4 ± 0.8 | 0.63 |
| WBC count, mean ± SD, k/μl | 11.9 ± 3.8 | 12.1 ± 3.8 | 11.5 ± 3.6 | 11.2 ± 2.3 | 11.9 ± 4.8 | 0.85 |
| Reticulocyte, mean ± SD, n = 146, % | 11.0 ± 5.3 | 11.2 ± 5.3 | 10.2 ± 4.0 | 11.3 ± 8.1 | 11.0 ± 5.3 | 0.89 |
| Has asthma, % | 28.6 | 23.1 | 41.7 | 50.0 | 33.3 | 0.12 |
| Early life ETS exposure, n = 144, % | 43.1 | 42.6 | 66.7 | 20.0 | 11.1 | 0.007§ |
| History of wheezing leading to shortness of breath, n = 145, % | 18.6 | 16.5 | 25.0 | 22.2 | 22.2 | 0.78 |
| On hydroxyurea, n = 146, % | 17.8 | 17.5 | 16.7 | 20.0 | 22.2 | 0.98 |
| On ICS, % | 22.4 | 19.2 | 29.2 | 50.0 | 11.1 | 0.13 |
| ACS rate retrospective, n = 147, events/yr | ||||||
| Median (IQR) | 0.13 (0.27) | 0.11 (0.25) | 0.18 (0.41) | 0.27 (0.29) | 0.13 (0.21) | 0.27† |
| Mean ± SD | 0.21 ± 0.26 | 0.20 ± 0.28 | 0.23 ± 0.21 | 0.30 ± 0.30 | 0.11 ± 0.10 | 0.43 |
| ACS rate prospective, n = 144, events/yr | ||||||
| Median (IQR) | 0.00 (0.31) | 0.00 (0.31) | 0.00 (0.31) | 0.20 (0.64) | 0.00 (0.18) | 0.42† |
| Mean ± SD | 0.23 ± 0.46 | 0.24 ± 0.49 | 0.23 ± 0.45 | 0.33 ± 0.36 | 0.76 ± 0.12 | 0.69 |
| Pain rate retrospective, n = 147, events/yr | ||||||
| Median (IQR) | 0.29 (0.63) | 0.30 (0.69) | 0.22 (0.38) | 0.47 (0.69) | 0.11 (0.32) | 0.22† |
| Mean ± SD | 0.48 ± 0.56 | 0.50 ± 0.58 | 0.40 ± 0.54 | 0.58 ± 0.50 | 0.26 ± 0.45 | 0.48 |
| Pain rate prospective, n = 145, events/yr | ||||||
| Median (IQR) | 0.47 (1.35) | 0.43 (1.54) | 0.39 (1.15) | 0.82 (1.31) | 0.49 (1.15) | 0.86† |
| Mean ± SD | 0.99 ± 1.41 | 1.07 ± 1.52 | 0.75 ± 1.14 | 1.00 ± 1.34 | 0.70 ± 0.83 | 0.68 |
Definition of abbreviations: ACS = acute chest syndrome; BMI = body mass index; ETS = environmental tobacco smoke; Hb = hemoglobin; HbSS = hemoglobin SS; ICS = inhaled corticosteroids; IQR = interquartile range; WBC = white blood cell.
Two participants were categorized as having a “mixed” restrictive and obstructive pattern and are not included in this table.
Chi-square tests for categorical variables, analysis of variance tests for continuous variables unless otherwise noted.
Kruskal–Wallis test.
Lower in the restricted group compared with the normal group (P = 0.02).
Higher frequency in obstruction compared with nonspecific pattern (P = 0.02).
Using the classification scheme as shown in Figure 1, 104 children (70%) had normal lung function and 45 (30%) had an abnormal lung function pattern. Of those with an abnormal pattern, 24 had obstruction, 10 had restriction, 2 had a mixed pattern of both restrictive and obstructive abnormalities, and 9 had a nonspecific pattern. When those with the nonspecific pattern were assessed with the slow vital capacity (SVC) maneuver, there was less than a 5% difference between FVC and SVC in seven of nine patients, with the FVC higher than SVC in four of nine patients.
There were significant differences across the lung function pattern groups (Tables 1 and 2) for ETS, FEV1% predicted, FVC % predicted, FEV1/FVC, FEF25–75%, having a positive BD response, and RV/TLC. However, there were no group differences for age, sex, hematologic parameters, frequency of asthma, or history of wheezing leading to shortness of breath (Table 1).
Table 2.
Lung function parameters of the study population
| Lung Function Parameter | All (N = 147) | Normal (n = 104) | Obstruction (n = 24) | Restriction (n = 10) | Nonspecific (n = 9) | P Value* |
|---|---|---|---|---|---|---|
| FEV1 % predicted | 88.3 ± 13.4 | 93.1 ± 10.4 | 80.9 ± 13.5 | 67.0 ± 7.9 | 75.3 ± 8.5 | <0.001† |
| FVC % predicted | 92.8 ± 14.1 | 96 4 ± 10.9 | 94.8 ± 14.6 | 67.2 ± 8.0 | 74.1 ± 5.2 | <0.001‡ |
| FEV1/FVC, actual | 0.84 ± 0.06 | 0.85 ± 0.04 | 0.76 ± 03 | 0.88 ± 0.04 | 0.90 ± 0.06 | <0.001§ |
| FEV1/FVC % predicted | 94.9 ± 6.5 | 96.2 ± 4.7 | 84.9 ± 3.0 | 99.5 ± 5.4 | 101.0 ± 6.2 | <0.001§ |
| FEF25–75% predicted | 73.2 ± 21.1 | 79.6 ± 20.0 | 49.2 ± 26.7 | 67.3 ± 15.6 | 71.5 ± 26.7 | <0.001|| |
| BD response ≥ 12.0, n = 143, % | 19.6 | 11.9 | 41.7 | 20.0 | 50.0 | 0.003¶ |
| RV/TLC ratio | 0.33 ± 0.07 | 0.31 ± 0.07 | 0.36 ± 0.07 | 0.34 ± 0.10 | 0.38 ± 0.06 | 0.003** |
| RV/TLC ratio Z-score†† | 0.76 ± 1.23 | 0.56 ± 1.17 | 1.26 ± 1.14 | 1.08 ± 1.63 | 1.84 ± 0.86 | 0.003‡‡ |
Definition of abbreviations: BD = bronchodilator; FEF25–75% = forced expiratory flow, midexpiratory phase; FEV = forced expiratory volume in 1 second; FVC = forced vital capacity; RV = residual volume; TLC = total lung capacity.
Data are presented as mean ± SD unless otherwise noted. Two participants were categorized as having a “mixed” restrictive and obstructive pattern and are not included in this table.
Chi-square tests for categorical variables, analysis of variance tests for continuous variables unless otherwise noted.
Highest in the normal group (P < 0.001), lower in restriction versus normal group (P = 0.005).
Higher in normal and obstruction groups versus restriction and nonspecific (P < 0.001 for all comparisons).
Lowest among the obstruction group versus all other groups (P < 0.001 for all), nonspecific group higher than normal (P = 0.02).
Lower in obstruction versus normal (P < 0.001) and nonspecific groups (P = 0.02).
Higher frequency in obstruction and nonspecific groups versus normal.
Higher in the obstruction (P = 0.04) and nonspecific groups (P = 0.03) versus the normal group.
Calculated based on normative data for height and sex (Mark Rosenthal, personal communication).
Higher in the obstruction (P = 0.04) and nonspecific groups (P = 0.03) versus the normal group.
Prior Pain and ACS Morbidity Is Not Associated with Lung Function Pattern in Children with SCA
Logistic regression models for obstruction were developed and are shown in Tables 3. Neither retrospective rates of ACS nor pain before the lung function testing session was associated with obstruction in the unadjusted model (Table 3, model 1) or in models adjusted for demographic factors, SCD factors, and respiratory-related factors (Table 3, models 2–4). As with obstruction, neither retrospective rates of ACS nor pain was associated with restriction or nonspecific patterns (Table 4 and 5, models 1–4). Alternating asthma with wheeze causing shortness of breath in the models for obstruction, restriction, and nonspecific patterns instead of wheezing did not change the results (data not shown).
Table 3.
Logistic regression model of the association between retrospective rates of acute chest syndrome and pain and having obstruction versus normal lung function in children with sickle cell anemia
| Model 1* | Model 2† | Model 3‡ | Model 4§ | |
|---|---|---|---|---|
| Retrospective ACS rate | 1.91 (0.35–10.35), 0.45 | 2.27 (0.38–13.60), 0.37 | 2.75 (0.44–17.31), 0.28 | 1.11 (0.12–10.38), 0.93 |
| Retrospective pain rate | 0.62 (0.24–1.59), 0.32 | 0.59 (0.23–1.55), 0.29 | 0.54 (0.19–1.51), 0.24 | 0.85 (0.32–2.21), 0.73 |
Definition of abbreviations: ACS = acute chest syndrome; CI = confidence interval; OR = odds ratio.
Data presented as OR (95% CI), P value. No. with complete data = 23 versus 91 with normal lung function.
Unadjusted model.
Model adjusted for age and sex.
Model adjusted for age, sex, and sickle cell disease factors (hemoglobin [g/dL], white blood cell count, and reticulocyte percentage).
Model adjusted for age, sex, and pulmonary factors of interest (has asthma, bronchodilator response > 12%, early life environmental tobacco smoke exposure, ln [IgE]).
Table 4.
Logistic regression model of the association between retrospective rates of acute chest syndrome and pain and having restriction versus normal lung function in children with sickle cell anemia
| Model 1* | Model 2† | Model 3‡ | Model 4§ | |
|---|---|---|---|---|
| Retrospective ACS rate | 2.23 (0.26–18.91), 0.46 | 5.12 (0.43–60.05), 0.19 | 5.30 (0.42–67.60), 0.20 | 6.94 (0.53–91.41), 0.14 |
| Retrospective pain rate | 0.97 (0.29–3.24), 0.97 | 0.76 (0.21–2.75), 0.68 | 0.77 (0.19–3.06), 0.71 | 0.63 (0.16–2.49), 0.51 |
Definition of abbreviations: ACS = acute chest syndrome; CI = confidence interval; OR = odds ratio.
Data presented as OR (95% CI), P value. No. with complete data = 9 versus 99 with normal lung function
Unadjusted model.
Model adjusted for age and sex.
Model adjusted for age, sex, and sickle cell disease factors (hemoglobin [g/dL], white blood cell count, and reticulocyte percentage).
Model adjusted for age, sex, and pulmonary factors of interest (history of wheezing with shortness of breath and early life environmental tobacco smoke exposure).
Table 5.
Logistic regression model of the association between retrospective rates of acute chest syndrome and pain and having nonspecific pattern versus normal lung function in children with sickle cell anemia
| Model 1* | Model 2† | Model 3‡ | Model 4§ | |
|---|---|---|---|---|
| Retrospective ACS rate | 0.15 (0.002–13.04), 0.40 | 0.15 (0.002–14.34), 0.42 | 0.17 (0.002–14.37), 0.43 | 0.14 (0.001–14.22), 0.40 |
| Retrospective pain rate | 0.34 (0.05–2.38), 0.28 | 0.33 (0.05–2.44), 0.28 | 0.24 (0.03–2.17), 0.20 | 0.33 (0.04–2.39), 0.27 |
Definition of abbreviations: ACS = acute chest syndrome; CI = confidence interval; OR = odds ratio.
Data presented as OR (95% CI), P value. No. with complete data = 9 versus 99 with normal lung function.
Unadjusted model.
Model adjusted for age and sex.
Model adjusted for age, sex, and sickle cell disease factors (hemoglobin [g/dL], white blood cell count, and reticulocyte percentage).
Model adjusted for age, sex, and wheeze with shortness of breath.
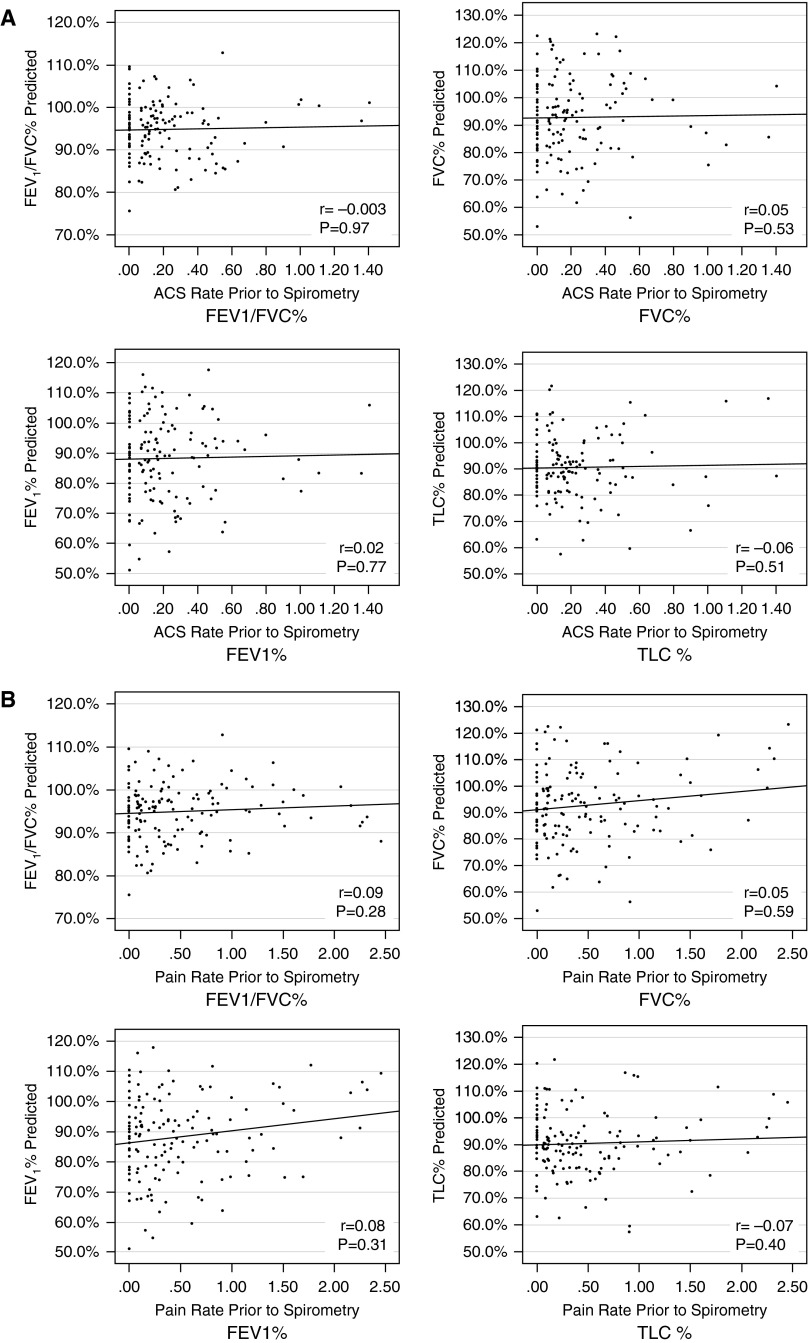
We further explored whether prior morbidity was associated with lung function parameters in children with SCA. Figure 2 depicts the correlations between retrospective ACS rate (Figure 2A) and pain rate (Figure 2B) (from birth to the date of the lung function test) and percent predicted values for FVC, FEV1, FEV1/FVC, and TLC. There were no observable relationships between prior rates of either ACS or pain events and baseline lung function parameters in our cohort.
Figure 2.
Scatter plots of the correlations between retrospective rates of acute chest syndrome (ACS) and pain on lung function parameters in children with sickle cell anemia. (A) Spearman correlations between rates of ACS (x-axis) and lung function parameters (y-axis). (B) Spearman correlations between rates of pain (x-axis) and lung function parameters (y-axis). TLC = total lung capacity.
Baseline Lung Function Does Not Predict Future Morbidity in Children with SCA
Baseline lung function patterns (obstruction, restriction, and nonspecific) were not associated with future ACS or pain events in screening or final models adjusted for demographic factors, SCD factors, and respiratory-related factors (Table 6). When analyses were restricted to the 121 participants who had 12 or more months of prospective follow up, results were unchanged: baseline lung function patterns were not associated with future rates of pain or ACS (see Table E1 in the online supplement). As expected, taking HU at the time of testing was associated with higher rates of both ACS and pain, consistent with clinical decisions to use HU for patients with more severe disease (see Table E3 for multivariable models examining associations between lung function pattern and prospective ACS and pain that include HU as a covariate).
Table 6.
Final negative binomial regression models for prospective rates of acute chest syndrome* and vasoocclusive pain† in children with sickle cell anemia
| IRR | 95% CI | P Value | |
|---|---|---|---|
| Prospective rates of ACS | |||
| Retrospective rate of ACS events/yr | 14.14 | 7.38–27.07 | <0.001 |
| Obstruction | 0.89 | 0.42–1.88 | 0.76 |
| Restriction | 1.01 | 0.48–2.11 | 0.98 |
| Nonspecific | 0.56 | 0.22–1.40 | 0.22 |
| Prospective rates of pain | |||
| Age, yr | 1.07 | 1.00–1.15 | 0.041 |
| Retrospective rate of pain events/yr | 2.25 | 1.80–2.80 | <0.001 |
| Obstruction | 0.70 | 0.36–1.37 | 0.30 |
| Restriction | 0.59 | 0.33–1.07 | 0.08 |
| Nonspecific | 0.92 | 0.42–2.02 | 0.84 |
Definition of abbreviations: ACS = acute chest syndrome; CI = confidence interval; IRR = incidence rate ratio.
Median length of follow up, 4.6 years; range 3 months–6.7 years. N = 136 with complete data. See Table E2 in the online supplement for analyses of the 121 participants with ≥12 months of prospective follow up.
Initial screening model of the association between lung function pattern and prospective ACS was adjusted for: sex, prior history of ACS, white blood cell count, reticulocyte count, asthma, history of wheezing leading to shortness of breath, bronchodilator responsiveness, and use of inhaled corticosteroids.
Initial screening model of the association between lung function pattern and prospective pain was adjusted for: age, sex, prior history of pain episodes, hemoglobin, reticulocyte count, white blood cell count, bronchodilator responsiveness, wheezing leading to shortness of breath, early life environmental tobacco smoke, and ln (IgE).
The value of including FEF25–75% as an indicator of obstruction was assessed by moving the 14 children with an FEV1/FVC greater than or equal to LLN but an FEF25–75% less than LLN from the normal group to the obstruction group. Analyses of characteristics of those with obstruction and the impact of obstruction on future morbidity were not changed from the analyses using just FEV1/FVC to define obstruction (Tables E3 and E4).
Discussion
We found that 70% of participants in our unselected cohort of 149 children with SCA had normal lung function at steady state. The most common abnormal pattern was obstruction (found in 16%), with fewer patients having restriction (7%). Given that lung disease is a major cause of morbidity and mortality among adults with SCA (30), we sought to establish whether sickle cell morbidity was related to abnormal lung function among children. In our retrospective cohort analysis, we found that incidence rates of prior pain or ACS events were not predictive of having an abnormal lung function pattern. Equally important, in a prospective cohort design, having an abnormal lung function pattern (obstructive, restrictive, nonspecific) was not associated with future pain or ACS events.
Prior studies have examined the prevalence and progression of lung function abnormalities in children (14). The Pulmonary Hypertension and the Hypoxic Response in Sickle Cell Disease (PUSH) study (29) enrolled a cohort for research purposes and used LLN criteria for spirometry and plethysmography to define abnormal patterns similar to our approach. They had similar findings to our study in terms of percentages of obstruction (19%) and restriction (9%) among the 97 children with SCA in their cohort (29). Intzes and colleagues (13) reported 44% with obstruction in a retrospective clinical cohort but defined obstruction with a cut-off for the FEV1/FVC ratio (<0.85), rather than the ATS/ERS guideline recommendation of FEV1/FVC below the LLN (26).
In contrast, MacLean and colleagues (4) reviewed longitudinal spirometry and plethysmography results obtained during routine PFT screening of their sickle cell clinic population. At age 8 years, 96.5% of their cohort had normal lung function, but there was progression to more abnormal lung function through adolescence such that by age 17 years, 19% of their patients had restriction defined by TLC less than 70% predicted (4). They found a much lower prevalence of obstruction (0.9% at age 8 years and 0% at age 17 years) among their patients, but their definition of obstruction was having an FEV1/FVC ratio below the specific threshold of 80% predicted. MacLean and colleagues reanalyzed their data using Hankinson’s LLN criteria and found that 20% of their sample had at least one FEV1/FVC measurement below the LLN (35).
Nine children (6% of our cohort) were categorized as having the nonspecific pattern of lung function in which the FVC was reduced but the TLC was in the normal range. This pattern has been described in adults attending clinics for lung disease (27, 28) but not previously described in individuals with SCA. Hyatt and colleagues (27) found the nonspecific pattern was present in adults with airway disease or obesity and speculated that it was due to volume derecruitment, with scattered, diseased airways closing at variable rates without effect on the overall FEV1/FVC ratios. Presence of nonspecific patterns could develop from decreases in expiratory muscle strength that would result in mismatch between TLC measured in a body plethysmograph and forced expiratory flows.
The recent findings by Ong and colleagues (36) of decreases in respiratory muscle force and the resultant decreases in maximal expiratory pressure in children with SCD could provide an explanation for the findings of nonspecific patterns. Muscle strength was not measured in our cohort; however, the finding of SVC not being higher than FVC does not support decreased muscle strength as a primary cause of nonspecific pattern in our population. In addition, the finding of RV/TLC not being higher in the nonspecific group than in the obstruction group does not provide evidence for limitation of FVC due to air trapping causing the nonspecific pattern. Further understanding of this nonspecific pattern in SCA could not be further explored, given the small number of children with this finding.
Previous studies have explored the association between prior history of ACS and lung function. In one of the earliest studies of lung function and morbidity, Pianosi and colleagues compared the lung function of 10 children with and 27 children without a history of ACS and did not find significant differences in spirometric indices or TLC between those with and without histories of ACS (9). Few investigators have examined the association between abnormal lung function and future risk of ACS or vasoocclusive pain.
Boyd and colleagues found that among 102 children with SCD, ATS/ERS–defined lower airway obstruction was associated with a significantly higher rate of prospective rate of vasoocclusive pain episodes than in those with normal lung function (2.22 vs. 1.01 events/yr, P =0.02). However, this was a single-institution cohort of children with all types of SCD referred for PFTs because of respiratory symptoms (10). As such, those children may not reflect the general population of children with SCD. It should be noted that our findings regarding a lack of association between morbidity and lung function abnormalities cannot be extrapolated to adult patients with SCD.
Knight-Madden and colleagues studied 80 young adults with SCD (all with HbSS), ranging in age from 19 to 27 years, and compared lung function between those with “recurrent” (two or more prior episodes) ACS to those with zero or one episode (17). They found that those with recurrent ACS had significantly lower percentage predicted values of FVC, FEV1, and TLC than those who did not have recurrent ACS (17).
Strengths and Limitations
Our study has a number of strengths, including ascertainment of lung function measures and centralized over-reading and interpretation of results using standard ATS/ERS criteria and both retrospective and prospective ascertainment of SCD morbidity.
Regarding limitations, our study cohort includes children from three tertiary care academic centers whose families agreed to participation in prospective longitudinal study. We are unable to control for selection factors into the cohort, such as parent willingness to participate in clinical research, severity of disease, or other unknown factors. Additionally, although we used a robust definition of asthma for our study (the commonly used definition of “parent-reported physician diagnosis” plus a medical record–confirmed prescription for an asthma medication), determining which children with SCA have asthma versus isolated features of recurrent wheezing and airway hyperreactivity remains a challenge for clinicians and researchers (37). The consistency of our lung function abnormality results with those of the PUSH study (29), however, suggests that our findings are representative of the majority of children with SCA.
Conclusions
More than two-thirds of children with SCA in our research cohort had normal lung function. The predominant lung function abnormality was obstruction. As in prior studies, lower airway obstruction determined by PFT was not associated with asthma. In our study, lung function abnormalities were not associated with prior rates of hospitalizations for pain or acute pain syndrome, nor were they associated with future risk of pain or acute chest syndrome during childhood.
Despite this being the largest study to date of children with SCA having paired spirometry and lung volumes along with longitudinal morbidity data from birth for almost 16 years, larger studies are needed to determine definitively that there is no effect of lung function abnormalities on prospective morbidity. Further research is also needed to assess whether lung function abnormalities detected in childhood are associated with longer-term risks of pulmonary function test abnormalities and ACS in adulthood. Additionally, given current recommendations against universal pulmonary function screening for patients with SCD (38) juxtaposed with the recently demonstrated association between lower airway obstruction and CT and echocardiographic evidence of increased pulmonary vascular volume (39, 40), future studies might explore whether lung function abnormalities are associated with other clinically relevant outcomes in adulthood, such as progressive dyspnea, vascular dysfunction, pulmonary hypertension, and early mortality.
Acknowledgments
Sleep Asthma Cohort investigative team:
Vanderbilt University School of Medicine, Nashville, TN: Michael DeBaun, M.D., M.P.H. (Principal Investigator); Washington University, St. Louis, MO: Robert Strunk, M.D. (Co-Principal Investigator), Lisa Garrett, R.N., C.C.R.P., Pamela Bates, C.R.T., R.P.F.T., P.R.S.G.T., Rick Talbert, R.P.S.G.T., Sabrina Lockett, R.P.S.G.T., Valerie Morgan, R.R.T., Yan Yan, M.D., Ph.D., Avril Adelman, M.A., Phillip Blanks, Tinishia Greene.
Case Western Reserve University, Cleveland, OH: Carol Rosen, M.D. (Principal Investigator), Susan Redline, M.D., M.P.H., Heather Rogers, R.P.S.G.T., Susan Surovec, B.A., Dan Craven, M.D., Nancy Scott, B.S., R.E.E.G./E.P.T., R.P.S.G.T., R.E.D.T., C.N.I.M., Sinziana Seicean, M.D., M.P.H., Mary DeBarr, R.N., B.S.N., Brad Casucci, M.A.
UCL Institute of Child Health and Great Ormond Street Hospital, London, UK: Fenella Kirkham, M.D., F.R.C.P.C.H. (Principal Investigator), Janet Stocks, Ph.D., Jane Kirkby, Ph.D., Satwinder Sahota, B.Sc., Liam Welsh, Ph.D., Ursula Johnson, R.N., Aidan Laverty, M.Sc., M.B.C.S., Johanna Gavlak, B.Sc., Anne Yardumian, M.D., F.R.C.P., Olu Wilkey, F.R.C.P.C.H., Marilyn Roberts-Harewood, M.R.C.P.C.H., Anne O’Reilly.
Imperial College, London, UK: Irene Roberts, M.D., F.R.C.P.C.H., John Warner, M.D., F.R.C.P.C.H.
Hull York Medical School, UK: Avijit Kumar Datta, M.D., M.R.C.P.
Medical College of Wisconsin, Milwaukee, WI: Kirk Pritchard, Ph.D. (Principal Investigator), Thom Feroah, Ph.D., Cheryl Hillery, M.D., Keith Oldham, M.D.
Johns Hopkins University, Baltimore, MD: James Casella, M.D. (Principal Investigator).
Footnotes
Supported by the National Institutes of Health (NIH) grant R01HL079937. The project described was supported, in part, by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant UL1 RR024989. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions: R.T.C. analyzed and interpreted the data, drafted and revised the manuscript, and approved the version to be published. R.C.S. conceived and designed the study, participated in acquiring and interpreting the data and drafting and revision of the manuscript, and approved the version to be published. C.L.R. and F.J.K. contributed to conception and design of the study, acquisition of the data, and critical revision of the manuscript, and approved the version to be published. J.K. contributed to conception and design of the study, interpretation of the data, and critical revision of the manuscript, and approved the version to be published. M.R. analyzed and interpreted the data, drafted and revised the manuscript, and approved the version to be published. M.R.D. conceived and designed the study, participated in acquisition and interpretation of the data and drafting and revision of the manuscript, and approved the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Castro O, Brambilla DJ, Thorington B, Reindorf CA, Scott RB, Gillette P, Vera JC, Levy PS The Cooperative Study of Sickle Cell Disease. The acute chest syndrome in sickle cell disease: incidence and risk factors. Blood. 1994;84:643–649. [PubMed] [Google Scholar]
- 2.Delclaux C, Zerah-Lancner F, Bachir D, Habibi A, Monin JL, Godeau B, Galacteros F. Factors associated with dyspnea in adult patients with sickle cell disease. Chest. 2005;128:3336–3344. doi: 10.1378/chest.128.5.3336. [DOI] [PubMed] [Google Scholar]
- 3.Gordeuk VR, Minniti CP, Nouraie M, Campbell AD, Rana SR, Luchtman-Jones L, Sable C, Dham N, Ensing G, Prchal JT, et al. Elevated tricuspid regurgitation velocity and decline in exercise capacity over 22 months of follow up in children and adolescents with sickle cell anemia. Haematologica. 2011;96:33–40. doi: 10.3324/haematol.2010.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLean JE, Atenafu E, Kirby-Allen M, MacLusky IB, Stephens D, Grasemann H, Subbarao P. Longitudinal decline in lung volume in a population of children with sickle cell disease. Am J Respir Crit Care Med. 2008;178:1055–1059. doi: 10.1164/rccm.200708-1219OC. [DOI] [PubMed] [Google Scholar]
- 5.Lunt A, McGhee E, Sylvester K, Rafferty G, Dick M, Rees D, Height S, Thein SL, Greenough A.Longitudinal assessment of lung function in children with sickle cell disease Pediatr Pulmonol[online ahead of print] 22 Dec 2015. DOI: 10.1002/ppul.23367 [DOI] [PubMed] [Google Scholar]
- 6.Miller AC, Gladwin MT. Pulmonary complications of sickle cell disease. Am J Respir Crit Care Med. 2012;185:1154–1165. doi: 10.1164/rccm.201111-2082CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sylvester KP, Patey RA, Milligan P, Dick M, Rafferty GF, Rees D, Thein SL, Greenough A. Pulmonary function abnormalities in children with sickle cell disease. Thorax. 2004;59:67–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Tassel C, Arnaud C, Kulpa M, Fleurence E, Kandem A, Madhi F, Bernaudin F, Delacourt C. Leukocytosis is a risk factor for lung function deterioration in children with sickle cell disease. Respir Med. 2011;105:788–795. doi: 10.1016/j.rmed.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Pianosi P, D’Souza SJ, Charge TD, Esseltine DE, Coates AL. Pulmonary function abnormalities in childhood sickle cell disease. J Pediatr. 1993;122:366–371. doi: 10.1016/s0022-3476(05)83418-3. [DOI] [PubMed] [Google Scholar]
- 10.Boyd JH, DeBaun MR, Morgan WJ, Mao J, Strunk RC. Lower airway obstruction is associated with increased morbidity in children with sickle cell disease. Pediatr Pulmonol. 2009;44:290–296. doi: 10.1002/ppul.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koumbourlis AC, Zar HJ, Hurlet-Jensen A, Goldberg MR. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. J Pediatr. 2001;138:188–192. doi: 10.1067/mpd.2001.111824. [DOI] [PubMed] [Google Scholar]
- 12.Liem RI, Nevin MA, Prestridge A, Young LT, Thompson AA. Tricuspid regurgitant jet velocity elevation and its relationship to lung function in pediatric sickle cell disease. Pediatr Pulmonol. 2009;44:281–289. doi: 10.1002/ppul.20996. [DOI] [PubMed] [Google Scholar]
- 13.Intzes S, Kalpatthi RV, Short R, Imran H. Pulmonary function abnormalities and asthma are prevalent in children with sickle cell disease and are associated with acute chest syndrome. Pediatr Hematol Oncol. 2013;30:726–732. doi: 10.3109/08880018.2012.756961. [DOI] [PubMed] [Google Scholar]
- 14.Koumbourlis AC. Lung function in sickle cell disease. Paediatr Respir Rev. 2014;15:33–37. doi: 10.1016/j.prrv.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Koumbourlis AC, Hurlet-Jensen A, Bye MR. Lung function in infants with sickle cell disease. Pediatr Pulmonol. 1997;24:277–281. doi: 10.1002/(sici)1099-0496(199710)24:4<277::aid-ppul6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Santoli F, Zerah F, Vasile N, Bachir D, Galacteros F, Atlan G. Pulmonary function in sickle cell disease with or without acute chest syndrome. Eur Respir J. 1998;12:1124–1129. doi: 10.1183/09031936.98.12051124. [DOI] [PubMed] [Google Scholar]
- 17.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. The impact of recurrent acute chest syndrome on the lung function of young adults with sickle cell disease. Lung. 2010;188:499–504. doi: 10.1007/s00408-010-9255-2. [DOI] [PubMed] [Google Scholar]
- 18.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 19.Field JJ, Stocks J, Kirkham FJ, Rosen CL, Dietzen DJ, Semon T, Kirkby J, Bates P, Seicean S, DeBaun MR, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139:563–568. doi: 10.1378/chest.10-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Kirkby J, Welsh L, Lum S, Fawke J, Rowell V, Thomas S, Marlow N, Stocks J EPICure Study Group. The EPICure study: comparison of pediatric spirometry in community and laboratory settings. Pediatr Pulmonol. 2008;43:1233–1241. doi: 10.1002/ppul.20950. [DOI] [PubMed] [Google Scholar]
- 22.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 23.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenthal M, Cramer D, Bain SH, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years: II--Single breath analysis and plethysmography. Thorax. 1993;48:803–808. doi: 10.1136/thx.48.8.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkby J, Bonner R, Lum S, Bates P, Morgan V, Strunk RC, Kirkham F, Sonnappa S, Stocks J. Interpretation of pediatric lung function: impact of ethnicity. Pediatr Pulmonol. 2013;48:20–26. doi: 10.1002/ppul.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 27.Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest. 2009;135:419–424. doi: 10.1378/chest.08-1235. [DOI] [PubMed] [Google Scholar]
- 28.Iyer VN, Schroeder DR, Parker KO, Hyatt RE, Scanlon PD. The nonspecific pulmonary function test: longitudinal follow-up and outcomes. Chest. 2011;139:878–886. doi: 10.1378/chest.10-0804. [DOI] [PubMed] [Google Scholar]
- 29.Arteta M, Campbell A, Nouraie M, Rana S, Onyekwere OC, Ensing G, Sable C, Dham N, Darbari D, Luchtman-Jones L, et al. Abnormal pulmonary function and associated risk factors in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol. 2014;36:185–189. doi: 10.1097/MPH.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67:66–76. [PubMed] [Google Scholar]
- 31.Cohen RT, Strunk RC, Field JJ, Rosen CL, Kirkham FJ, Redline S, Stocks J, Rodeghier MJ, DeBaun MR. Environmental tobacco smoke and airway obstruction in children with sickle cell anemia. Chest. 2013;144:1323–1329. doi: 10.1378/chest.12-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudry RA, Rosenthal M, Bush A, Crowley S. Reduced forced expiratory flow but not increased exhaled nitric oxide or airway responsiveness to methacholine characterises paediatric sickle cell airway disease. Thorax. 2014;69:580–585. doi: 10.1136/thoraxjnl-2013-204464. [DOI] [PubMed] [Google Scholar]
- 33.Quanjer PH, Weiner DJ, Pretto JJ, Brazzale DJ, Boros PW. Measurement of FEF25-75% and FEF75% does not contribute to clinical decision making. Eur Respir J. 2014;43:1051–1058. doi: 10.1183/09031936.00128113. [DOI] [PubMed] [Google Scholar]
- 34.Lukic KZ, Coates AL. Does the FEF25-75 or the FEF75 have any value in assessing lung disease in children with cystic fibrosis or asthma? Pediatr Pulmonol. 2015;50:863–868. doi: 10.1002/ppul.23234. [DOI] [PubMed] [Google Scholar]
- 35.MacLean J, Grasseman H, Subbarao P. Changes in lung function in children with sickle cell disease. Am J Respir Crit Care Med. 2009;180:377–378, author reply 377–378. doi: 10.1164/ajrccm.180.4.377. [DOI] [PubMed] [Google Scholar]
- 36.Ong BA, Caboot J, Jawad A, McDonough J, Jackson T, Arens R, Marcus CL, Smith-Whitley K, Mason TB, Ohene-Frempong K, et al. Respiratory muscle force and lung volume changes in a population of children with sickle cell disease. Br J Haematol. 2013;163:112–117. doi: 10.1111/bjh.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen RT, Klings ES, Strunk RC. Sickle cell disease: wheeze or asthma? Asthma Res Pract. 2015;1:14. doi: 10.1186/s40733-015-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 39.Wedderburn CJ, Rees D, Height S, Dick M, Rafferty GF, Lunt A, Greenough A. Airways obstruction and pulmonary capillary blood volume in children with sickle cell disease. Pediatr Pulmonol. 2014;49:724. doi: 10.1002/ppul.22952. [DOI] [PubMed] [Google Scholar]
- 40.Lunt A, Desai SR, Wells AU, Hansell DM, Mushemi S, Melikian N, Shah AM, Thein SL, Greenough A. Pulmonary function, CT and echocardiographic abnormalities in sickle cell disease. Thorax. 2014;69:746–751. doi: 10.1136/thoraxjnl-2013-204809. [DOI] [PubMed] [Google Scholar]