Abstract
Rationale: Primary ciliary dyskinesia (PCD), a genetically heterogeneous, recessive disorder of motile cilia, is associated with distinct clinical features. Diagnostic tests, including ultrastructural analysis of cilia, nasal nitric oxide measurements, and molecular testing for mutations in PCD genes, have inherent limitations.
Objectives: To define a statistically valid combination of systematically defined clinical features that strongly associates with PCD in children and adolescents.
Methods: Investigators at seven North American sites in the Genetic Disorders of Mucociliary Clearance Consortium prospectively and systematically assessed individuals (aged 0–18 yr) referred due to high suspicion for PCD. The investigators defined specific clinical questions for the clinical report form based on expert opinion. Diagnostic testing was performed using standardized protocols and included nasal nitric oxide measurement, ciliary biopsy for ultrastructural analysis of cilia, and molecular genetic testing for PCD-associated genes. Final diagnoses were assigned as “definite PCD” (hallmark ultrastructural defects and/or two mutations in a PCD-associated gene), “probable/possible PCD” (no ultrastructural defect or genetic diagnosis, but compatible clinical features and nasal nitric oxide level in PCD range), and “other diagnosis or undefined.” Criteria were developed to define early childhood clinical features on the basis of responses to multiple specific queries. Each defined feature was tested by logistic regression. Sensitivity and specificity analyses were conducted to define the most robust set of clinical features associated with PCD.
Measurements and Main Results: From 534 participants 18 years of age and younger, 205 were identified as having “definite PCD” (including 164 with two mutations in a PCD-associated gene), 187 were categorized as “other diagnosis or undefined,” and 142 were defined as having “probable/possible PCD.” Participants with “definite PCD” were compared with the “other diagnosis or undefined” group. Four criteria-defined clinical features were statistically predictive of PCD: laterality defect; unexplained neonatal respiratory distress; early-onset, year-round nasal congestion; and early-onset, year-round wet cough (adjusted odds ratios of 7.7, 6.6, 3.4, and 3.1, respectively). The sensitivity and specificity based on the number of criteria-defined clinical features were four features, 0.21 and 0.99, respectively; three features, 0.50 and 0.96, respectively; and two features, 0.80 and 0.72, respectively.
Conclusions: Systematically defined early clinical features could help identify children, including infants, likely to have PCD.
Clinical trial registered with ClinicalTrials.gov (NCT00323167).
Keywords: primary ciliary dyskinesia, clinical features, laterality defects, neonatal respiratory distress
Primary ciliary dyskinesia (PCD) is an inherited disorder characterized by motile ciliary dysfunction resulting in an array of clinical manifestations, including chronic rhinosinusitis, middle ear effusions, laterality defects, infertility, and chronic bronchitis leading to bronchiectasis. The incidence of PCD is estimated at 1 in 15,000–20,000 live births, based on the prevalence of situs inversus totalis and bronchiectasis (1, 2). Few patients carry a well-established diagnosis, which reflects the limited ability to identify and definitively diagnose affected individuals. Therefore, the actual incidence may be higher than these estimates. While the clinical spectrum of PCD has been described for decades, recent literature continues to redefine the phenotype (3–10).
Historically, the diagnosis of PCD relied on the combination of clinical features and ultrastructural analysis of respiratory cilia (11–13), which requires specialized expertise for tissue collection, processing, and performing and interpreting transmission electron micrography. Ultrastructural changes in cilia may be specific for PCD, but they can also overlap with acquired changes due to exposure to viral infection or environmental pollutants. Moreover, nearly one-third of affected individuals have normal ciliary ultrastructure (8). The clinical phenotype of PCD has characteristics in common with other conditions, which can cause diagnostic delays, especially if the patients do not have the classical features, such as organ laterality defects, and this delay may lead to poorer outcomes for patients (3).
Past clinical criteria for diagnosis were based on available evidence and expert opinion (14). Recent studies have advanced understanding of the spectrum of clinical phenotypes and have identified new diagnostic tests, including the emergence of genetic testing for PCD (15). The development of our large multicenter collaborative group in North America provides an opportunity for diagnostic standardization in children and adolescents. In this paper, we describe a prospective study that delineates characteristic early childhood clinical features, alone or in combination, that are predictive of PCD among infants and children referred with high suspicion of PCD. We propose that systematic evaluation of these clinical markers helps discriminate individuals, including infants, likely to have PCD from unaffected children, thereby identifying individuals who require more definitive diagnostic genetic and physiologic testing (4).
Methods
Study Sites and Participants
Investigators at seven North American sites in the Genetic Disorders of Mucociliary Clearance Consortium systematically and prospectively evaluated pediatric participants who were referred because of high suspicion for PCD. Some participants had a preexisting diagnosis of PCD but had not had a full systematic assessment. Evaluation included comprehensive collection of clinical information using standardized case report forms. Standard operating procedures defined all details of evaluation, including performance of diagnostic tests and assignment into diagnostic categories.
The investigators defined the clinical questions and corresponding answers for the clinical report form based on expert opinion before initiation of the study. Specific queries from case report forms were used to define criteria for PCD-specific clinical features, including neonatal respiratory distress, otitis media, cough, nasal congestion, and laterality defects. Bronchiectasis and sinusitis could not be systematically assessed, because computed tomography of the chest and paranasal sinuses was not performed in all children. Institutional review board approval was obtained at all sites, and informed consent and assent were acquired from parents and from participants, as appropriate. An observational safety monitoring board reviewed the protocol.
Diagnostic Testing
Diagnostic testing included nasal nitric oxide measurement, ciliary biopsy for electron microscopy, and molecular genetic testing for PCD-associated genes. Nasal nitric oxide measurements were performed using standardized protocols (16, 17). Molecular genetic analyses and electron microscopy were performed by researchers at one site (University of North Carolina), who communicated results to the local sites.
Ciliated epithelial cells were collected from the inferior surface of the inferior nasal turbinates and processed for transmission electron microscopy, as previously described (4, 6, 18). Three reviewers evaluated transmission electron micrographs in a blinded fashion for PCD-specific ciliary defects, specifically (1) absent or truncated outer dynein arms alone, (2) absent outer and inner dynein arms, (3) absent inner dynein arms with microtubular disorganization, and (4) central apparatus or radial spoke defects with absent or off-center central pair associated with transpositions of peripheral doublet. Isolated inner dynein arm defects were not considered diagnostic, except when apparent on repeat biopsies.
Genetic testing included screening for the coding regions and splice junctions for 26 PCD-associated genes using established techniques (6). Loss-of-function variants, including nonsense, frameshift, and splice site variants, were considered pathogenic mutations. Missense variants or in-frame deletions were considered to be pathogenic if they were present in the cases carrying loss-of-function mutations and if the ultrastructural defects were congruent with the mutated gene. Missense variants or in-frame deletions were initially checked for rarity using public databases and if predicted to be pathogenic using in silico programs such as MutationTaster (19). Then they were checked for conservation (across species and/or being part of the functional domains). Segregation analysis was performed when possible to determine the phase. Missense variants on both alleles were considered pathogenic only if previous reports showed that these variants caused functional defects or had been seen in multiple cases.
To assess for alternative diagnoses, results from prior clinical tests were recorded in the case report forms, including sweat chloride test, CFTR mutation analysis, pH probe and imaging studies to assess aspiration or gastroesophageal reflux, and imaging studies of the chest and sinuses. Additional clinical testing performed as part of our research protocol, if not done before the study visit, included sweat chloride test, complete blood count with leukocyte differential, and chest radiograph.
Diagnostic Categories
Final diagnoses were assigned by the investigator at the local site on the basis of specific findings as directed by standard operating protocols: “definite PCD” (hallmark ultrastructural cilia defects and/or two pathogenic gene mutations), “probable/possible PCD” (no definitive structural defect or genetic diagnosis, but a compatible clinical phenotype and reduced nasal nitric oxide level in the PCD range), “other diagnosis” (another etiology identified), or “undefined” (no etiology identified). Researchers at the central site (University of North Carolina) reviewed the assigned diagnostic category to ensure that ultrastructural and genetic results from the central site were accurately reflected in the database. Challenging cases were discussed in biweekly telephone conference calls.
Statistical Analysis
Data were collated and entered in a web-based database managed by the data management coordinating center. Participants with definite PCD were compared with those with “other diagnosis” and “undefined,” combined (hereafter referred to as other/undefined). Fisher’s exact test or the Wilcoxon rank-sum test were used to compare demographics and clinical features between the PCD and other/undefined groups. Logistic regression was used to assess the associations of the five specifically defined clinical criteria (Figure 1) with PCD.
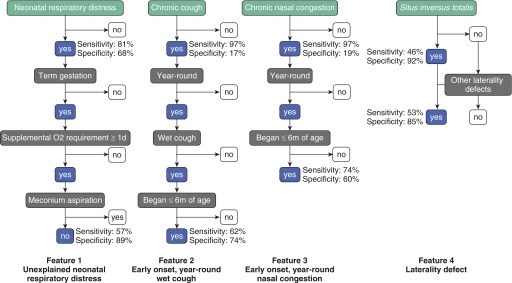
Figure 1.
Schematic diagram showing general clinical features and series of questions to define criteria-defined clinical features. The four criteria-defined clinical features most predictive of primary ciliary dyskinesia were unexplained neonatal respiratory distress, early onset, year-round wet cough, early onset, year-round nasal congestion, and laterality defects. The sensitivity and specificity to identify children and adolescents with primary ciliary dyskinesia are shown for each general clinical feature and its respective criteria-defined clinical feature. Early onset is defined as onset before 6 months of age; year-round is defined as occurring in all 12 months of the year; and wet cough is defined as sounds productive even if unable to expectorate sputum.
Diagnostic performance of clinical criteria was evaluated by assessing sensitivity, specificity, and weighted and unweighted receiver operating characteristic curves. Sensitivity analyses were conducted to explore external validity (by including subjects with probable/possible PCD in the analyses) and to determine whether diagnostic accuracy would be different in children with versus without laterality defects (by testing interactions between each symptom-based criterion and laterality status in a logistic regression model). P values less than 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
Participants
Between June 2006 and September 2012, a total of 534 participants (aged ≤18 yr) were enrolled at seven consortium sites across North America: Chapel Hill, North Carolina, the lead site (254 participants); St. Louis, Missouri (84 participants); Toronto, Ontario, Canada (70 participants); Aurora, Colorado (43 participants); Seattle, Washington (36 participants); Palo Alto, California (31 participants); and Bethesda, Maryland (16 participants). Of 534 participants evaluated, 205 were identified as having “definite PCD” (48% female; mean age, 7.8 yr), 187 (46% female; mean age, 7.0 yr) were categorized as “other disease” (n = 60, including immunodeficiency, gastroesophageal reflux, asthma, allergic rhinitis, sinusitis alone, chronic otitis media alone, and laterality defect alone) or “undefined” (n = 127) (Table 1), and 142 were categorized as having “probable/possible” PCD (46% female; mean age, 5.8 yr).
Table 1.
Demographics and nasal nitric oxide values at enrollment for definite primary ciliary dyskinesia and other/undefined groups
| PCD (n = 205) | Other/Undefined (n = 187) | P Value | |
|---|---|---|---|
| Female sex | 99 (48%) | 86 (46%) | 0.686 |
| Race, white | 171 (83%) | 168 (90%) | 0.076 |
| Ethnicity, non-Hispanic | 179 (87%) | 168 (90%) | 0.526 |
| Age | |||
| Median (range), yr | 8 (0–18) | 7 (0–18) | 0.191 |
| Mean ± SD, yr | 7.8 ± 5.4 | 7.0 ± 4.5 | 0.127 |
| ≤5 yr, n (%) | 75 (37%) | 76 (41%) | 0.467 |
| Nasal nitric oxide* | |||
| Mean ± SD, nl/min | 20.9 ± 21.8 (n = 121) | 258.3 ± 146.9 (n = 102) | <0.0001 |
| Below cutoff of 77 nl/min† | 117 (97%) | 1 (0.9%) | <0.0001 |
Definition of abbreviation: PCD = primary ciliary dyskinesia.
Limited to participants older than 5 years of age who were able to cooperate with the maneuver.
Prior studies established a cutoff of 77 nl/min with sensitivity of 98% for PCD (16).
The diagnosis in the 205 participants with “definite PCD” was based on a hallmark ultrastructural defect (n = 178) or two mutations in a PCD-associated gene (n = 164, not mutually exclusive), including 28 participants who had genetic diagnoses alone (10 with DNAH11 mutations and 18 with genes associated with either outer dynein arm or central apparatus defects but inadequate or indeterminate ultrastructural analyses) (Table 2). Of the 164 participants in the definite PCD group with two mutations in an associated PCD gene, 128 had two loss-of-function mutations, 28 had a loss-of-function mutation with either a missense variant or an in-frame deletion fulfilling criteria for pathogenic mutations, and 8 had missense mutations on both alleles fulfilling criteria for pathogenic mutations based on the previous reports (20–22). Molecular analysis of the 26 genes was performed for 174 of the 187 participants in the other/undefined group. None harbored biallelic mutations, and eight participants carried one loss-of-function mutant allele; however, the gene mutation was not congruent with the ultrastructural phenotype.
Table 2.
Distribution of ciliary ultrastructural defects and associated primary ciliary dyskinesia genes with two pathogenic mutations in the 205 participants with “definite” primary ciliary dyskinesia
| Ciliary Defect | Mutated Gene | Number of Participants (%) | |
|---|---|---|---|
| Absent or truncated outer dynein arms, alone | DNAH5 | 60 | |
| DNAI1 | 14 | ||
| DNAI2 | 5 | ||
| CCDC114 | 3 | ||
| ARMC4 | 2 | ||
| Not identified | 18 | ||
| Total | 102 (49.8%) | ||
| Absent outer and inner dynein arms | LRRC6 | 3 | |
| HEATR2 | 6 | ||
| SPAG1 | 7 | ||
| DNAAF1 (LRRC50) | 1 | ||
| DNAAF2 (KTU) | 2 | ||
| ZMYND10 | 2 | ||
| DYX1C1 | 3 | ||
| Not identified | 6 | ||
| Total | 30 (14.6%) | ||
| Absent inner dynein arms with microtubular disorganization | CCDC39 | 15 | |
| CCDC40 | 24 | ||
| Not identified | 15 | ||
| Total | 54 (26.3%) | ||
| Central apparatus or radial spoke defects; or inner dynein arm defect, alone | RSPH4A | 6 | |
| RSPH9 | 1 | ||
| Not identified | 2 | ||
| Total | 9 (4.4%) | ||
| Normal or indeterminate | DNAH11 | 10 | |
| Total | 10 (4.9%) |
Demographic and nasal nitric oxide values recorded at the time of enrollment are shown in Table 1. There were no differences in sex, race, ethnicity, or age for PCD versus other/undefined group. General clinical features prevalent (>80%) in both groups were chronic cough, chronic nasal congestion, and recurrent otitis. Neonatal respiratory distress was reported much more frequently among children with PCD (81% vs. 32%) (Table 3).
Table 3.
Prevalence of general clinical features and criteria-defined clinical features among participants with primary ciliary dyskinesia versus other/undefined groups and multiple logistic regression model for criteria-defined clinical features
| Features | Group | |||
|---|---|---|---|---|
| General clinical features | PCD (n = 205) | Other/Undefined (n = 187) | Adjusted Odds Ratio (95% Confidence Interval)* | P Value |
| Neonatal respiratory distress | 166 (81%) | 59 (32%) | 7.8 (4.6–13.4) | <0.0001 |
| Chronic cough | 198 (97%) | 155 (83%) | 2.4 (0.8–6.7) | 0.103 |
| Chronic nasal congestion | 198 (97%) | 151 (81%) | 6.2 (2.3–16.9) | 0.0004 |
| Situs inversus totalis | 95 (46%) | 15 (8%) | 12.0 (5.6–25.5) | <0.0001 |
| Recurrent otitis | 180 (88%) | 152 (81%) | 1.5 (0.7–3.2) | 0.246 |
| Criteria-defined clinical features (see definitions in Figure 1) | PCD (n = 204†) | Other/Undefined (n = 185‡) | Adjusted Odds Ratio (95% Confidence Interval)* | P Value |
| Unexplained neonatal respiratory distress (feature 1) | 116 (57%) | 21 (11%) | 6.6 (3.5–12.3) | <0.0001 |
| Early-onset, year-round wet cough (feature 2) | 128 (62%) | 48 (26%) | 3.1 (1.7–5.5) | 0.0001 |
| Early-onset, year-round nasal congestion (feature 3) | 151 (74%) | 74 (40%) | 3.4 (1.9–6.3) | <0.0001 |
| Laterality defect (feature 4) | 109 (53%) | 28 (15%) | 7.7 (4.0–14.9) | <0.0001 |
| Multiple ear infections in first 2 yr of life with sequelae | 89 (43%) | 66 (35%) | 1.0 (0.6–1.8) | 0.981 |
Definition of abbreviation: PCD = primary ciliary dyskinesia.
Age was adjusted in the model.
One participant with PCD was missing data for the criteria.
Two other/undefined participants were missing data for the criteria.
To systematically define clinical features associated with PCD, we asked additional questions related to each of the general clinical features to generate more specific criteria for each (Figure 1). The specific criteria-defined clinical features were tested for ability to better discriminate PCD from the other/undefined group (Table 4). With the exception of laterality defects, fulfillment of the specific criteria-defined clinical feature, compared with the respective general feature, was associated with a decrease in the prevalence of the characteristic in both groups, which was disproportionately greater for the other/undefined group. Likewise, the more specific criteria-defined clinical features had decreased sensitivity but greater specificity than the general clinical features (Figure 1).
Table 4.
Sensitivity and specificity for each general and criteria-defined clinical feature
| Features | Sensitivity | Specificity |
|---|---|---|
| General clinical features (PCD, n = 205; other/undefined, n = 187) | ||
| Neonatal respiratory distress | 81% | 68% |
| Chronic cough | 97% | 17% |
| Chronic nasal congestion | 97% | 19% |
| Situs inversus totalis | 46% | 92% |
| Recurrent otitis | 88% | 19% |
| Criteria-defined clinical features (see definitions in Figure 1) (PCD, n = 204*; other/undefined, n = 185†) | ||
| Unexplained neonatal respiratory distress (feature 1) | 57% | 89% |
| Early-onset, year-round wet cough (feature 2) | 62% | 74% |
| Early-onset, year-round nasal congestion (feature 3) | 74% | 60% |
| Laterality defect (feature 4) | 53% | 85% |
| Multiple ear infections in first 2 yr of life with sequelae | 43% | 65% |
Definition of abbreviation: PCD = primary ciliary dyskinesia.
One participant with PCD was missing data for the criteria.
Two other/undefined participants were missing data for the criteria.
For the more systematically defined laterality defect, inclusion of laterality defects other than situs inversus totalis increased prevalence in both groups compared with the general query of situs inversus totalis, resulting in a modest increase in sensitivity but a slight decrease in specificity. The four criteria-defined features that were noted significantly (P < 0.001) more frequently in PCD compared with the other/undefined group were (1) unexplained neonatal respiratory distress (57% vs. 11%); (2) early-onset, year-round wet cough (62% vs. 26%); (3) early-onset, year-round nasal congestion (74% vs. 40%); and (4) laterality defects (53% vs. 15%) (Table 3). Adjusted odds ratio estimates for each of these individual criteria-defined clinical features were strong, ranging from 3.1 to 7.7 (Table 3).
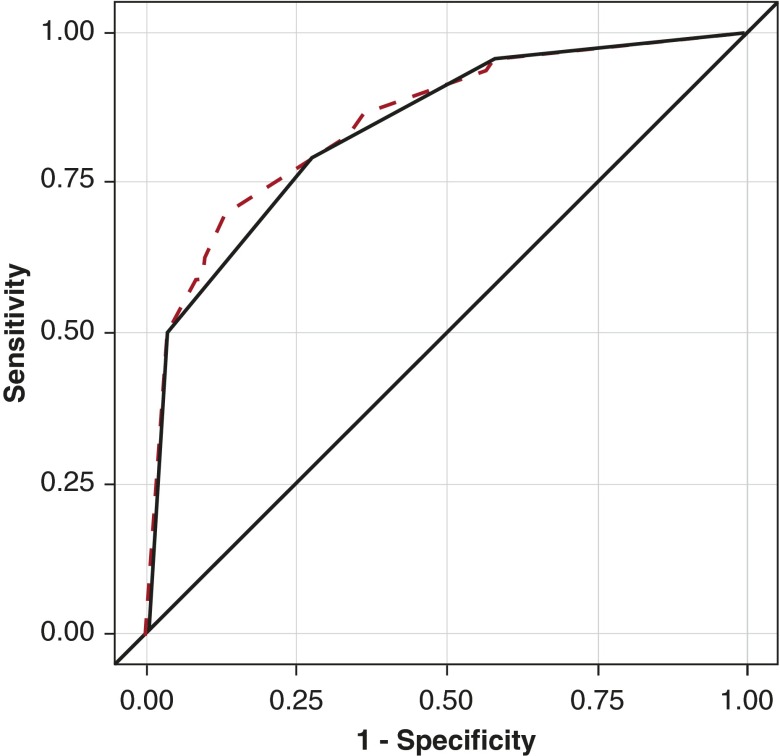
The sensitivity and specificity analysis based on number of criteria-defined clinical features is shown in Table 5 and Figure 2. The unweighted receiver operating characteristic curve suggests that if a subject had any three or four of those criteria-defined clinical features, the diagnosis of PCD would be more than 96% specific but less than 50% sensitive (Table 5). Of note, combination of the two most predictive criteria-based clinical features based on logistic regression, feature 1 (unexplained neonatal respiratory distress), and feature 4 (laterality defect) is predictive of PCD with a sensitivity of 34% and specificity of 95%. Figure 2 shows receiver operating characteristic curves based on number of criteria-defined clinical features that were present. The unweighted curve for the four significant criteria-defined clinical features overlaps well with the weighted curve, based on the logistic regression coefficient for all five criteria-defined clinical features, indicating that assignment of a relative weight to each feature does not improve diagnostic accuracy.
Table 5.
Sensitivity and specificity based on number of general clinical features versus criteria-defined clinical features: performance as test
| Features | Sensitivity | Specificity |
|---|---|---|
| Number of general clinical features | ||
| 4 | 0.37 | 0.97 |
| 3 | 0.84 | 0.74 |
| 2 | 0.99 | 0.22 |
| 1 | 1.00 | 0.04 |
| 0 | 1.00 | 0.00 |
| Number of criteria-defined clinical features | ||
| 4 | 0.21 | 0.99 |
| 3 | 0.50 | 0.96 |
| 2 | 0.80 | 0.72 |
| 1 | 0.96 | 0.41 |
| 0 | 1.00 | 0.00 |
Figure 2.
Receiver operating characteristic curves for the criteria-defined clinical features. The unweighted receiver operating characteristic curve (solid curved line) uses a uniform weight for each of the four significant criteria-defined clinical features. For the weighted curve (dashed line), each clinical feature is weighted by its logistic regression parameter estimate from Table 3 (2.05 for laterality defect, 1.89 for neonatal respiratory distress, 1.28 for nasal congestion, 1.13 for chronic cough and 0.01 for multiple ear infections). The areas under the curve were 0.86 (95% confidence interval, 0.82–0.89) for the weighted receiver operating characteristic curve and 0.84 (95% confidence interval, 0.81–0.88) for the unweighted receiver operating characteristic curve. The weighted curve was minimally better than the unweighted curve (P = 0.019), but the shape was similar except for the region of sensitivity (50–80%).
In terms of sensitivity analyses, when participants with probable and possible PCD were included in the PCD group, sensitivity and specificity results were similar, though with lower sensitivity (see Table E1 in the online supplement). No interaction was found between laterality status and the association between the remaining three clinical criteria and PCD case status (P > 0.10 for each), indicating that the diagnostic accuracy of these three criteria is similar among children with and without laterality defects (data not shown).
Discussion
In this prospective study, we defined specific clinical features apparent in early childhood that, alone or in combination, are predictive of PCD. The best performing clinical criteria for PCD included (1) unexplained neonatal respiratory distress with supplemental oxygen requirement more than 24 hours in term infants; (2) early-onset, year-round wet cough; (3) early-onset, year-round nasal congestion; and (4) laterality defects. If three or more of these rigorously defined clinical features are met, specificity for the diagnosis is greater than 96%. Moreover, meeting only one or two of the criteria-defined clinical features may still warrant diagnostic testing. Using these four systematically defined but simple clinical features, the general clinician may be able to effectively screen for PCD in early childhood, thereby leading to earlier referral for specialized diagnostic testing at a PCD center while avoiding unnecessary and costly evaluation of patients unlikely to have the disease.
The clinical features of PCD can overlap with other conditions, which historically has made diagnosis of PCD challenging. Consortium investigators evaluated over 500 symptomatic children who were referred because of high suspicion for PCD to identify 205 individuals with definite disease, which underscores the diagnostic confusion among physicians. This uncertainty is due largely to relative inexperience with this rare disease and lack of familiarity with the limitations of diagnostic testing, including transmission electron microscopy, ciliary motility studies, genetic panels, and nasal nitric oxide testing. For these reasons, all subjects evaluated at the participating sites underwent identical comprehensive testing with rigorous diagnostic standards to ensure that the “definite PCD” group did not include participants who may have had other respiratory disorders.
The prevalence of clinical features in children with PCD enrolled in this collaborative study was similar to that in previous studies (3–5, 10); however, none of those studies assessed use of clinical features to determine the likelihood of PCD. The only published prospective study (23) in which researchers examined clinical features to identify patients likely to have PCD also identified neonatal respiratory distress and laterality defects as strong indicators, though the authors’ approach was not as systematic about defining the clinical features or diagnosis. Our study population significantly differs from the clinical population seen by a general pediatric pulmonologist, since many were referred specifically for PCD diagnostic testing. Inclusion criteria for consortium studies limited enrollment to individuals for whom referring physicians had a high suspicion of PCD; consequently, a high proportion (40.5%) of total participants had “definite” PCD. Further, the “other disease” group also had a high prevalence of clinical features that could be suggestive of PCD, including laterality defects.
To achieve the best discrimination of clinical features to predict which individuals are likely to have PCD, further definition of these general clinical features, based on use of a series of detailed questions, was required. For instance, chronic cough alone was a poor discriminator, since most participants referred for evaluation presented with cough. However, children with PCD characteristically had a year-round, wet or productive cough that began as a prominent symptom before 6 months of age. Thus, seasonal, intermittent, or dry cough is inconsistent with the diagnosis of PCD.
The characteristic chronic cough in PCD likely compensates for the lack of effective mucociliary clearance and thereby leads to some protection for the airways and lung. Physiologic data have shown that “effective” coughs may result in lung clearance almost equal to normal mucociliary clearance over short time periods, even in subjects with PCD who lack clearance at baseline (24). Chronic airway infection and inflammation lead to bronchiectasis in almost all patients by early adulthood, and pulmonary function testing typically shows progressive, airflow-obstructive defects. Clinical and radiographic features of bronchiectasis develop as the disease progresses, even in some children at a very young age (25).
The general clinical feature “neonatal respiratory distress” was more prevalent in the “definite PCD” group than in the “other” group (81% vs. 32%; P < 0.0001; specificity, 68%). By limiting neonatal respiratory distress to those requiring supplemental oxygen for more than 24 hours and eliminating other causes, such as prematurity and meconium aspiration (4, 7), the difference between PCD and other diagnoses led to a more specific discriminator (56.6% vs. 11.2%; P < 0.0001; specificity, 89%). Furthermore, the combination of neonatal respiratory distress with a laterality defect was an even stronger predictor, with specificity of 95%.
In past studies, investigators have reported that patients with PCD present in the newborn period with significant respiratory distress, suggesting a potential link between effective ciliary function and the clearance of lung liquid in the neonatal period (26, 27). However, the association of PCD with newborn respiratory distress requiring supplemental oxygen has been underappreciated. Thus, the diagnosis is often delayed, as described in a retrospective review in which the investigators reported that the mean age of diagnosis was older than 4 years of age (3).
Frequently, the neonatal respiratory symptoms in infants with PCD are attributed to an erroneous etiology, such as transient tachypnea of the newborn, neonatal pneumonia, or meconium aspiration, based on limited features of these disorders and lack of an alternative diagnosis in the newborn period (7). Therefore, clinicians need to carefully scrutinize the neonatal history and chest imaging findings of patients suspected to have PCD. Further delineation of PCD-associated neonatal respiratory features for pediatricians and neonatologists could lead to an earlier diagnosis.
Another striking phenotypic feature that may be detected at birth is a laterality defect (28). Situs inversus totalis is not genetically predetermined in patients with PCD, but occurs as a random phenomenon due to nodal ciliary dysfunction during embryogenesis (29, 30). More recently, investigators have found an association between PCD and heterotaxy (situs ambiguus or organ laterality defects other than situs inversus totalis) (31–33), which is associated with increased morbidity due to complex cardiovascular anomalies. Therefore, patients with anomalies consistent with situs ambiguus or heterotaxy, including congenital heart disease, who also have neonatal respiratory distress or chronic respiratory tract symptoms should be considered for PCD evaluation.
Our data show that the remaining three criteria-defined clinical features (features 1–3), including neonatal respiratory distress, are equally predictive of PCD in patients with and without laterality defects. Of note, patients who have central apparatus defects, such as those with biallelic mutations in RSPH4A or RSPH9, do not have laterality defects, presumably due to preserved left-to-right flow across the embryonic node (8).
The upper respiratory tract is almost universally involved, and early onset (age ≤6 mo) of year-round nasal congestion is a feature that distinguishes PCD from non-PCD. Rhinosinusitis and otitis media begin in early infancy in children with PCD and are responsible for morbidity early in life (3, 4). Many children with PCD have a history of recurrent or persistent otitis media requiring multiple antibiotic courses, often with sequelae that include conductive hearing loss and/or intervention with repeated myringotomy tube placement. However, chronic or recurrent otitis media is prominent in children without PCD as well. Therefore, middle ear disease did not clearly discriminate subjects with PCD from those without it in this study, though absence of middle ear disease during early childhood is inconsistent with a diagnosis.
Many PCD cases are not recognized until late childhood or adulthood, after bronchiectasis from chronic airway infections occurs. Family history, particularly in an older sibling, may prompt diagnostic testing in infancy. However, most patients do not have a positive family history, as is true for other autosomal recessive disorders. All four criteria-defined clinical features in our panel are apparent in the first year of life; therefore, specialists evaluating children with chronic respiratory symptoms or laterality defects could use this panel to promote earlier recognition of infants and young children likely to have PCD, which would lead to earlier diagnosis. For example, a pulmonologist evaluating a young child for chronic respiratory symptoms may recognize that a child with early-onset wet cough and early-onset nasal congestion may have PCD, even in the absence of neonatal respiratory distress or laterality defect. Similarly, a patient with all four criteria-defined clinical features has a high likelihood of PCD, even if diagnostic testing is inconclusive. With further validation and refinement, a panel based on clinical features may have utility as a diagnostic tool.
Strengths and Limitations
The strength of our findings is that we designed a multicenter prospective study with standardized data collection of the clinical information, followed by rigorous uniform application of a reference diagnosis for PCD, including electron microscopy of ciliary ultrastructure and molecular genetic analyses. These diagnostic tests can provide firm evidence for a PCD diagnosis, but they may miss some cases. Molecular genetic diagnosis is challenging because of the extensive genetic heterogeneity. Currently, mutations in 35 genes are associated with PCD and account for approximately 66% of all cases. Thus, pathogenic mutations in novel genes or in the regions not interrogated by testing laboratories may be missed. Additionally, not all of the 35 PCD-associated genes are part of the panels that are being offered in clinical molecular genetic testing laboratories.
One limitation of our approach is that the application of a rigorous reference diagnosis resulted in exclusion of a large proportion of our cohort (26%) that we strongly suspect has PCD on the basis of nasal nitric oxide testing. However, we demonstrated in a sensitivity analysis that inclusion of these unclassified subjects as PCD cases yielded slightly lower sensitivities but in similar ranges, increasing confidence in the external validity of our findings. A second limitation is that we evaluated the accuracy of the clinical criteria in a cohort referred for suspicion of PCD. Thus, our results are not necessarily generalizable to the general population. Finally, owing to the rarity of this disease, we have not yet applied our clinical diagnostic criteria to a validation cohort; however, this is a goal of future research.
Conclusions
Four criteria-defined clinical features (unexplained neonatal respiratory distress with persistent supplemental oxygen requirement in term infants; early-onset, year-round wet cough; early-onset, year-round nasal congestion; and laterality defects) in combination are highly effective in discriminating children and adolescents who are likely to have PCD. Conversely, the absence of these clinical features should reduce the clinician’s index of suspicion. The sensitivity and specificity of these four criteria among children referred for suspicion of PCD indicate that they can be used to identify at-risk children and direct decisions regarding further diagnostic testing.
Acknowledgments
Acknowledgment
The authors are grateful to the patients with PCD and their families for their participation. The authors thank Michele Manion and the U.S. Primary Ciliary Dyskinesia Foundation; all additional investigators; and the coordinators of the Genetic Disorders of Mucociliary Clearance Consortium (GDMCC), which is part of the Rare Diseases Clinical Research Network, including Tanya Glaser, Meghan O’Connell, Heather Root, Andrea Henkel, and Caroline Smith (National Institute of Allergy and Clinical Infectious Diseases, NIH, Bethesda, MD); Jane Quante (Washington University, St. Louis, MO); Donna Parker, Shelley Mann, and Carol Kopecky (Children’s Hospital Colorado, Aurora, CO); Sharon McNamara, Liz Cochrane, Molly Elliott, Jennifer Soper, and Robert Johnson (Children’s Hospital, Seattle, WA); Jacquelyn Zirbes (Stanford University Medical Center, Stanford, CA); Donna Wilkes and Melody Miki (Hospital for Sick Children, Toronto, ON, Canada); and Susan Minnix, Katherine Thurlow, and Caroline LaFave (University of North Carolina at Chapel Hill). The authors thank Kimberly Burns, Whitney Wolf, Rhonda Pace, and Michael Patrone for technical assistance; Elizabeth Godwin for administrative support; and Alexandra Infanzon for editorial assistance.
Footnotes
Supported by (1) National Institutes of Health (NIH) grants 5 U54 HL09640958-11 and 5R01 HL071798, funded by the Office of Rare Diseases Research (ORDR) of the National Center for Advancing Translational Sciences (NCATS), and by the National Heart, Lung, and Blood Institute (NHLBI); (2) Clinical and Translational Science Awards (CTSA) NIH/NCATS UNC ULTR000083; (3) CTSA NIH/NCATS Colorado UL1TR000154; and (4) the Intramural Research Program of the NHLBI, NIH. The Genetic Disorders of Mucociliary Clearance Consortium (5 U54HL096458) is a part of the NIH Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the NIH ORDR at the NCATS, and the NIH NHLBI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions: M.W.L. and M.R.K.: designed the study; M.W.L., M.R.K., T.W.F., and S. D. Davis: prepared the manuscript; H.-S.L.: performed biostatistical analysis; M.J.H.: developed standard operating procedures for nasal nitric oxide measurements; J.L.C.: reviewed transmission electron micrographs; M.W.L., M.R.K., S. D. Davis, S. D. Dell, T.W.F., K.N.O., S.D.S., A.J.S, J.E.P., M.R., and C.M.: recruited and evaluated patients at consortium sites; K.M.S., H.-S.L. and J.K.: coordinated web-based data entry; and M.A.Z.: coordinated and validated genetic studies.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Torgersen J. Situs inversus, asymmetry, and twinning. Am J Hum Genet. 1950;2:361–370. [PMC free article] [PubMed] [Google Scholar]
- 2.Katsuhara K, Kawamoto S, Wakabayashi T, Belsky JL. Situs inversus totalis and Kartagener’s syndrome in a Japanese population. Chest. 1972;61:56–61. doi: 10.1378/chest.61.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Coren ME, Meeks M, Morrison I, Buchdahl RM, Bush A. Primary ciliary dyskinesia: age at diagnosis and symptom history. Acta Paediatr. 2002;91:667–669. doi: 10.1080/080352502760069089. [DOI] [PubMed] [Google Scholar]
- 4.Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 5.Storm van’s Gravesande K, Omran H. Primary ciliary dyskinesia: clinical presentation, diagnosis and genetics. Ann Med. 2005;37:439–449. doi: 10.1080/07853890510011985. [DOI] [PubMed] [Google Scholar]
- 6.Davis SD, Ferkol TW, Rosenfeld M, Lee HS, Dell SD, Sagel SD, Milla C, Zariwala MA, Pittman JE, Shapiro AJ, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med. 2015;191:316–324. doi: 10.1164/rccm.201409-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullowney T, Manson D, Kim R, Stephens D, Shah V, Dell S. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics. 2014;134:1160–1166. doi: 10.1542/peds.2014-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia: recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowles MR, Ostrowski LE, Leigh MW, Sears PR, Davis SD, Wolf WE, Hazucha MJ, Carson JL, Olivier KN, Sagel SD, et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am J Respir Crit Care Med. 2014;189:707–717. doi: 10.1164/rccm.201311-2047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boon M, Smits A, Cuppens H, Jaspers M, Proesmans M, Dupont LJ, Vermeulen FL, Van Daele S, Malfroot A, Godding V, et al. Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J Rare Dis. 2014;9:11. doi: 10.1186/1750-1172-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Iongh RU, Rutland J. Ciliary defects in healthy subjects, bronchiectasis, and primary ciliary dyskinesia. Am J Respir Crit Care Med. 1995;151:1559–1567. doi: 10.1164/ajrccm.151.5.7735615. [DOI] [PubMed] [Google Scholar]
- 12.Bush A, Cole P, Hariri M, Mackay I, Phillips G, O’Callaghan C, Wilson R, Warner JO. Primary ciliary dyskinesia: diagnosis and standards of care. Eur Respir J. 1998;12:982–988. doi: 10.1183/09031936.98.12040982. [DOI] [PubMed] [Google Scholar]
- 13.Papon JF, Coste A, Roudot-Thoraval F, Boucherat M, Roger G, Tamalet A, Vojtek AM, Amselem S, Escudier E. A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2010;35:1057–1063. doi: 10.1183/09031936.00046209. [DOI] [PubMed] [Google Scholar]
- 14.Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, Bartoloni L, Eber E, Escribano A, Haarman E, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–1276. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 15.Zariwala MA, Knowles MR, Leigh MW.Primary ciliary dyskinesia Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K.editors. GeneReviews®. Seattle, WA: University of Washington; 1993–2016[updated 2015 Sep 3; accessed 2016 Apr 23]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1122/ [PubMed] [Google Scholar]
- 16.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 17.Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, Lavange LM, Horton BJ, Qaqish B, Carson JL, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013;10:574–581. doi: 10.1513/AnnalsATS.201305-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olin JT, Burns K, Carson JL, Metjian H, Atkinson JJ, Davis SD, Dell SD, Ferkol TW, Milla CE, Olivier KN, et al. Genetic Disorders of Mucociliary Clearance Consortium. Diagnostic yield of nasal scrape biopsies in primary ciliary dyskinesia: a multicenter experience. Pediatr Pulmonol. 2011;46:483–488. doi: 10.1002/ppul.21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz JM, Cooper DN, Schuelke M, Seelow D. Mutation Taster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 20.Horani A, Druley TE, Zariwala MA, Patel AC, Levinson BT, Van Arendonk LG, Thornton KC, Giacalone JC, Albee AJ, Wilson KS, et al. Whole-exome capture and sequencing identifies HEATR2 mutation as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2012;91:685–693. doi: 10.1016/j.ajhg.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guichard C, Harricane MC, Lafitte JJ, Godard P, Zaegel M, Tack V, Lalau G, Bouvagnet P. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome) Am J Hum Genet. 2001;68:1030–1035. doi: 10.1086/319511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall CR, Scherer SW, Zariwala MA, Lau L, Paton TA, Stockley T, Jobling RK, Ray PN, Knowles MR, Hall DA, et al. FORGE Canada Consortium. Whole-exome sequencing and targeted copy number analysis in primary ciliary dyskinesia. G3 (Bethesda) 2015;5:1775–1781. doi: 10.1534/g3.115.019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noll EM, Rieger CH, Hamelmann E, Nüsslein TG. Questionnaire to preselect patients with a high probability of primary ciliary dyskinesia. Klin Padiatr. 2011;223:22–26. doi: 10.1055/s-0030-1263136. [DOI] [PubMed] [Google Scholar]
- 24.Noone PG, Bennett WD, Regnis JA, Zeman KL, Carson JL, King M, Boucher RC, Knowles MR. Effect of aerosolized uridine-5′-triphosphate on airway clearance with cough in patients with primary ciliary dyskinesia. Am J Respir Crit Care Med. 1999;160:144–149. doi: 10.1164/ajrccm.160.1.9806146. [DOI] [PubMed] [Google Scholar]
- 25.Brown DE, Pittman JE, Leigh MW, Fordham L, Davis SD. Early lung disease in young children with primary ciliary dyskinesia. Pediatr Pulmonol. 2008;43:514–516. doi: 10.1002/ppul.20792. [DOI] [PubMed] [Google Scholar]
- 26.Bhutta ZA. Primary ciliary dyskinesia: a cause of neonatal respiratory distress. J Pak Med Assoc. 1995;45:70–73. [PubMed] [Google Scholar]
- 27.Holzmann D, Felix H. Neonatal respiratory distress syndrome--a sign of primary ciliary dyskinesia? Eur J Pediatr. 2000;159:857–860. doi: 10.1007/pl00008354. [DOI] [PubMed] [Google Scholar]
- 28.Kartagener M, Stucki P. Bronchiectasis with situs inversus. Arch Pediatr. 1962;79:193–207. [PubMed] [Google Scholar]
- 29.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [Published erratum appears in Cell 1999;99:117.] [DOI] [PubMed] [Google Scholar]
- 30.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left–right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Schidlow DV, Katz SM, Turtz MG, Donner RM, Capasso S. Polysplenia and Kartagener syndromes in a sibship: association with abnormal respiratory cilia. J Pediatr. 1982;100:401–403. doi: 10.1016/s0022-3476(82)80439-3. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, Robinson BV, Minnix SL, Olbrich H, Severin T, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007;115:2814–2821. doi: 10.1161/CIRCULATIONAHA.106.649038. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro AJ, Davis SD, Ferkol T, Dell SD, Rosenfeld M, Olivier KN, Sagel SD, Milla C, Zariwala MA, Wolf W, et al. Laterality defects other than situs inversus totalis in primary ciliary dyskinesia: insights into situs ambiguus and heterotaxy. Chest. 2014;146:1176–1186. doi: 10.1378/chest.13-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]