Abstract
Rationale: Patients with autoimmune lymphoproliferative syndrome (ALPS), a disorder of impaired lymphocyte apoptosis, often undergo radiographic chest imaging to evaluate the presence and progression of lymphadenopathy. These images often lead to parenchymal and interstitial lung findings of unclear clinical significance.
Objectives: To characterize the pulmonary findings associated with ALPS and to determine if lung abnormalities present on computed tomographic (CT) imaging of the chest correlate with infection or functional status.
Methods: Patients with lung abnormalities observed on chest CT scans were retrospectively identified from the largest known ALPS cohort. Lung computed tomography findings were characterized and correlated with medical records, bronchoalveolar lavage, biopsy, and lung function.
Measurements and Main Results: CT images of the chest were available for 234 (92%) of 255 of the patients with ALPS. Among patients with a chest CT scan, 18 (8%) had lung abnormalities on at least one CT scan. Fourteen (78%) of those 18 were classified as having ALPS with undetermined genetic defect. Most patients (n = 16 [89%]) with lung lesions were asymptomatic. However, two (11%) of them had associated dyspnea and/or desaturation on room air. Immunosuppressive treatment was administered for lung disease in nine (50%) cases, and all were followed for clinical outcomes.
Conclusions: Patients with ALPS can develop chest radiographic findings with protean manifestations that may mimic pulmonary infection. Management of patients with ALPS with incidental lung lesions identified by CT imaging should be guided by clinical correlation. Symptomatic patients may benefit from chest CT imaging and lesion biopsy to exclude infection and guide administration of immunosuppressive therapy.
Keywords: pulmonary function, imaging, natural history
The autoimmune lymphoproliferative syndrome (ALPS) is a disorder of impaired lymphocyte apoptosis associated with defects in the CD95 signaling cascade (1, 2). Lymphadenopathy, splenomegaly, and autoimmune cytopenias are typical in the clinical presentation of ALPS and are caused by unregulated lymphocyte proliferation. Lymphoproliferation in ALPS is not directly related to the development of infection or malignancy, though patients are at increased risk for B-cell lymphomas (3, 4). ALPS typically presents in early childhood, and patients may experience fatigue, pallor, icterus associated with hemolytic anemia, or bruising and hemorrhages due to thrombocytopenia. Lung manifestations of ALPS have not previously been well described.
Criteria for the diagnosis of ALPS were created by consensus in 1999 and revised in 2010 by a group of international investigators. The diagnostic criteria are summarized in Table 1 (2). The immunophenotypical hallmark of ALPS is the expansion of “double-negative” T-cell receptor αβ+ T lymphocytes that lack both CD4 and CD8 markers such that they compose more than 1.5% of all circulating lymphocytes. The exact pathophysiological role for double-negative T-cell receptor αβ+ T lymphocytes in the development of ALPS is unknown, but because their presence is seldom seen in other hematological disorders, they are thought to be pathognomonic for ALPS (5).
Table 1.
2009 Revised diagnostic criteria for autoimmune lymphoproliferative syndrome
| A diagnosis of ALPS is based on the presence of both required criteria and at least one primary accessory criterion. A probable case of ALPS is suspected in the presence of both required criteria and one secondary accessory criterion. |
|---|
| Required criteria |
| 1. More than 6 mo of nonmalignant, noninfectious lymphadenopathy and/or splenomegaly |
| 2. Elevated CD3+TCRαβ+CD4−CD8− double-negative cells (≥1.5% of total lymphocytes or 2.5% of CD3+ lymphocytes) |
| Accessory criteria |
| Primary |
| 1. Evidence of defective lymphocyte apoptosis |
| 2. Somatic or germline pathogenic mutation in FAS, FASLG, or CASP10 genes |
| Secondary |
| 1. sFASL levels >200 pg/ml or |
| a. Plasma IL-10 levels >20 pg/ml or |
| b. Vitamin B12 levels >1,500 ng/L or |
| c. Plasma IL-18 levels >500 pg/ml |
| 2. Typical immunohistological findings as reviewed by an experienced hematopathologist |
| 3. Autoimmune cytopenias in the presence of elevated IgG levels |
| 4. Family history of nonmalignant and/or noninfectious lymphoproliferation with or without accompanying autoimmunity |
Definition of abbreviations: ALPS = autoimmune lymphoproliferative syndrome; sFASL = soluble FAS ligand; TCR = T-cell receptor.
Adapted by permission from Reference 31.
Genetic diagnostic techniques have allowed for ALPS to be divided into multiple subclassifications based on the identification of specific defects in the apoptotic pathway. ALPS-FAS involves either homozygous or heterozygous germline mutations in the FAS gene; ALPS-sFAS involves somatic mutations to FAS; ALPS-FASLG involves mutations to the FAS ligand; and ALPS-CASP10 involves germline mutations to the gene encoding for caspase 10. Autoimmune lymphoproliferative syndrome with undetermined genetic defect (ALPS-U) is a term used to categorize patients who fulfill the diagnostic criteria for ALPS but have an as yet undetermined genetic mutation. Mutations in the FAS gene associated with defects in lymphocyte apoptosis are the most commonly recognized causes of ALPS, and most published experience related to ALPS stems from the natural history studies of these patients (4). While ALPS is rare, its true prevalence is unknown because many cases are likely undiagnosed due to variable phenotypic expression (6).
Despite the extensive underlying lymphoproliferative abnormalities and perturbations of the immune system, the clinical course of ALPS is typically relatively benign. With the exception of patients who undergo splenectomy, serious infectious consequences of ALPS-associated immunosuppression are surprisingly uncommon (4, 7, 8). A significant number of patients with ALPS do not require intervention for the asymptomatic lymphadenopathy and splenomegaly seen in this population. In fact, both of these conditions often appear to improve with age (9). Despite this, patients with ALPS are regularly followed for evidence of disease progression (including development of lymphoma) and often undergo serial radiography. During such routine monitoring, abnormal radiographic results with unclear clinical significance can be encountered.
In this article, we report lung manifestations based on routine and clinically indicated computed tomographic (CT) scans of the chest in the largest known cohort of patients with ALPS. We also report the histopathological findings associated with CT scan findings and the associated pulmonary signs, symptoms, and clinical courses. Most of the patients with ALPS with lung lesions described here do not have a FAS mutation to account for their symptoms.
Methods
Subjects
Patients with chest CT scan abnormalities were identified from a database of patients with ALPS enrolled in a natural history protocol approved by an institutional review board of the National Institutes of Health (www.clinicaltrials.gov identifier NCT00001350). The ALPS cohort at the National Institutes of Health is the largest collection of patients assembled for study of this disease. Of 839 patients followed in the cohort, 584 are either healthy relatives of patients with confirmed ALPS or have an unclassified disease with ALPS-like features.
Patients in the ALPS cohort were followed at regular intervals (one or two visits yearly and as needed for evaluation of clinical change) that included clinical and laboratory evaluations. In addition, CT scans of the neck, chest, abdomen, and pelvis were obtained approximately every 2 years to monitor for the development of splenomegaly, mediastinal lymphadenopathy, and evidence of lymphoma. CT scans were also obtained for clinical indication at the discretion of care providers. CT examinations were acquired with the following scanners, based on availability: SOMATOM Definition Flash (Siemens Healthcare, Malvern, PA), Brilliance 16 (Philips Healthcare, Bothell, WA), Aquilion One (Toshiba America Medical Systems, Tustin, CA), Siemens Definition (Siemens Healthcare USA, Malvern, PA), or LightSpeed (GE Healthcare, Chicago, IL). We conducted a retrospective review of the data available from January 1, 1993, to December 31, 2013, of the 234 patients with clinical data and a confirmed diagnosis of ALPS.
Data Extraction
Demographic and clinical information were collected by chart review. Clinical information, including documentation of bronchoalveolar lavage (BAL), lung biopsy, culture (blood, sputum, and/or lavage) and sensitivity, respiratory symptoms, 6-minute walk tests (6MWTs), and pulmonary function tests (PFTs) performed for clinical indications, was reviewed. Lung function was assessed and categorized as obstructive, restrictive, mixed, or no significant abnormality according to the American Thoracic Society/European Respiratory Society recommendations (10, 11). Respiratory manifestations were captured and categorized according to chart documentation. Patient management was ascertained as immunosuppression, antibiotics, or observation. Outcome was assessed as improved, unchanged, or worsened. Findings were retrospectively reviewed in multidisciplinary sessions with radiology, pathology, pulmonology, and infectious disease physicians.
Chest Imaging
Abnormal CT images from patients with ALPS were identified for further study. A radiologist (L.R.F.) reviewed all abnormal scans in a random fashion and rated each scan with a subjective severity scale of 0–3 (0 = not present, 1 = mild, 2 = moderate, 3 = severe) (12) for the following characteristics: ground-glass opacities, tree-in-bud nodules, larger nodules (3–10 mm and not in tree-in-bud distribution), consolidation, septal and nonseptal lines, cavitation, cysts, bronchiectasis, and overall disease. The radiologist was blinded to identifying information, including patient information and scan date. Additionally, five randomly selected scans were inserted into the reviewer’s queue and repeated for internal validation.
Statistical Analysis
Baseline characteristics between subjects with ALPS with pulmonary abnormalities and those without pulmonary abnormalities on CT scans were compared using Fisher’s exact test (for discrete variables) and Wilcoxon’s rank-sum test (for continuous variables). Among the subjects with pulmonary abnormalities, results of baseline PFTs and computed tomography were summarized. Spearman’s correlations were estimated to evaluate the relationship between chest CT scan patterns and the percent predicted diffusing capacity of the lung for carbon monoxide (DlCO) corrected for Hb. A Bonferroni correction was applied to account for multiplicity.
To explore the clinical course, the proportion of patients managed with antibiotics, immunosuppression, or observation, as well as the proportion of patients whose clinical status improved, did not change, or worsened, was determined. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.1.2 software.
Results
Clinical data, including CT images of the chest, were available for 234 (92%) of 255 of the patients with ALPS. Approximately 8% (n = 18) of patients in our cohort had at least one chest CT examination with abnormal lung findings that necessitated further clinical evaluation. The time from onset of ALPS-defining symptoms to first abnormal chest CT examination ranged from 1 to 37 years, with an average of 12.7 years.
Characteristics of Patients with Abnormal Chest Imaging
Table 2 shows the characteristics of each of the 18 patients with ALPS with abnormal chest computed tomography findings. Four were female, and 14 were male. Fourteen carried a diagnosis of ALPS-U, three patients had ALPS-FAS, and one had ALPS-FASLG. Notably, 78% of patients with lung lesions had ALPS-U, compared with only 14% of patients without lung lesions (Table 3). Sixteen of the 18 patients with lung lesions were asymptomatic; 2 (11%) had associated dyspnea and/or desaturation on room air. All 18 were nonsmokers.
Table 2.
Patient characteristics
| Patient | Sex | ALPS Subtype | Age at First Abnormal CT (yr) | DlCO Corrected for Hb* (% predicted) | Lung Physiology | Lung CT Patterns | Pathology Findings | Presenting Symptoms | Treatment for Pulmonary Disease | Outcome (yr of follow-up) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | ALPS-U | 21 | 96 | Obstructive† | Bronchiectasis, cyst, nodules not TIB, septal lines | NA | Asymptomatic | Continued on same dose of MMF | No change (18) |
| 2 | M | ALPS-U | 14 | 59 | No significant abnormality | Bronchiectasis, consolidation, GGO, nodules not TIB, septal lines, TIB | Interstitial lymphoplasmacytic infiltrate‡ (TTB) | Asymptomatic | None | No change (12) |
| 3 | M | ALPS-U | 11 | 58 | Restrictive | Bronchiectasis, GGO, nodules not TIB, septal lines, TIB | NA | Asymptomatic | None | No change (27) |
| 4 | M | ALPS-U | 17 | 68 | Restrictive | Bronchiectasis, cyst, GGO, nodules not TIB, septal lines, TIB | Patchy lymphocytic infiltrate‡ (TBB, BAL) | Asymptomatic | Continued on same dose of MMF, given pulse dose steroids | CT-based improvement (27) |
| 5 | F | ALPS-U | 10 | 61 | Restrictive | Bronchiectasis, consolidation, GGO, nodules not TIB, septal lines, TIB | Organizing pneumonia with BOOP-like changes‡ (TBB) | Asymptomatic | Prednisone, azathioprine, MMF | Persistent diffusion defect, 6MWT and CT-based improvement (6) |
| 6 | M | ALPS-U | 15 | 57 | Restrictive† | Bronchiectasis, cavitation, consolidation, GGO, nodules not TIB, septal lines, TIB | Follicular bronchiolitis‡ (TBB) | Asymptomatic | Continued on same dose of MMF and methotrexate, given pulse dose steroids | No change in symptoms, progressive decline in DlCO (24) |
| 7 | F | ALPS-U | 7 | NA | NA | GGO, TIB, nodules not TIB | Follicular bronchiolitis, pneumonitis characterized by inflammation and lymphocytes in bronchioles, BOOP-like changes, EBER-negative‡ (TTB) | Asymptomatic | None | Pulmonary outcome unknown; patient deceased (4) |
| 8 | F | ALPS-FAS | 39 | 72 | No significant abnormality | Bronchiectasis, consolidation, cyst, GGO, nodules not TIB, septal lines, TIB | Granulomatous and chronic inflammation (TTB) | Asymptomatic | None | No change (53) |
| 9 | M | ALPS-FAS | 36 | NA | NA | Cyst, GGO | NA | Asymptomatic | None | No change (47) |
| 10 | M | ALPS-U | 14 | 70 | No significant abnormality | GGO, TIB, nodules not TIB, bronchiectasis | NA | Asymptomatic | Continued on same dose of MMF | No change (16) |
| 11 | M | ALPS-U/STAT3 GOF mutation | 22 | 50 | Restrictive | Bronchiectasis, consolidation, GGO, nodules not TIB, septal lines, TIB | Dense fibrous scar tissue, minimal chronic inflammation (TTB) | Asymptomatic | Continued on same dose of MMF | No change in symptoms, DlCO improved (21) |
| 12 | M | ALPS-U | 14 | 62 | No significant abnormality | GGO, TIB, nodules not TIB, bronchiectasis | NA | Asymptomatic | Continued on same dose of sirolimus | No change in symptoms, CT-based improvement (3) |
| 13 | M | ALPS-U | 30 | 60 | No significant abnormality | Bronchiectasis, consolidation, GGO, nodules not TIB, septal lines, TIB | Mild chronic inflammation of bronchial wall, mild chronic interstitial pneumonitis (TBB) | Asymptomatic | None | No change (8) |
| 14 | F | ALPS-U | 12 | 103 | Obstructive | Bronchiectasis, consolidation, GGO, nodules not TIB, septal lines, TIB | Patchy lymphocytic infiltrate, no evidence of double-negative T cells‡ (TBB, BAL) | DOE | MMF increased | DlCO improved (15) |
| 15 | M | ALPS-U | 6 | NA | NA | GGO, TIB, septal lines | Focally necrotizing granulomatous pneumonitis, interstitial lymphocytic infiltrate, small necrotizing granulomas (TBB) | Asymptomatic | None | NA (4) |
| 16 | M | ALPS-FAS | 22 | 77 | No significant abnormality | Nodules not TIB | NA | Asymptomatic | None | NA (22) |
| 17 | M | ALPS-U | 13 | 41 | Restrictive† | GGO, nodules not TIB, septal lines, TIB | Patchy T-cell–predominant lymphocytic infiltrate‡ (TBB, BAL) | Dyspnea, desaturation on room air | MMF, prednisone | DlCO and symptoms wax and wane (14) |
| 18 | M | ALPS-FASLG | 16 | 64 | No significant abnormality | Bronchiectasis, GGO, nodules not TIB, septal lines, TIB | Nondiagnostic‡ (TBB, BAL) | Asymptomatic | None | No change in symptoms, progressive decline in DlCO (24) |
Definition of abbreviations: ALPS-U = autoimmune lymphoproliferative syndrome with undetermined genetic defect; BAL = bronchoalveolar lavage; BOOP = bronchiolitis obliterans organizing pneumonia; CT = computed tomographic scan; DlCO = diffusing capacity of the lung for carbon monoxide; DOE = dyspnea on exertion; EBER = Epstein-Barr virus–encoded small RNA; GGO = ground-glass opacity; GOF = gain of function; MMF = mycophenolate mofetil; 6MWT = 6-minute walk test; NA = not available; STAT3 = signal transducer and activator of transcription 3; TBB = transbronchial biopsy; TIB = tree-in-bud pattern; TTB = transthoracic biopsy.
One patient was reclassified from ALPS-U to STAT3 gain-of-function mutation.
First DlCO measurement was obtained after CT abnormalities were detected.
Exertional dyspnea greater than 3% in 6-minute walk test.
Pathology reviewed at the National Institutes of Health.
Table 3.
Demographics of patients with confirmed autoimmune lymphoproliferative syndrome
| Patient Characteristics | All Patients with ALPS (n = 234) | Patients with ALPS without Lung Lesions (n = 216) | Patients with ALPS with Lung Lesions (n = 18) | P Value |
|---|---|---|---|---|
| Sex |
0.3069 | |||
| Male | 151 (64.5%) | 137 (63.4%) | 14 (77.8%) | |
| Female | 83 (35.5%) | 79 (36.6%) | 4 (22.2%) | |
| Median age at presentation, yr (range) | 14 (1–63) | 13 (1–63) | 14 (6–39) | |
| ALPS subclass |
0.0000 | |||
| ALPS-U | 45 (19.2%) | 31 (14.4%) | 14 (77.8%) | |
| ALPS-FAS | 163 (69.6%) | 160 (74.1%) | 3 (16.7%) | |
| ALPS-FASLG | 1 (0.4%) | 0 (0.0%) | 1 (5.6%) | |
| ALPS-sFAS | 19 (8.1%) | 19 (8.8%) | 0 (0.0%) | |
| ALPS-CASP10 | 6 (2.6%) | 6 (2.8%) | 0 (0.0%) | |
Definition of abbreviations: ALPS = autoimmune lymphoproliferative syndrome; ALPS-CASP10 = ALPS with mutations in caspase 10; ALPS-FAS = ALPS with homozygous or heterozygous mutations in FAS; ALPS-FASLG = ALPS with mutations in the FAS ligand; ALPS-sFAS = ALPS with somatic mutations in FAS; ALPS-U = autoimmune lymphoproliferative syndrome with undetermined genetic defect.
Data are presented as count (percent) or median (range).
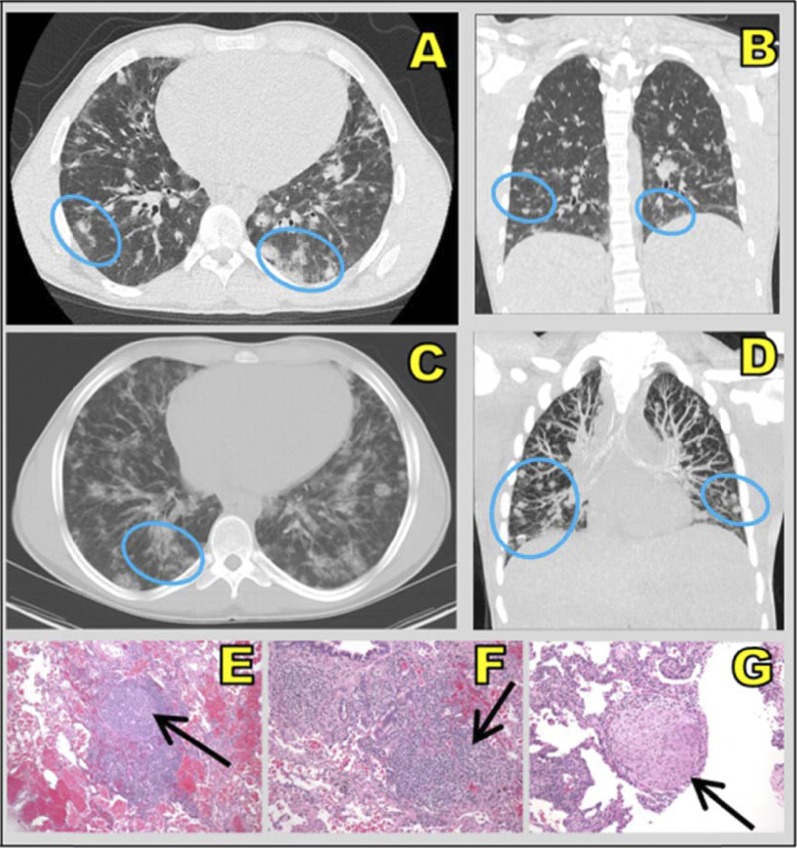
Of the 18 patients with radiographic lung lesions, 12 patients had pathology results from pulmonary specimens. Specimens were collected when (1) DlCO was low, (2) there was concern about progressive pulmonary deterioration, or (3) there was concern about infection in the setting of immunosuppressive administration. Eight patients contributed multiple biopsy and/or BAL specimens. Histopathology revealed five patients with lymphocytic infiltrates, three with pneumonitis, three with chronic inflammation, two with organizing pneumonia, two with follicular bronchiolitis, and two with granulomatous changes. Multiple findings were possible for each biopsy. An example of corresponding chest computed tomography and histopathology findings is shown in Figure 1.
Figure 1.
(A and C) Axial and (B and D) coronal computed tomographic scans show ill-defined, diffuse ground-glass nodules (blue ovals), which are common in fungal infection, in the lower lobes. Right lung biopsy (hematoxylin and eosin stain; original magnification, ×10) shows (E) a nodular lymphoid infiltrate with a prominent “naked” reactive germinal center, (F) a peribronchial lymphocytic infiltrate, and (G) a nonnecrotizing granuloma composed of epithelioid and rare multinucleated giant cells (black arrows) associated with interstitial lymphocytic infiltrate. This patient (patient 6 from Table 2) denied pulmonary symptoms and is an active hockey player.
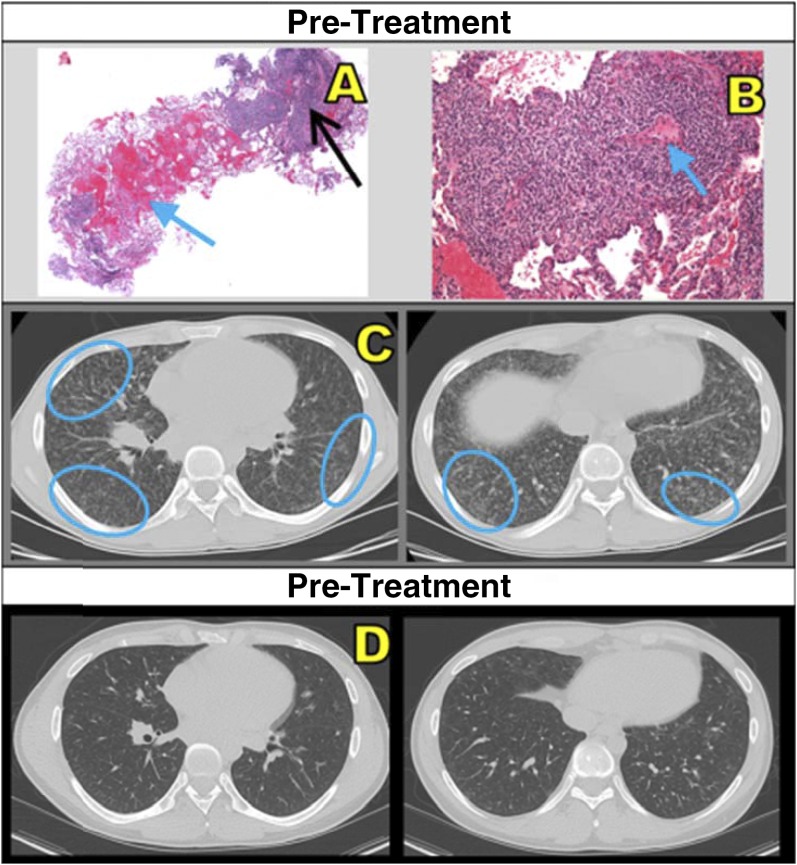
None of the patients demonstrated concurrent infection on initial workup or during follow-up. Evaluations included bacterial, fungal, and mycobacterial cultures; viral polymerase chain reaction of BAL fluid; and cytopathology. None of the patients were treated with antibiotics. Immunosuppressive medications, including corticosteroids, mycophenolate mofetil, and azathioprine, were safely used to manage nine (50%) of the patients during the study period. An example of pre- and post-treatment findings is shown in Figure 2. Improvement in computed tomography findings and symptoms was not always permanent; waxing and waning of lesions was observed.
Figure 2.
(A) Core lung biopsy (hematoxylin and eosin stain; original magnification, ×2) shows intraalveolar hemorrhage (blue arrow) and patchy peribronchial/perivascular lymphocytic infiltrate (black arrow). (B) Dense lymphocytic infiltrate and interstitial widening, sparing blood vessels (blue arrow) (original magnification, ×10). (C) Pretreatment computed tomographic scans show miliary-like, small, ill-defined, diffuse micronodular ground-glass nodules (blue ovals) in right middle and bilateral lower lobes. (D) This patient (patient 4 from Table 2) showed interval improvement after treatment with mycophenolate mofetil and high-dose methylprednisolone.
Fifteen of the 18 patients had PFTs ordered for clinical indications. PFTs were not consistently coordinated with CT scanning; seven patients had their PFTs performed on the same day as a CT scan, whereas six patients had their testing performed more than 1 year after a chest CT scan. All 15 patients who underwent testing had available spirometry, and 14 had lung volumes; 6 showed a restrictive pattern, 2 had obstructive patterns, and 7 had no significant abnormality. Of the 15 patients with DlCO, 12 (67%) had abnormal values. Eleven patients contributed 6MWT data, and four of those manifested significant oxyhemoglobin desaturation (>3% decline) with exertion (13). Baseline pulmonary function and 6MWT data are summarized in Table 4. Three asymptomatic patients had no PFTs done.
Table 4.
Summary of baseline pulmonary function test results of patients with lung lesions and autoimmune lymphoproliferative syndrome
| Number of Patients Tested | Mean Values | 95% CI | |
|---|---|---|---|
| FEV1, L/s | 15 | 2.94 | (2.17–3.71) |
| Percent predicted | 84.87 | (74.77–94.96) | |
| FVC, L/s | 15 | 3.51 | (2.52–4.50) |
| Percent predicted | 86.73 | (75.69–97.77) | |
| FEV1/FVC ratio | 15 | 0.86 | (0.81–0.91) |
| Total lung capacity, L | 14 | 4.96 | (3.72–6.19) |
| Percent predicted | 89.14 | (82.61–95.68) | |
| Residual volume, L | 14 | 1.29 | (0.91–1.67) |
| Percent predicted | 102.07 | (79.79–124.35) | |
| DlCO-corrected Hb | 15 | 23.06 | (18.01–28.12) |
| Percent predicted | 69.77 | (60.32–79.22) | |
| 6-minute walk distance, m | 11 | 539.82 | (486.32–593.32) |
| Prewalk saturation, % | 11 | 98 | (96.35–99.65) |
| Postwalk saturation, % | 11 | 95 | (90.67–99.33) |
Definition of abbreviations: CI = confidence interval; DlCO = diffusing capacity of the lung for carbon monoxide.
Chest CT Image Findings
A total of 60 chest CT scans were obtained on the 18 patients with interstitial lung lesions. The following patterns were seen in these patients: ground-glass opacities (n = 16), larger nodules (n = 16), tree-in-bud nodules (n = 15), bronchiectasis (n = 14), septal thickening (n = 13), consolidation (n = 7), cysts (n = 4), and cavitation (n = 1). The average overall severity score was 1.65 (range, 0–3). Data summarizing the presence or absence of each pattern, along with distribution of severity scores, are shown in Table 5. Most scans demonstrated multiple patterns. When all patterns were considered, six patients had a maximum severity of 1, six had a maximum severity of 2, and six had a maximum severity of 3. Repeat scoring of five randomly selected scans revealed acceptable internal consistency: 64% (29 of 45) of scored features remained unchanged, with the severity of the remaining 36% (16 of 45) increasing or decreasing by only 1 unit.
Table 5.
Severity scores for chest computed tomography findings
| Pattern | Presence on Any Scan (n = 18 patients) | Highest Severity Score (n = 18 patients) |
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Bronchiectasis | 14 (77.8) | 4 (22.2) | 11 (61.1) | 3 (16.7) | 0 (0.0) | |
| Cavitation | 1 (5.6) | 17 (94.4) | 1 (5.6) | 0 (0.0) | 0 (0.0) | |
| Consolidation | 7 (38.9) | 11 (61.1) | 2 (11.1) | 4 (22.2) | 1 (5.6) | |
| Cyst | 4 (22.2) | 14 (77.8) | 3 (16.7) | 1 (5.6) | 0 (0.0) | |
| Ground-glass opacity | 16 (88.9) | 2 (11.1) | 7 (38.9) | 7 (38.9) | 2 (11.1) | |
| Larger nodules | 16 (88.9) | 2 (11.1) | 7 (38.9) | 4 (22.2) | 5 (27.8) | |
| Septal thickening | 13 (72.2) | 5 (27.8) | 9 (50.0) | 3 (16.7) | 1 (5.6) | |
| Tree-in-bud nodules | 15 (83.3) | 3 (16.7) | 8 (44.4) | 6 (33.3) | 1 (5.6) | |
Sixty scans were available for the 18 patients with lung lesions; each patient could have multiple scans. The first column shows how many patients manifested a particular pattern on any one of their computed tomographic scans. The severity score columns show the highest severity score for each pattern for each patient across all of their scans. Severity was rated on a scale of 0–3 for each pattern on each chest computed tomographic scan. If all scans for a patient had a severity of 0 for a particular pattern, that pattern was considered absent. If any scan had had a nonzero score for a pattern, that pattern was considered present.
Among patients with lung lesions, no correlation was found between the degree of abnormality of lesion characteristics visualized by chest computed tomography and pulmonary function status. Spearman’s correlations were all nonsignificant.
Discussion
In this retrospective evaluation of a large cohort of patients with ALPS, we characterized the pulmonary manifestations in patients with ALPS with chest CT scan abnormalities. Most patients had no pulmonary symptoms. Those with evidence of symptomatic progression improved while on immunosuppressive therapy. Ground-glass opacities and larger nodules were the most common computed tomography findings, followed by tree-in-bud nodules, then bronchiectasis and septal thickening. Despite presenting with computed tomography findings that can be suggestive of infection, none of the 18 patients with ALPS with chest computed tomography changes had clinical evidence including systemic symptoms to support active infection, even while receiving immunosuppressive medications. Our data show that the disease course of ALPS is variable. Observed lymphoid infiltrates are likely a nonspecific host response and reflect lymphoproliferation due to an overactive immune system.
Our findings do not necessarily mirror what has previously been reported in ALPS, as previous descriptions have been focused on ALPS-FAS (4). The ALPS-U subtype showed a disproportionate predominance among those with lung lesions. It is likely that ALPS-U includes as yet unidentified genetic diseases associated with lung lesions. To this point, one patient classified as ALPS-U during this study period was subsequently found to have a signal transducer and activator of transcription 3 (STAT3) gain-of-function (GOF) mutation. The patients in the recently described early-onset lymphoproliferation and autoimmunity associated with STAT3 GOF mutations had a predominance of autoimmune cytopenias, lymphadenopathy, and hepatosplenomegaly similar to patients with ALPS, but also with striking liver disease with nodular regenerative hyperplasia and arthritis. Four of the 13 patients had lung manifestations ranging from nodules to lymphocytic interstitial pneumonitis. However, there was also a predominance of severe or recurring pulmonary and extrapulmonary infections not seen in the patients we describe here (14).
Other recently described ALPS-like disorders of immune regulation associated with lung manifestations result from mutations in the phosphatidylinositol-3-OH kinase (PIK3CD), cytotoxic T-lymphocyte antigen 4 (CTLA4), and LPS-responsive and beige-like anchor (LRBA) genes. GOF mutations in PIK3CD encoding for PI3K110δ or in-frame deletions of PIK3R1 leading to GOF in PI3K110δ were manifested clinically by recurring sinopulmonary infections, lymphadenopathy, cytopenias, cytomegalovirus and/or Epstein-Barr viremia, and predisposition to lymphoma (15–17). Nodular lymphoid hyperplasia of mucosal surfaces including the airways, bronchiectasis, atelectasis, and consolidation related to airway narrowing and recurring infection are also seen in this disease. In a lesser number of patients, alveolitis features and lymphocytic infiltrates have been recognized.
Patients with CTLA4 deficiency have presented with autoimmune cytopenias; organomegaly; hypogammaglobulinemia; endocrinopathies; and lymphocytic infiltration of the gut, brain, and lungs. Recurring respiratory tract infections and granulomatous lymphocytic interstitial lung disease have been reported in a majority of patients, while bronchiectasis has been less common (18, 19). Finally, patients with LRBA deficiency, which leads to defective and diminished CTLA-4 expression, typically have evidence of immune dysregulation, organomegaly, and hypogammaglobulinemia, with about one-half presenting with lung involvement. The latter included persistent lymphocytic infiltrates, bronchiectasis, and granulomatous inflammation; about one-third were diagnosed with granulomatous lymphocytic interstitial lung disease. Recurring infections were seen in 15 (71%) of 22 patients, including 10 with upper and 9 with lower respiratory tract infections (20).
Among patients with ALPS with lung lesions, computed tomography–based lesion classification did not correlate with functional status. Decisions as to whether it is appropriate to observe a patient, administer antibiotics, or administer immunosuppression must take into consideration the patient’s other ALPS manifestations, such as cytopenias, clinical status, and progression of symptoms or lung function abnormalities. In many cases, treatment administered for other disease aspects (e.g., pulse doses of corticosteroids and mycophenolate mofetil for cytopenias) resulted in improvement of radiographic abnormalities.
Imaging can influence management needed to address pulmonary symptoms in addition to describing the extent or progression of lung lesions over time (21); computed tomography findings in combination with pathology results were essential in ruling out infection and thus in guiding administration of immunosuppressive therapy in our cohort. However, the need for treatment (pulse dose prednisone followed by steroid-sparing measures) was ultimately determined by clinical status and symptoms. While all patients with suspected lymphoproliferative disorders of uncertain etiology should undergo baseline imaging to determine distribution and extent of lung involvement, routine and repeated chest CT scans are unlikely to affect management in the absence of signs or symptoms.
Radiographic findings seen in patients with ALPS are also seen in other disorders of the immune system. Common variable immune deficiency (CVID), also characterized by lymphoproliferation and autoimmune cytopenias, can be associated with chronic pulmonary disease (22–24). Patients with CVID may have similar, asymptomatic chest radiographic findings. In their study of adult patients with CVID, Maarschalk-Ellerbroek and colleagues found that almost all patients screened with chest computed tomography displayed previously unknown evidence of pulmonary abnormalities (25). Although those authors found that a diverse number of abnormal chest computed tomography findings correlated with specific T- and B-cell profiles, the computed tomography findings did not correlate with PFT results. van de Ven and colleagues also reported that abnormal immune function may contribute to development of radiographic changes in the pediatric CVID population. In that study, patients with CVID with a history of chronic structural airway disease and radiographic evidence of interstitial and/or parenchymal lung disease had distinct immunological profiles despite having no differences in PFT results (26). While an aberrant immune response may provide a plausible explanation for CT scan abnormalities in patients with CVID (27), a similar rationale could also be applied to explain the presence of chest computed tomography changes in patients with ALPS.
Some patients with ALPS can have varying degrees of hypogammaglobulinemia similar to those of CVID patients or following exposure to rituximab, an anti-CD20 monoclonal antibody (28). Five of our 18 patients with ALPS and lung lesions had IgG levels lower than 5 g/L, a required criterion for CVID, at one or more time points. While such IgG levels are consistent with a possible diagnosis of CVID (29), evaluation of complete CVID criteria was not possible, owing to data limitations. It should also be noted that 16 of our 18 patients received intravenous immunoglobulin and 6 received rituximab during the study period. Prospective collection of such data may provide insight to the link between ALPS and CVID or point toward one of the recently described genetically defined immune regulation disorders. Research suggesting that patients with CVID and patients with ALPS may share characteristic B-cell abnormalities provides further evidence that CVID and ALPS may have immunological overlap and may represent different aspects of a similar pathological entity (30).
Limitations
This study was limited intrinsically by the small sample size of the ALPS population with CT scans. Although our institution has the largest ALPS cohort, ALPS remains an orphan disease. Furthermore, this was a retrospective rather than a prospective study. A priori standardization of CT scan frequency and technique, a standardized procedure to rule out suspected infections, and blinded CT scan interpretation criteria could produce more robust results. Evolving ALPS disease classification also engenders uncertainties; with recognition of new ALPS-related immune disorder groups as additional genetic data becomes available, some patients may be reclassified and thus impact the correlations.
Last, PFT data were not available for all patients with ALPS, because routine PFTs were not part of the ALPS natural history cohort protocol; PFTs were collected per clinical indication, and thus their presence in the database is correlated with symptoms. For instance, 9 of the 12 patients with follow-up DlCO values manifested a subsequent decrease. It may have been that these patients had a clinical decline that precipitated DlCO evaluation or that the change reflected progression of their lung pathology related to ALPS. Thus, PFT comparisons cannot be made between patients with and without lung lesions or between patients with and without symptoms, owing to inherent bias. Standardized prospective collection of PFT data in all patients would have facilitated this evaluation.
Conclusions
Patients with ALPS can develop protean chest radiographic findings, which may overlap with those seen in pulmonary infection. Lung lesions are likely a manifestation of the overall aberrant lymphoproliferation rather than of specific pulmonary disease. Most patients with radiographic abnormalities were classified as ALPS-U, were asymptomatic (89%), and had decreased diffusing capacity. The possibility of other genetically defined immune dysregulation disorders with similar lung manifestations should be considered. Management of patients with ALPS with incidental lung lesions identified by computed tomography should be guided by clinical status. Symptomatic patients or those with declining lung function may benefit from chest computed tomography and lesion biopsy to rule out infection and guide administration of immunosuppressive therapy.
Acknowledgments
Acknowledgment
This work is supported by the intramural research programs at the National Institute of Allergy and Infectious Diseases; the National Heart, Lung, and Blood Institute; and the National Institutes of Health Clinical Center. The authors are gratefully indebted to the patients and the families of patients who have participated in the ALPS Natural History Study. The authors also thank Fred Gill, Helen Su, Joie Davis, and Gulbu Uzel for their valuable contributions to this research.
Footnotes
This research was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases; the National Heart, Lung, and Blood Institute; and the National Institutes of Health Clinical Center. This project was funded in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E (K.P.). The content of this publication does not necessarily reflect the views or policies of the National Institutes of Health, the Department of Health and Human Services, or the U.S. government.
Author Contributions: C.-Y.L. and A.D.M.: had full access to the study data and take responsibility for the integrity of the data and analysis; J.W. and L.E.D.: contributed to the analysis; K.P., S. Price, and S.W.: participated in data collection; S. Pittaluga: provided pathological analysis; L.R.F.: evaluated the computed tomographic scans. V.K.R. and K.N.O.: managed the patients and served as lead investigators; and C.-Y.L., A.D.M., J.W., L.E.D., L.R.F., V.K.R., and K.N.O.: contributed to the writing of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bidère N, Su HC, Lenardo MJ. Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol. 2006;24:321–352. doi: 10.1146/annurev.immunol.24.021605.090513. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, Rieux-Laucat F, Siegel RM, Su HC, Teachey DT, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–e40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rösen-Wolff A, Peters AM, Sneller MC, Hallahan CW, Wang J, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 4.Price S, Shaw PA, Seitz A, Joshi G, Davis J, Niemela JE, Perkins K, Hornung RL, Folio L, Rosenberg PS, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123:1989–1999. doi: 10.1182/blood-2013-10-535393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell TB, Kurre P. Double-negative T cells are non-ALPS-specific markers of immune dysregulation found in patients with aplastic anemia. Blood. 2010;116:5072–5073. doi: 10.1182/blood-2010-09-306910. [DOI] [PubMed] [Google Scholar]
- 6.Turbyville JC, Rao VK. The autoimmune lymphoproliferative syndrome: a rare disorder providing clues about normal tolerance. Autoimmun Rev. 2010;9:488–493. doi: 10.1016/j.autrev.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Rao VK. Approaches to managing autoimmune cytopenias in novel immunological disorders with genetic underpinnings like autoimmune lymphoproliferative syndrome. Front Pediatr. 2015;3:65. doi: 10.3389/fped.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neven B, Bruneau J, Stolzenberg MC, Meyts I, Magerus-Chatinet A, Moens L, Lanzarotti N, Weller S, Amiranoff D, Florkin B, et al. Defective anti-polysaccharide response and splenic marginal zone disorganization in ALPS patients. Blood. 2014;124:1597–1609. doi: 10.1182/blood-2014-02-553834. [DOI] [PubMed] [Google Scholar]
- 9.Rao VK, Oliveira JB. How I treat autoimmune lymphoproliferative syndrome. Blood. 2011;118:5741–5751. doi: 10.1182/blood-2011-07-325217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy MP, Noone PG, Leigh MW, Zariwala MA, Minnix SL, Knowles MR, Molina PL. High-resolution CT of patients with primary ciliary dyskinesia. AJR Am J Roentgenol. 2007;188:1232–1238. doi: 10.2214/AJR.06.0965. [DOI] [PubMed] [Google Scholar]
- 13.Casanova C, Cote C, Marin JM, Pinto-Plata V, de Torres JP, Aguirre-Jaíme A, Vassaux C, Celli BR. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134:746–752. doi: 10.1378/chest.08-0520. [DOI] [PubMed] [Google Scholar]
- 14.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, Lyons JJ, Engelhardt KR, Zhang Y, Topcagic N, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125:591–599. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angulo I, Vadas O, Garçon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deau MC, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, Cavazzana M, Picard C, Durandy A, Fischer A, et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest. 2015;125:1764–1765. doi: 10.1172/JCI81746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, Schickel JN, Tran DQ, Stoddard J, Zhang Y, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, Bulashevska A, Petersen BS, Schäffer AA, Grüning BA, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gámez-Díaz L, August D, Stepensky P, Revel-Vilk S, Seidel MG, Noriko M, Morio T, Worth AJ, Blessing J, Van de Veerdonk F, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016;137:223–230. doi: 10.1016/j.jaci.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Kami M, Tanaka Y, Kanda Y, Ogawa S, Masumoto T, Ohtomo K, Matsumura T, Saito T, Machida U, Kashima T, et al. Computed tomographic scan of the chest, latex agglutination test and plasma (1AE3)-β-d-glucan assay in early diagnosis of invasive pulmonary aspergillosis: a prospective study of 215 patients. Haematologica. 2000;85:745–752. [PubMed] [Google Scholar]
- 22.Martínez García MA, de Rojas MD, Nauffal Manzur MD, Muñoz Pamplona MP, Compte Torrero L, Macián V, Perpiñá Tordera M. Respiratory disorders in common variable immunodeficiency. Respir Med. 2001;95:191–195. doi: 10.1053/rmed.2000.1020. [DOI] [PubMed] [Google Scholar]
- 23.Kainulainen L, Varpula M, Liippo K, Svedström E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 1999;104:1031–1036. doi: 10.1016/s0091-6749(99)70085-0. [DOI] [PubMed] [Google Scholar]
- 24.Maglione PJ, Overbey JR, Radigan L, Bagiella E, Cunningham-Rundles C. Pulmonary radiologic findings in common variable immunodeficiency: clinical and immunological correlations. Ann Allergy Asthma Immunol. 2014;113:452–459. doi: 10.1016/j.anai.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maarschalk-Ellerbroek LJ, de Jong PA, van Montfrans JM, Lammers JW, Bloem AC, Hoepelman AI, Ellerbroek PM. CT screening for pulmonary pathology in common variable immunodeficiency disorders and the correlation with clinical and immunological parameters. J Clin Immunol. 2014;34:642–654. doi: 10.1007/s10875-014-0068-6. [DOI] [PubMed] [Google Scholar]
- 26.van de Ven AA, de Jong PA, Hoytema van Konijnenburg DP, Kessels OA, Boes M, Sanders EA, Terheggen-Lagro SW, van Montfrans JM. Airway and interstitial lung disease are distinct entities in paediatric common variable immunodeficiency. Clin Exp Immunol. 2011;165:235–242. doi: 10.1111/j.1365-2249.2011.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JE, Beal I, Dilworth JP, Tormey V, Haddock J. The HRCT appearances of granulomatous pulmonary disease in common variable immune deficiency. Eur J Radiol. 2005;54:359–364. doi: 10.1016/j.ejrad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Campagnoli MF, Garbarini L, Quarello P, Garelli E, Carando A, Baravalle V, Doria A, Biava A, Chiocchetti A, Rosolen A, et al. The broad spectrum of autoimmune lymphoproliferative disease: molecular bases, clinical features and long-term follow-up in 31 patients. Haematologica. 2006;91:538–541. [PubMed] [Google Scholar]
- 29.Ameratunga R, Woon ST, Gillis D, Koopmans W, Steele R. New diagnostic criteria for common variable immune deficiency (CVID), which may assist with decisions to treat with intravenous or subcutaneous immunoglobulin. Clin Exp Immunol. 2013;174:203–211. doi: 10.1111/cei.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rensing-Ehl A, Warnatz K, Fuchs S, Schlesier M, Salzer U, Draeger R, Bondzio I, Joos Y, Janda A, Gomes M, et al. Clinical and immunological overlap between autoimmune lymphoproliferative syndrome and common variable immunodeficiency. Clin Immunol. 2010;137:357–365. doi: 10.1016/j.clim.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Oliveria JB, Blessing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, Rieux-Laucat F, Siegel RM, Su HC, Teachey DT, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–e40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]