Abstract
Rationale: Neuroendocrine cell hyperplasia of infancy (NEHI) is a diffuse lung disease that presents in infancy and improves during childhood. Long-term outcomes have not previously been described. In one familial cohort, we have reported that NEHI is associated with a heterozygous variant of NKX2.1/TTF1.
Objectives: Our objective was to determine whether pulmonary abnormalities persist in adults with NEHI, to aid in elucidating the natural history of this disease.
Methods: Four adult relatives with heterozygous NKX2.1 mutation and with clinical histories compatible with NEHI enrolled in a prospective study that included questionnaires, pulmonary function tests, and chest computed tomography scans.
Measurements and Main Results: Mild radiologic abnormalities including mosaicism were seen in all four cases. Three individuals had obstruction on pulmonary function tests, two had marked air trapping, and three had symptomatic impairments with exercise intolerance.
Conclusions: Although clinical improvement occurs over time, NEHI may result in lifelong pulmonary abnormalities in some cases. Further studies are required to better describe the natural history of this disease and would be facilitated by additional delineation of genetic mechanisms to enable improved case identification.
Keywords: neuroendocrine cell hyperplasia of infancy, childhood interstitial lung disease, thyroid transcription factor-1
Neuroendocrine cell hyperplasia of infancy (NEHI) is a diffuse lung disease of childhood that typically presents in the first year of life. The common presenting features include tachypnea, retractions, hypoxemia, crackles, and failure to thrive (1–3). Affected individuals improve gradually during childhood years (2, 4), although continuation of symptoms well into childhood has been described (5). Furthermore, older children have been reported to experience exacerbations of NEHI symptoms even after their baseline symptoms improve (6). However, as the definitive report was published in 2005 (2), phenotypes and long-term outcomes in adults with NEHI have not previously been characterized.
Chest computerized tomography (CT) findings in infants with NEHI have been well described; relatively homogeneous ground-glass opacities are present centrally and in the right middle lobe and lingula without other parenchymal abnormalities (7). These imaging findings, in conjunction with an appropriate clinical context, are now considered sufficient for NEHI diagnosis, per American Thoracic Society clinical guidelines (8). Although these radiologic findings are believed to be specific for NEHI when observed and have been used to retrospectively identify cases (9), atypical imaging is present in some cases subsequently diagnosed by biopsy (7). Chest CT patterns in adults with NEHI have not been characterized to date.
Pulmonary function testing in affected infants demonstrates reduction in forced expiratory volume in 0.5 seconds and FVC, with marked air trapping (10, 11). There are no published data on pulmonary function testing patterns in adults with NEHI, and thus it is unclear if the pattern of air trapping continues throughout life. Likewise, although symptoms appear to improve throughout early childhood, ongoing symptoms in adulthood have not been described.
A heterozygous variant in the homeodomain of NKX2.1/TTF1 (c.572G>T, p.Arg191Leu) was previously identified in association with NEHI in one familial cohort (12). Adults with this mutation in the familial cohort were evaluated for ongoing pulmonary manifestations to aid in elucidating the natural history of this disease.
Methods
This prospective study was designed to identify ongoing manifestations of NEHI in adults and was approved by the Institutional Review Board at Vanderbilt University (#131026). Eligibility was determined based on clinical history and/or genetic diagnosis of familial NEHI when available. Subjects provided written informed consent. Written and phone questionnaires, high-resolution inspiratory noncontrast chest CT scans, and pulmonary function tests were performed per American Thoracic Society criteria. Chest CT scans were reviewed by an adult chest radiologist (J.A.W.) with expertise in interstitial lung disease.
Results
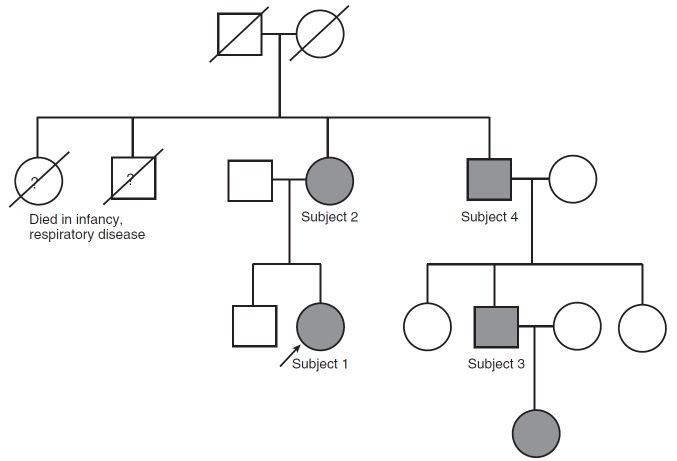
A familial cohort (Figure 1) of four adult relatives and one infant with newly diagnosed NEHI were enrolled in this research study. The proband (Subject 1) was diagnosed with NEHI by clinical history, chest CT findings, and by lung biopsy in infancy (12). As previously reported, this individual was heterozygous for an NKX2.1 mutation, as were three adult relatives (Subjects 2–4) with a clinical history compatible with NEHI in infancy and early childhood, including pulmonary manifestations and failure to thrive (12). In addition, an infant in the family, the daughter of Subject 3 and granddaughter of Subject 4, has been subsequently diagnosed with NEHI. A description of her presentation is also reviewed below.
Figure 1.
Pedigree demonstrating neuroendocrine cell hyperplasia of infancy (NEHI) and lung disease in association with an NKX2.1 mutation. The proband (arrow) was diagnosed with NEHI on the basis of lung biopsy performed in infancy. Multiple other family members had pulmonary symptoms and failure to thrive as infants. The proband and other family members affected by the NKX2.1 missense mutation are identified in gray.
Symptoms and clinical histories in adulthood are displayed in Table 1. Variable exercise intolerance was noted in three subjects. Two subjects experienced pneumonia as adults that required hospital admission and use of supplemental oxygen. None of the participants required chronic supplemental oxygen beyond 18 years of age.
Table 1.
Clinical characteristics in adulthood of subjects with familial neuroendocrine cell hyperplasia of infancy
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | |
|---|---|---|---|---|
| Age, yr | 25 | 63 | 33 | 62 |
| Current signs/symptoms | ||||
| Exercise intolerance | Yes* | Yes* | None | Minimal† |
| Shortness of breath | Yes, exertion | Yes, exertion | None | None |
| Cough | None | None | None | None |
| Wheeze | None | Yes | None | None |
| Crackles | Yes | None | None | None |
| Pneumonia as an adult | Yes (3 episodes) | Bronchitis requiring antibiotics, approximately annually from ages 38–50 yr | None | Yes (2 episodes) |
| If absent, at what age did symptoms resolve? | n/a | n/a | 20 yr | n/a |
| Hospitalizations as an adult | ||||
| Age | 20 yr | None | None | 29 yr |
| Duration | 3 d | n/a | n/a | 2 wk |
| Treatments | Antibiotics, oxygen | n/a | n/a | Antibiotics, oxygen |
| Medication history | ||||
| Inhaled medications | PRN albuterol | PRN inhaled corticosteroids | None | None |
| Systemic steroids | 2 oral courses | None | None | None |
| Supplemental oxygen | Continuous use in early childhood; nighttime oxygen until age 18 yr | During extended hospitalizations in early childhood | Continuous use in early childhood; nighttime oxygen until age 4 yr | During hospitalizations in childhood |
| Comorbidities | ||||
| Thyroid disorder | None | Hypothyroidism | None | None |
| Nutritional concerns | Resolved as child | Resolved as child | Testosterone for growth in adolescence | None |
| Smoking/exposures | None | Former smoker | None | None |
| Other | None | GERD, hypercholesterolemia, | Hypercholesterolemia | Hypercholesterolemia |
Definition of abbreviations: GERD = gastroesophageal reflux; n/a = not applicable; PRN = as needed.
Reported as dyspnea with walking on incline and climbing stairs.
Reported as limitation in distance able to run despite training.
Three of the four subjects had abnormal pulmonary function tests (Table 2). The proband, Subject 1, had FEV1 of 54% predicted, FVC of 48% predicted, and air trapping with residual volume of 174% predicted at 25 years of age. Subject 2 (aged 61 yr) had normal pulmonary function tests. Subject 3 (32 yr) demonstrated reduction in both FEV1 and FVC, with marked air trapping (residual volume, 207% predicted). Subject 4 (aged 61 yr) had mild obstruction on spirometry, with FEV1/FVC ratio of 0.64 and FEV1 of 73% predicted. He had normal lung volumes without evidence of air trapping.
Table 2.
Pulmonary function test results in adult subjects with familial neuroendocrine cell hyperplasia of infancy
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | |
|---|---|---|---|---|
| Age at testing, yr | 25 | 61 | 32 | 61 |
| FEV1, L (% predicted) | 1.54 (54) | 2.3 (98) | 2.92 (73) | 2.97 (73) |
| FVC, L (% predicted) | 1.72 (48) | 2.91 (99) | 3.65 (74) | 4.63 (86) |
| FEV1/FVC ratio | 0.89 | 0.79 | 0.8 | 0.64 |
| TLC, L (% predicted) | 4.08 (89) | 4.86 (99) | 6.9 (110) | 6.75 (86) |
| RV, L (% predicted) | 2.23 (174) | 1.89 (97) | 3.13 (207) | 2.12 (85) |
| DlCO, ml/min/mm Hg (% predicted) | 18.8 (83) | 19.3 (102) | 32.71 (97) | 27.13 (91) |
Definition of abbreviations: DlCO = diffusing capacity of the lung for carbon monoxide; RV = residual volume; TLC = total lung capacity.
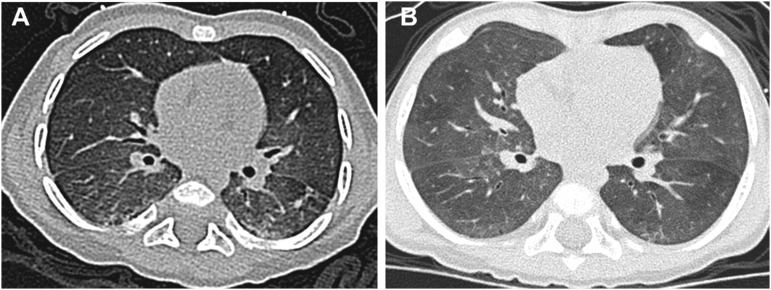
All four adult subjects had subtle radiologic abnormalities including mosaicism (Figure 2). Subject 1’s chest CT scan completed at 25 years of age demonstrated multiple areas of ground-glass opacity and mosaicism most prominent in the right middle lobe, lingula, and medial aspect of lower lobes as well as upper lobe septal lines (Figures 2A and 2B). Chest CT scan for Subject 2 demonstrated upper lobe smooth septal lines (not shown) and subtle mosaicism in the lower lobes, lingula, and middle lobe (Figures 2C and 2D). Subject 3 had several small subpleural and calcified nodules with pleural thickening of the left major fissure. There was subtle mosaicism throughout all lobes (Figures 2E and 2F). Chest CT scan for Subject 4 was significant for marked asymmetric pectus excavatum, several subpleural nodules and calcifications, and pleural abnormalities with thickening along the major fissure on the right and nodularity of the pleura in the upper lobes bilaterally. He had upper lobe smooth septal lines and subtle mosaicism at the bases (Figures 2G and 2H).
Figure 2.
Inspiratory high-resolution chest computerized tomography (CT) scans of adult subjects with neuroendocrine cell hyperplasia of infancy by history and NKX2.1 mutations. (A and B) CT images from Subject 1 (proband) at 25 years of age demonstrate mosaicism most prominently in right middle lobe, lingula, and medial aspects of lower lobes. (C and D) Subject 2 at 61 years of age with subtle mosaicism. (E and F) Subject 3 at 32 years of age with subtle mosaicism and small subpleural nodule (arrow). (G and H) Subject 4 at 61 years of age with subtle mosaicism, upper lobe smooth septal lines, and small subpleural nodule (arrow).
The newly identified infant in the family cohort initially presented with failure to thrive, retractions, and hypoxemia from 2 to 4 months of age. Other etiologies of disease were excluded, chest CT scan (Figure 3) and clinical course were consistent with NEHI, and genetic sequencing confirmed that she carried the same NKX2.1/TTF1 variant previously reported in other family members.
Figure 3.
Inspiratory high-resolution chest computerized tomography (CT) scans of infant subject with neuroendocrine cell hyperplasia of infancy. (A) Infant at 5 months of age with very subtle mosaicism and dependent atelectasis. (B) Imaging for the same infant at 11 months of age shows mosaicism with ground-glass opacities most prominent in the right middle lobe and lingula.
Discussion
NEHI has been described as a diffuse lung disease in childhood that improves over time. Here we report the first data on formal pulmonary assessments of adults with a history of NEHI. This familial series demonstrates that disease manifestations, such as exercise intolerance, pulmonary function testing abnormalities, and imaging findings, can persist well into adulthood. Thus, long-term follow-up studies will be required to more fully describe the natural history of this disease.
Although the classic NEHI distribution of ground-glass opacities centrally and in the right middle lobe and lingula was noted in Subject 1 at age 25 years, the chest CT abnormalities differed somewhat in the other three adults in this cohort. Mosaicism involving other lobes was present in Subjects 2 to 4 (aged 32–61 yr). Other radiologic findings included subtle septal thickening and subpleural pulmonary nodules, findings not previously reported in NEHI cases in childhood. It is unknown whether these abnormalities including the specific regional areas of involvement were previously present in these individuals. Patients with other genetic mutations resulting in interstitial lung diseases, such as surfactant protein C gene mutations (SFPTC), have been previously reported to have different imaging findings in childhood compared with older adults, with remarkable evolution of radiologic abnormalities over time (13, 14).
The chest CT findings noted in the adult patients in this series partially overlap with those reported in the recently described adult-onset disorder diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH). This diagnosis is most commonly seen in middle-aged women with symptoms of cough and dyspnea. In DIPNECH, there are obstructive abnormalities seen with pulmonary function testing, with chest CT findings demonstrating pulmonary nodules, ground-glass opacities and mosaicism with air trapping, and bronchiectasis (15, 16). Two of the adults in this familial NEHI cohort had small subpleural nodules of uncertain significance. However, these cases remain distinct from DIPNECH on the basis of both the clinical history and the lack of bronchiectasis. Furthermore, the degree of mosaicism was relatively milder than that reported in most DIPNECH cases, although expiratory imaging was not performed in our study, which might have aided in more detailed assessment of the mosaicism. Future studies will be needed to determine whether there is molecular or other etiologic overlap between NEHI and DIPNECH.
Prominent air trapping has been demonstrated in NEHI through infant pulmonary function tests, although only anecdotal experience exists about pulmonary physiology in older children with NEHI (6). In this context, we noted with great interest that the younger adults in this familial cohort continued to demonstrate similar air-trapping physiology, with proportionate reduction of FEV1 and FVC and marked air trapping, although these findings were not seen in the two subjects older than 60 years. No generalized management guidelines regarding frequency for chest CT scans or pulmonary function tests in adults with NEHI can be drawn from these cases; however, it is informative that in this cohort, some abnormalities in both imaging and pulmonary function testing persist despite minimal symptoms. Thus, in addition to spirometry, assessment of lung volumes for patients with NEHI into adulthood may be appropriate for initial screening.
The identification of the infant with NKX2.1 mutation and diagnosis of NEHI is highly significant in considering the apparent phenotypic spectrum in this extended family that we previously reported (12). Subjects 2, 3, and 4 had history consistent with a diagnosis of NEHI, but available data from childhood did not allow us to conclude whether these individuals truly had typical NEHI phenotypes. Thus, the typical NEHI presentation in infancy of this subsequent generation strengthens the association of this NKX2.1 mutation with NEHI and the relevance of the findings in the other adults in this cohort. The evolution of her imaging findings between the first and second CT scans over a 6-month interval is of interest and may have broader implications for clinicians evaluating infants with suspected NEHI.
Delineation of additional genetic mechanisms would allow for further understanding of pathogenesis and also facilitate identification of affected individuals and evaluation of the clinical course and spectrum of NEHI. A limitation of our study is that it is unknown whether these findings in individuals heterozygous for the NKX2.1 mutation will be generalizable to the entire NEHI population or even other familial occurrences identified (17). Furthermore, it is unknown whether there may be genotype–phenotype correlations in NEHI that could explain the variation in the pattern of clinical findings and natural history of NEHI that is increasingly being observed.
In conclusion, a relative paucity of information exists regarding the long-term impact of NEHI on lung function and pulmonary status into adulthood. This familial series demonstrates that some symptoms, imaging findings, and pulmonary function abnormalities continue into adulthood and may be lifelong.
Footnotes
Supported by grants from the American Thoracic Society/ChILD Foundation/chILD (Lung) Foundation-UK (L.R.Y.); National Institutes of Health grants HL 119503 (L.R.Y.), HL 092870 (T.S.B. and J.A.W.), HL 54703 (L.M.N.); T32HD060554 (R.J.N.) and the ATS/ChILD Foundation (L.M.N.).
Author Contributions: L.R.Y. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. R.J.N. contributed to the data acquisition and analysis, drafted the manuscript, provided critical input into the final version of the manuscript, and approved the final version of the manuscript. E.T.G. and R.L.M. contributed to the data acquisition and analysis, provided critical input into the final version of the manuscript, and approved the final version of the manuscript. J.A.W., L.M.N., and T.S.B. contributed to study inception and data acquisition and analysis, provided critical input and helped revise the final version of the manuscript, and approved the final version of the manuscript. L.R.Y. contributed to study inception and data acquisition and analysis, drafted the manuscript, provided critical input and helped revise the final version of the manuscript, and approved the final version of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Deterding RR, Fan LL, Morton R, Hay TC, Langston C.Persistent tachypnea of infancy (PTI): a new entity Pediatr Pulmonol 2001. Suppl 23:72–73. [PubMed] [Google Scholar]
- 2.Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40:157–165. doi: 10.1002/ppul.20243. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor MG, Wurth M, Young LR. Rare becomes more common: recognizing neuroendocrine cell hyperplasia of infancy in everyday pulmonary consultations. Ann Am Thorac Soc. 2015;12:1730–1732. doi: 10.1513/AnnalsATS.201507-422LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, Brody AS, Nogee LM, Trapnell BC, Langston C, et al. Pathology Cooperative Group; ChILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukkarinen H, Pelkonen A, Lohi J, Malmström K, Malmberg LP, Kajosaari M, Lindahl H, Föhr A, Ruuskanen O, Mäkelä MJ. Neuroendocrine cell hyperplasia of infancy: a prospective follow-up of nine children. Arch Dis Child. 2013;98:141–144. doi: 10.1136/archdischild-2012-302115. [DOI] [PubMed] [Google Scholar]
- 6.Houin PR, Deterding RR, Young LR. Exacerbations in neuroendocrine cell hyperplasia of infancy are characterized by increased air trapping. Pediatr Pulmonol. 2016;51:E9–E12. doi: 10.1002/ppul.23347. [DOI] [PubMed] [Google Scholar]
- 7.Brody AS, Guillerman RP, Hay TC, Wagner BD, Young LR, Deutsch GH, Fan LL, Deterding RR. Neuroendocrine cell hyperplasia of infancy: diagnosis with high-resolution CT. AJR Am J Roentgenol. 2010;194:238–244. doi: 10.2214/AJR.09.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, Castile RG, Dell S, Fan LL, Hamvas A, Hilman BC, et al. American Thoracic Society Committee on Childhood Interstitial Lung Disease (chILD) and the chILD Research Network. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188:376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soares JJ, Deutsch GH, Moore PE, Fazili MF, Austin ED, Brown RF, Sokolow AG, Hilmes MA, Young LR. Childhood interstitial lung diseases: an 18-year retrospective analysis. Pediatrics. 2013;132:684–691. doi: 10.1542/peds.2013-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerby GS, Wagner BD, Popler J, Hay TC, Kopecky C, Wilcox SL, Quinones RR, Giller RH, Accurso FJ, Deterding RR. Abnormal infant pulmonary function in young children with neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2013;48:1008–1015. doi: 10.1002/ppul.22718. [DOI] [PubMed] [Google Scholar]
- 11.Young LR, Brody AS, Inge TH, Acton JD, Bokulic RE, Langston C, Deutsch GH. Neuroendocrine cell distribution and frequency distinguish neuroendocrine cell hyperplasia of infancy from other pulmonary disorders. Chest. 2011;139:1060–1071. doi: 10.1378/chest.10-1304. [DOI] [PubMed] [Google Scholar]
- 12.Young LR, Deutsch GH, Bokulic RE, Brody AS, Nogee LM. A mutation in TTF1/NKX2.1 is associated with familial neuroendocrine cell hyperplasia of infancy. Chest. 2013;144:1199–1206. doi: 10.1378/chest.13-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abou Taam R, Jaubert F, Emond S, Le Bourgeois M, Epaud R, Karila C, Feldmann D, Scheinmann P, de Blic J. Familial interstitial disease with I73T mutation: a mid- and long-term study. Pediatr Pulmonol. 2009;44:167–175. doi: 10.1002/ppul.20970. [DOI] [PubMed] [Google Scholar]
- 14.van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, van Es HW, van den Bosch JM, Grutters JC. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a Dutch cohort. Am J Respir Crit Care Med. 2010;182:1419–1425. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 15.Nassar AA, Jaroszewski DE, Helmers RA, Colby TV, Patel BM, Mookadam F. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: a systematic overview. Am J Respir Crit Care Med. 2011;184:8–16. doi: 10.1164/rccm.201010-1685PP. [DOI] [PubMed] [Google Scholar]
- 16.Carr LL, Chung JH, Duarte Achcar R, Lesic Z, Rho JY, Yagihashi K, Tate RM, Swigris JJ, Kern JA. The clinical course of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. Chest. 2015;147:415–422. doi: 10.1378/chest.14-0711. [DOI] [PubMed] [Google Scholar]
- 17.Popler J, Gower WA, Mogayzel PJ, Jr, Nogee LM, Langston C, Wilson AC, Hay TC, Deterding RR. Familial neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2010;45:749–755. doi: 10.1002/ppul.21219. [DOI] [PubMed] [Google Scholar]