Abstract
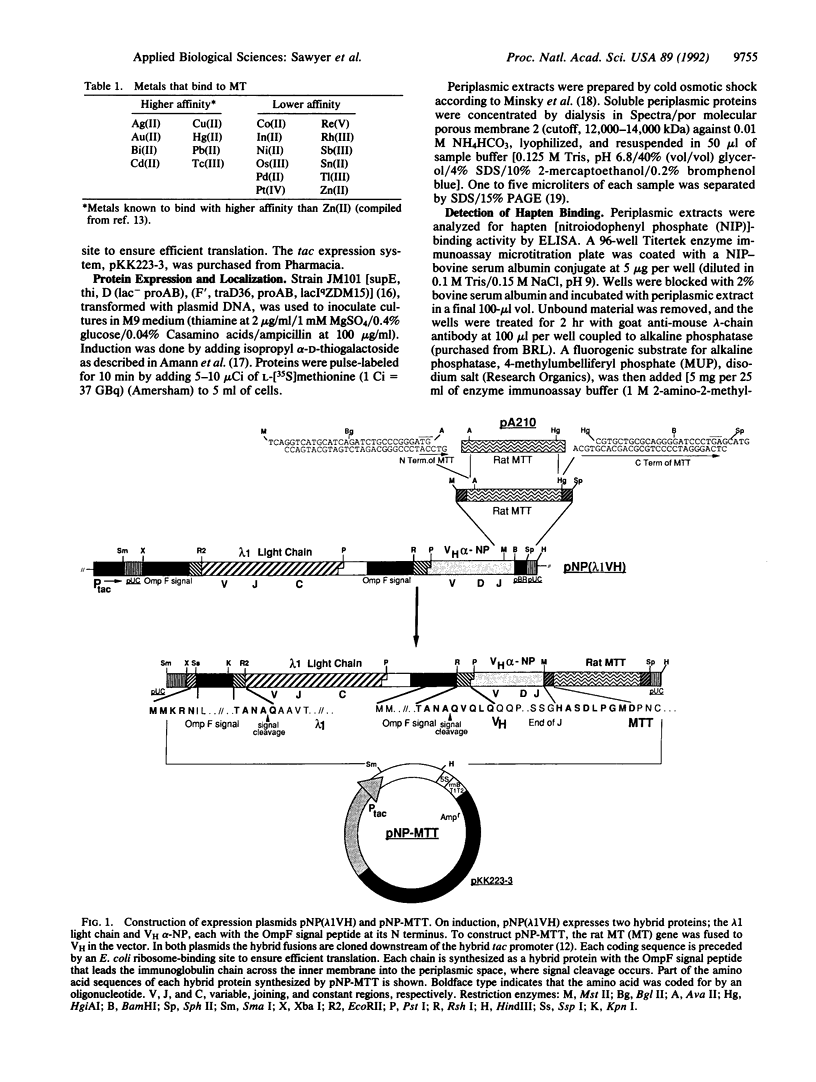
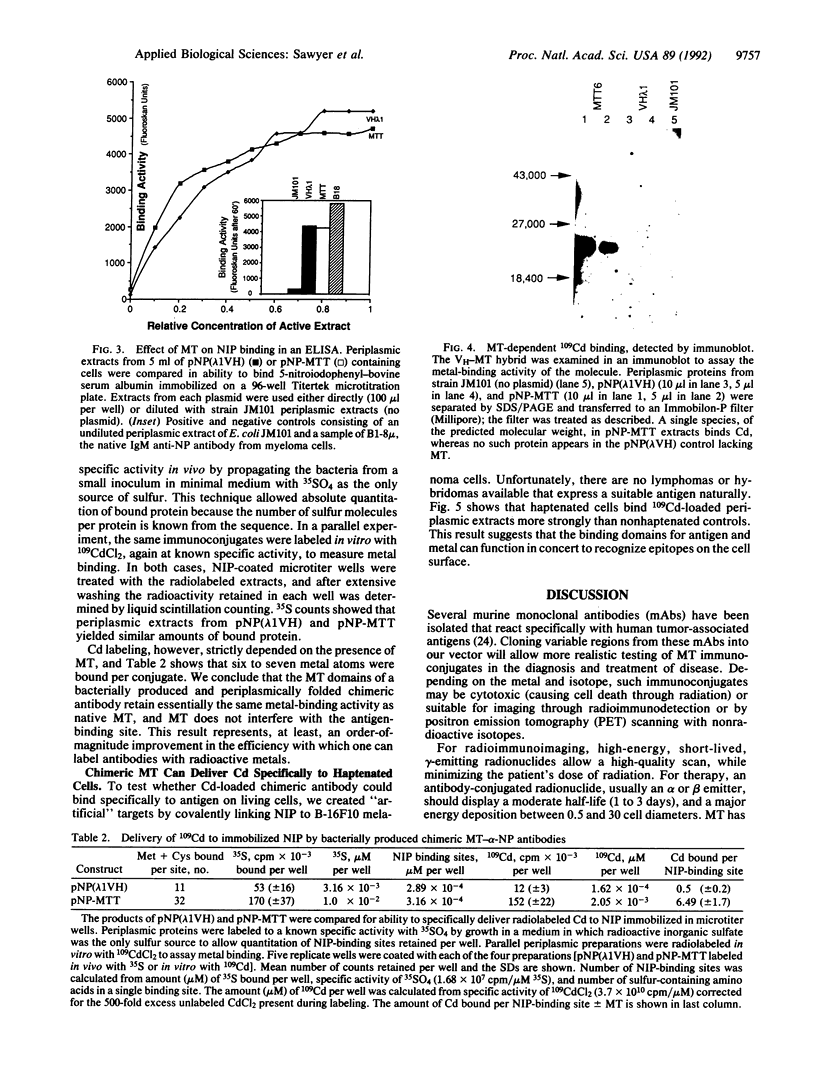
Metallothionein, a well-characterized biological chelator of metals, has been genetically fused to the binding domain of an antibody and expressed in the periplasm of Escherichia coli. Specific delivery of 109Cd to immobilized hapten or to haptenated cells was demonstrated directly in periplasmic extracts. This approach is potentially useful for targeted radiotherapy and diagnostic imaging. We find six to seven atoms of metal per active antigen-combining site. Absence of the Fc portion of the immunoglobulin along with low immunogenicity of metallothionein-metal complexes should reduce immunologic reactions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Andersen R. D., Birren B. W., Ganz T., Piletz J. E., Herschman H. R. Molecular cloning of the rat metallothionein 1 (MT-1) mRNA sequence. DNA. 1983;2(1):15–22. doi: 10.1089/dna.1.1983.2.15. [DOI] [PubMed] [Google Scholar]
- Barbosa M. S., Lowy D. R., Schiller J. T. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989 Mar;63(3):1404–1407. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Chang C. P., Robinson R. R., Horwitz A. H. Escherichia coli secretion of an active chimeric antibody fragment. Science. 1988 May 20;240(4855):1041–1043. doi: 10.1126/science.3285471. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Boss M. A., Kenten J. H., Wood C. R., Emtage J. S. Assembly of functional antibodies from immunoglobulin heavy and light chains synthesised in E. coli. Nucleic Acids Res. 1984 May 11;12(9):3791–3806. doi: 10.1093/nar/12.9.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. A., Drozynski C. A., Dearborn C. B., Hadjian R. A., Liberatore F. A., Tulip T. H., Tolman G. L., Haber S. B. Conjugation of metallothionein to a murine monoclonal antibody. Anal Biochem. 1988 Jul;172(1):22–28. doi: 10.1016/0003-2697(88)90406-x. [DOI] [PubMed] [Google Scholar]
- Burchiel S. W., Hadjian R. A., Hladik W. B., Drozynski C. A., Tolman G. L., Haber S. B., Gallagher B. M. Pharmacokinetic evaluation of technetium-99-metallothionein-conjugated mouse monoclonal antibody B72.3 in rhesus monkeys. J Nucl Med. 1989 Aug;30(8):1351–1357. [PubMed] [Google Scholar]
- Cabilly S., Riggs A. D., Pande H., Shively J. E., Holmes W. E., Rey M., Perry L. J., Wetzel R., Heyneker H. L. Generation of antibody activity from immunoglobulin polypeptide chains produced in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3273–3277. doi: 10.1073/pnas.81.11.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Batra J. K., Gallo M. G., Willingham M. C., FitzGerald D. J., Pastan I. A rapid method of cloning functional variable-region antibody genes in Escherichia coli as single-chain immunotoxins. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1066–1070. doi: 10.1073/pnas.87.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Queen C., Junghans R. P., Waldmann T. A., FitzGerald D. J., Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989 Jun 1;339(6223):394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- Gierasch L. M. Signal sequences. Biochemistry. 1989 Feb 7;28(3):923–930. doi: 10.1021/bi00429a001. [DOI] [PubMed] [Google Scholar]
- Hochman J., Inbar D., Givol D. An active antibody fragment (Fv) composed of the variable portions of heavy and light chains. Biochemistry. 1973 Mar 13;12(6):1130–1135. doi: 10.1021/bi00730a018. [DOI] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. T., Dear P. H., Foote J., Neuberger M. S., Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. 1986 May 29-Jun 4Nature. 321(6069):522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Minsky A., Summers R. G., Knowles J. R. Secretion of beta-lactamase into the periplasm of Escherichia coli: evidence for a distinct release step associated with a conformational change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4180–4184. doi: 10.1073/pnas.83.12.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelock M. M., Tolman G. L. Metallothionein: a bifunctional chelator for the radiolabeling of biologically active molecules. Experientia Suppl. 1987;52:247–253. doi: 10.1007/978-3-0348-6784-9_19. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Fox R. O. Recombinant antibodies possessing novel effector functions. Nature. 1984 Dec 13;312(5995):604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- Schnee J. M., Runge M. S., Matsueda G. R., Hudson N. W., Seidman J. G., Haber E., Quertermous T. Construction and expression of a recombinant antibody-targeted plasminogen activator. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6904–6908. doi: 10.1073/pnas.84.19.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J., Givol D. Preparation of Fv fragment from the mouse myeloma XRPC-25 immunoglobulin possessing anti-dinitrophenyl activity. Biochemistry. 1976 Apr 6;15(7):1591–1594. doi: 10.1021/bi00652a033. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Neuberger M. S. Production of antibody-tagged enzymes by myeloma cells: application to DNA polymerase I Klenow fragment. Gene. 1986;43(3):319–324. doi: 10.1016/0378-1119(86)90223-4. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]






