Abstract
Invagination of epithelium into the surrounding mesenchyme is a critical step that marks the developmental onset of many ectodermal organs. In this issue, Ahtiainen et al. (2016. J. Cell. Biol. http://dx.doi.org/10.1083/jcb.201512074) use the mouse incisor as a model to advance our understanding of the cellular mechanisms underlying ectodermal organ morphogenesis.
Nonsensory organs that form on the body surface, such as hair follicles, cutaneous glands, and teeth, use similar molecular and cellular processes during their development from the ectodermal germ layer of the embryo (Pispa and Thesleff, 2003). Morphogenesis of these organs takes place when a flat epithelium thickens into the placode stage and is then sculpted into a complex three-dimensional structure through a series of events (Biggs and Mikkola, 2014). Although the molecular underpinnings of tooth morphogenesis are relatively well characterized, our understanding of how interactions at a cellular level are coordinated within a tissue to carry out the tasks of tooth development is still limited.
Hypotheses about cellular mechanisms that mediate tooth morphogenesis have traditionally been studied using histological analyses at different time points. However, the two-dimensional static snapshots obtained in this manner have inherent limitations when studying a three-dimensional process that progresses over time. To overcome this technical issue, several recent studies have attempted to address key questions about various cellular processes that take place during the early steps of tooth development by using live imaging, lineage tracing, and explant culturing. Prochazka et al. (2015) showed that, before any visible sign of molar development, a group of cells within the epithelium actively migrate anteriorly to the location where the molar tooth placode will form and contribute to the starting material of the cheek teeth. Li et al. (2016b) and Panousopoulou and Green (2016) revealed that vertical cell divisions in the molar basal layer cause thickening (stratification) of the placode. The cells in the covering suprabasal cell layer, together with surrounding, but not underlying, basal layer cells converge and actively stack on top of and in between one another in a convergence-extension mechanism where elongation is accompanied by narrowing and intercalation. Together, these cellular processes drive the invagination (Fig. 1).
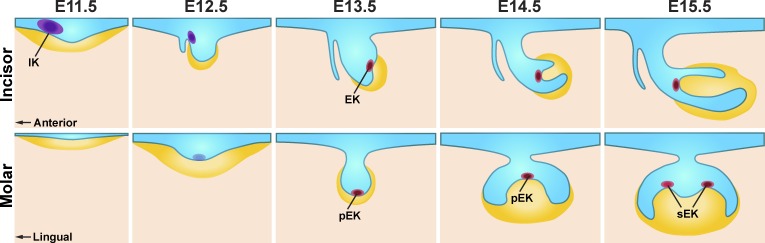
Figure 1.
Signaling centers coordinate tooth morphogenesis. The stages of mouse incisor (top) and molar (bottom) development are depicted in sagittal and frontal views, respectively. Tooth development begins with the appearance of a localized thickening of the oral epithelium (blue) to form the dental placode, which continues to thicken and then invaginates into the underlying dental mesenchyme (yellow), forming a tooth bud. The subsequent folding of the tooth bud into a cap-shaped enamel organ and the continued condensation of surrounding mesenchyme form the tooth germ. Ahtiainen et al. (2016) identified the cellular mechanisms by which the early signaling center of the incisor (IK; purple) formed, and they established the relationship between the IK and the better-studied EK (red) of the incisor. The early signaling center has not yet been examined in detail in the molar, which, unlike the incisor, has both primary (pEK) and secondary EKs (sEK).
The aforementioned morphological events, along with many other developmental processes, are regulated by signaling molecules, and embryonic signaling centers have long been recognized as the source of these ligands. In tooth development, several sets of signaling centers were originally suggested (Dassule and McMahon, 1998; Keränen et al., 1998): an early signaling center and later structures called enamel knots (EKs; Fig. 1). In contrast to the EKs, which are well-studied clusters of cells that express growth factors and regulate tooth morphogenesis (Jernvall et al., 1994), the initial placodal early signaling center has been largely neglected. In this issue, Ahtiainen et al. bring this earlier signaling center, which the authors have named the initiation knot (IK), back into focus. Through a series of exciting findings, they show that the IK controls the size of the tooth bud and that it may also affect the size of the tooth at later stages.
Ahtiainen et al. (2016) first explore the cellular events during early incisor morphogenesis between the placode stage and bud stage using live imaging and quantitative analysis with a fluorescent ubiquitination–based cell cycle indicator (Fucci) mouse (Sakaue-Sawano et al., 2008), which was chosen for its ability to clearly distinguish between stages of the cell cycle in vivo. The authors identified a subset of G1/G0 epithelial cells at embryonic day 11.5 (E11.5) that initially intermix with other dividing cells but later condense toward the anterior border of the forming tooth germ between E12 and 13 through directional cell migration. The resultant IK controls the proliferation of the neighboring non-IK placodal cells, and in this manner it presumably promotes placode stratification and subsequent bud formation.
Importantly, Ahtiainen et al. (2016) studied invagination in the incisor, in contrast to the two aforementioned recent studies, which analyzed a similar stage of tooth development in the molar (Li et al., 2016b; Panousopoulou and Green, 2016). Incisors and molars share many features and develop through comparable stages, yet they are distinct tooth types with unique function and shape. Therefore, it is expected that some differences will be present in their morphogenesis at equivalent stages. For example, development of the mouse incisor precedes development of the molar by ∼24 h, the incisor bud develops asymmetrically along the anterior–posterior and lingual–labial axes when compared with the symmetric molar, and the molar has both primary and secondary EKs whereas only a single EK is present in the incisor (Fig. 1). From the work of Ahtiainen et al. (2016), it appears that proliferation of basal layers of the placode is the process that drives the invagination during incisor development. It will be interesting in the future to assess whether, in addition to the proliferation of the basal layers of the placode, incisor invagination is also actively propelled by a cellular mechanism of convergence-extension as seen in molars (Panousopoulou and Green, 2016).
The nondividing nature of the IK reported by Ahtiainen et al. (2016) is a common feature of many embryonic signaling centers, including the apical ectodermal ridge of the limb bud and the neural tube floor plate. Specifically in ectodermal organs, a quiescent signaling center is seen in the EK of the tooth germ (Jernvall et al., 1994) and in the placode region of hair follicles (Ahtiainen et al., 2014). Therefore, a generalizable mechanism for the formation of signaling centers may be in place to induce cells to exit the cell cycle and become specialized in signal production. Based on their previous study in the hair follicle (Ahtiainen et al., 2014), and the ability of augmented Wnt activity to induce ectopic signaling centers in teeth (Järvinen et al., 2006), Ahtiainen et al. (2016) speculate that Wnt signaling may similarly control cell cycle cessation in the IK. In addition, it has recently been shown that several signaling centers, including the incisor EK, appear to use a mechanism by which αE-catenin prevents the transcriptional regulators YAP and TAZ from entering the nucleus, thereby promoting p21 expression and arrest of proliferation (Li et al., 2016a). It will be interesting to determine if the IK exploits a similar mechanism.
Another recurrent theme during ectodermal organ morphogenesis is directional cell migration. For example, active migration of cells drives mammary gland branching (Ewald et al., 2008), and cells in the hair follicle placode actively migrate toward the center region of the placode (Ahtiainen et al., 2014). In this new study, Ahtiainen et al. (2016) found that directional cell migration, as driven by actomyosin contraction, also contributes to the condensation of the IK. The compaction of the signaling center results in a narrower or more confined region for ligand secretion. An important question to address in the future is whether this cell migration is guided by chemoattractant directional cues, similar to migration of epithelial cells toward a Shh-expressing region observed during initiation of the molar field (Prochazka et al., 2015), or by other mechanisms, such as through a repulsive signal or force from surrounding tissue that corrals the IK cells together. Regardless, it is intriguing to speculate that concentrating a group of post-mitotic signal-secreting cells within a single confined region is beneficial for developing organs, as tighter control of ligand concentration and distribution may be achieved.
In an attempt to shed light on the mechanism that regulates the formation of the IK, Ahtiainen et al. (2016) used a mouse model to demonstrate that ectodysplasin (EDA), a TNF family ligand that triggers one of the central pathways regulating ectodermal organ development (Mikkola, 2008), controls the size of the IK to achieve proper formation of the tooth bud. The current study did not explore in detail the mechanism by which EDA signaling functions, but it does suggest that EDA, together with other genes, inhibit cell cycle progression in cells of the IK. Notably, in the hair follicle placode the same group found that overexpression of EDA causes an increase in cell motility without affecting cell proliferation (Ahtiainen et al., 2014). This disparity could be caused by the different methods that were used to manipulate Eda or, more interestingly, may point to a discrepancy in the developmental program for the two ectodermal organs, where the same set of signaling pathways could instruct a wide range of cellular processes to shape different organs.
Compared with the ancestral mammalian dentition, mouse dentition has been dramatically reduced to just one incisor and three molars in each jaw quadrant, and the mouse also has only one generation of teeth, without the replacement of deciduous (“baby”) teeth. Nevertheless, mouse embryos retain the potential to make more teeth than are present in the wild-type adult. Tooth rudiments are evident in the anterior regions of both the incisor (Hovorakova et al., 2011) and the first molar (Viriot et al., 2002), and these are marked by transient signaling centers that appear before the functional signaling centers and express several common molecular markers (Prochazka et al., 2010). Therefore, one might speculate that the IK identified by Ahtiainen et al. (2016) belonged to a residual or deciduous tooth initially and later became coopted into the formation of incisors. However, this is probably not the case, as rudiment signaling centers often arrest and regress in the early bud stage, whereas the IK continues to be present and has an instructive function in bud morphogenesis.
Several signaling centers appear transiently at different stages of tooth development before they undergo apoptosis and are removed. One question that is important from both developmental and evolutionary perspectives is whether a former signaling center contributes physically to a latter one. For example, it is still debated whether or not during molar development a few cells of the primary EK, which appears at the end of the bud stage, escape apoptosis and migrate to contribute to the buccal portion of the secondary EKs, which appear at the cusp stage (Matalova et al., 2005; Cho et al., 2007). Ahtiainen et al. (2016) show that the IK and the EK in the incisor are two separate signaling centers that are spatiotemporally disconnected. By culturing explants from Fucci mice and tracking every cell, the authors determined that the IK cells stay localized to the epithelial surface within the boundaries of the early signaling center and do not proliferate. In contrast, the EK signaling center emerges at E13.5 as a novel cluster of cells at the tip of the maturing bud and at a distance from the IK. One potential drawback of explant culturing is that removing the tissue from its normal surroundings can cause unexpected consequences, which may lead to artifacts. Thus, a future complementary approach would be to label the IK cells in vivo using a Cre-ER system and trace their fate over time. However, this system has its own caveats; in particular, the half-life of tamoxifen can confound temporal interpretations. Additional approaches including cell ablation and cell type–specific genetic modification may help to answer this open question.
Teeth share many regulatory molecules with other ectodermal appendages and develop through similar steps of initiation and morphogenesis. However, it is noteworthy that the ability to regenerate long after embryonic life is maintained by hair follicles, feathers, nails, claws, and mammary glands, whereas in many mammalian species the teeth cannot do so. A better understanding of tooth morphogenesis by studies such as the one by Ahtiainen et al. (2016) may facilitate future attempts toward therapeutic regeneration of the dentition and even other organs.
Acknowledgments
We thank Jeremy Green, Jan Prochazka, and Jimmy Hu for helpful discussions and Adriane Joo for drawing the figure.
The authors were funded by the National Institute of Dental and Craniofacial Research.
The authors declare no competing financial interests.
References
- Ahtiainen L., Lefebvre S., Lindfors P.H., Renvoisé E., Shirokova V., Vartiainen M.K., Thesleff I., and Mikkola M.L.. 2014. Directional cell migration, but not proliferation, drives hair placode morphogenesis. Dev. Cell. 28:588–602. 10.1016/j.devcel.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Ahtiainen L., Uski S.I., Thesleff I., and Mikkola M.L.. 2016. Early epithelial signaling center governs tooth budding morphogenesis. J. Cell Biol. 10.1083/jcb.201512074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs L.C., and Mikkola M.L.. 2014. Early inductive events in ectodermal appendage morphogenesis. Semin. Cell Dev. Biol. 25-26:11–21. 10.1016/j.semcdb.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Cho S.-W., Lee H.-A., Cai J., Lee M.-J., Kim J.-Y., Ohshima H., and Jung H.-S.. 2007. The primary enamel knot determines the position of the first buccal cusp in developing mice molars. Differentiation. 75:441–451. 10.1111/j.1432-0436.2006.00153.x [DOI] [PubMed] [Google Scholar]
- Dassule H.R., and McMahon A.P.. 1998. Analysis of epithelial–mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev. Biol. 202:215–227. 10.1006/dbio.1998.8992 [DOI] [PubMed] [Google Scholar]
- Ewald A.J., Brenot A., Duong M., Chan B.S., and Werb Z.. 2008. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell. 14:570–581. 10.1016/j.devcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovorakova M., Prochazka J., Lesot H., Smrckova L., Churava S., Boran T., Kozmik Z., Klein O., Peterkova R., and Peterka M.. 2011. Shh expression in a rudimentary tooth offers new insights into development of the mouse incisor. J. Exp. Zoolog. B Mol. Dev. Evol. 316:347–358. 10.1002/jez.b.21408 [DOI] [PubMed] [Google Scholar]
- Järvinen E., Salazar-Ciudad I., Birchmeier W., Taketo M.M., Jernvall J., and Thesleff I.. 2006. Continuous tooth generation in mouse is induced by activated epithelial Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. USA. 103:18627–18632. 10.1073/pnas.0607289103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J., Kettunen P., Karavanova I., Martin L.B., and Thesleff I.. 1994. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int. J. Dev. Biol. 38:463–469. [PubMed] [Google Scholar]
- Keränen S.V., Aberg T., Kettunen P., Thesleff I., and Jernvall J.. 1998. Association of developmental regulatory genes with the development of different molar tooth shapes in two species of rodents. Dev. Genes Evol. 208:477–486. 10.1007/s004270050206 [DOI] [PubMed] [Google Scholar]
- Li C.-Y., Hu J., Lu H., Lan J., Du W., Galicia N., and Klein O.D.. 2016a αE-catenin inhibits YAP/TAZ activity to regulate signalling centre formation during tooth development. Nat. Commun. 7:12133 10.1038/ncomms12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chatzeli L., Panousopoulou E., Tucker A.S., and Green J.B.A.. 2016b Epithelial stratification and placode invagination are separable functions in early morphogenesis of the molar tooth. Development. 143:670–681. 10.1242/dev.130187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalova E., Antonarakis G.S., Sharpe P.T., and Tucker A.S.. 2005. Cell lineage of primary and secondary enamel knots. Dev. Dyn. 233:754–759. 10.1002/dvdy.20396 [DOI] [PubMed] [Google Scholar]
- Mikkola M.L. 2008. TNF superfamily in skin appendage development. Cytokine Growth Factor Rev. 19:219–230. 10.1016/j.cytogfr.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Panousopoulou E., and Green J.B.A.. 2016. Invagination of ectodermal placodes is driven by cell intercalation-mediated contraction of the suprabasal tissue canopy. PLoS Biol. 14:e1002405 10.1371/journal.pbio.1002405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa J., and Thesleff I.. 2003. Mechanisms of ectodermal organogenesis. Dev. Biol. 262:195–205. 10.1016/S0012-1606(03)00325-7 [DOI] [PubMed] [Google Scholar]
- Prochazka J., Pantalacci S., Churava S., Rothova M., Lambert A., Lesot H., Klein O., Peterka M., Laudet V., and Peterkova R.. 2010. Patterning by heritage in mouse molar row development. Proc. Natl. Acad. Sci. USA. 107:15497–15502. 10.1073/pnas.1002784107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka J., Prochazkova M., Du W., Spoutil F., Tureckova J., Hoch R., Shimogori T., Sedlacek R., Rubenstein J.L., Wittmann T., and Klein O.D.. 2015. Migration of founder epithelial cells drives proper molar tooth positioning and morphogenesis. Dev. Cell. 35:713–724. 10.1016/j.devcel.2015.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue-Sawano A., Kurokawa H., Morimura T., Hanyu A., Hama H., Osawa H., Kashiwagi S., Fukami K., Miyata T., Miyoshi H., et al. 2008. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 132:487–498. 10.1016/j.cell.2007.12.033 [DOI] [PubMed] [Google Scholar]
- Viriot L., Peterková R., Peterka M., and Lesot H.. 2002. Evolutionary implications of the occurrence of two vestigial tooth germs during early odontogenesis in the mouse lower jaw. Connect. Tissue Res. 43:129–133. 10.1080/03008200290001168 [DOI] [PubMed] [Google Scholar]