Abstract
We developed a protocol to procure lungs from uncontrolled donors after circulatory determination of death (NCT02061462). Subjects with cardiovascular collapse, treated on scene by a resuscitation team and transferred to the emergency room, are considered potential donors once declared dead. Exclusion criteria include unwitnessed collapse, no‐flow period of >15 min and low flow >60 min. After death, lung preservation with recruitment maneuvers, continuous positive airway pressure, and protective mechanical ventilation is applied to the donor. After procurement, ex vivo lung perfusion (EVLP) is performed. From November 2014, 10 subjects were considered potential donors; one of these underwent the full process of procurement, EVLP, and transplantation. The donor was a 46‐year‐old male who died because of thoracic aortic dissection. Lungs were procured 4 h and 48 min after death, and deemed suitable for transplantation after EVLP. Lungs were then offered to a rapidly deteriorating recipient with cystic fibrosis (lung allocation score [LAS] 46) who consented to the transplant in this experimental setting. Six months after transplantation, the recipient is in good condition (forced expiratory volume in 1 s 85%) with no signs of rejection. This protocol allowed procurement of lungs from an uncontrolled donor after circulatory determination of death following an extended period of warm ischemia.
Short abstract
The authors describe the feasibility of a novel, in situ lung preservation strategy developed to procure lungs from uncontrolled donors after circulatory determination of death. See the editorial from Egan on page 1051.
Abbreviations
- CPAP
continuous positive airway pressure
- CPR
cardiopulmonary resuscitation
- DCDD
circulatory determination of death
- EVLP
ex vivo lung perfusion
- PEEP
positive end‐expiratory pressure
- uDCDD
uncontrolled DCDD
Introduction
As a measure to increase the number of organs available for transplantation, many countries worldwide have adopted organ procurement from donors after circulatory death 1, 2, 3. The majority of organs so far have been procured from controlled donors, according to the Maastricht definition 4, 5, mainly in Belgium, The Netherlands, the United Kingdom, Australia, and the United States. However, despite being an indisputable source of organs, as emphasized by the World Health Organization 6, this procedure has been considered with caution. Indeed, the possibility that a number of potential donors after brain death (DBD) turn into donors after circulatory determination of death (DCDD) within the controlled category of donors has caused skepticism 7. If true, this would eventually lead to a lesser number of organs procured, since the numbers of organs procured from DCDD are significantly lower than those procured from DBD 8. Conversely, recovery of organs from uncontrolled DCDD (uDCDD) would increase the pool of organs available for transplantation 9. However, donation from this category adds logistical, ethical, and legal complexity to the donation process in many countries 10. In fact, far fewer organs have been recovered from uncontrolled donors, mainly in France and Spain 11.
Preclinical data show that lungs have the potential to better tolerate warm ischemia relative to other solid organs 12, 13, 14, 15. This could make the uDCDD process safer in the lungs compared to other solid organs. However, although deceased donors are considered a valuable strategy to procure lungs 16, there are few reports on lung donation from uDCDD 17, 18, 19.
We are currently investigating the safety/efficacy of a clinical protocol designed to procure lungs from uncontrolled donors after determination of death with circulatory criteria. In this report, we present the first case recruited, and discuss some peculiarities of our protocol.
Materials and Methods
The trial was approved by the Ethics Committee of the Fondazione IRCCS Ca’ Granda and of the San Gerardo Hospital (NCT02061462).
Subjects with cardiovascular collapse, treated by an advanced life support crew on scene first, then transferred to the emergency room (San Gerardo Hospital, Monza), are considered to be potential donors if declared dead after advanced cardiac life support attempts failed. Unwitnessed collapse, no‐flow period of >15 min, or low flow >60 min are among exclusion criteria. After clinical diagnosis of death (5 min of no touch), a recruitment maneuver (RM) is performed (RM: positive end‐expiratory pressure, PEEP 5; inspiratory/expiratory [I/E] ratio 1:1; respiratory rate, RR 10/min; Pressure controlled + 25 ×2, PC + 30 ×2, PC + 35 ×4) and continuous positive end‐expiratory pressure (CPAP 10 cmH2O, 100% FiO2) is applied until death is confirmed according to circulatory criteria (20 min of flat electrocardiogram in our country). After next of kin consent to donation is obtained, heparin is given (10 000 U endovenous push, followed by 3 min of cardiopulmonary resuscitation, CPR), a new RM is performed and ventilation is started (respiratory rate 4/min, tidal volume 6 mL/kg, PEEP 8 cmH2O, fraction of inspired oxygen [FiO2] 100%, I/E ratio 1:1). If chest radiograph is negative, the subject is transferred to the operating room. If bronchoscope evaluation is negative, lungs are perfused in situ with a fibrinolytic agent (15 mg recombinant tissue plasminogen activator [rTPA]), flushed with preservation solution (Perfadex® [XVIVO Perfusion AB, Göteborg, Sweden], 60 mL/kg antegrade, 250 ×4 mL retrograde) and cold stored on ice. Once transferred to the Fondazione Ca’ Granda (25 km away from Monza), lung function is evaluated after ex vivo lung perfusion (EVLP), run with a low‐flow, open atrium and low hematocrit technique, as previously described 20.
An overview of the protocol flow is shown in Figure 1.
Figure 1.
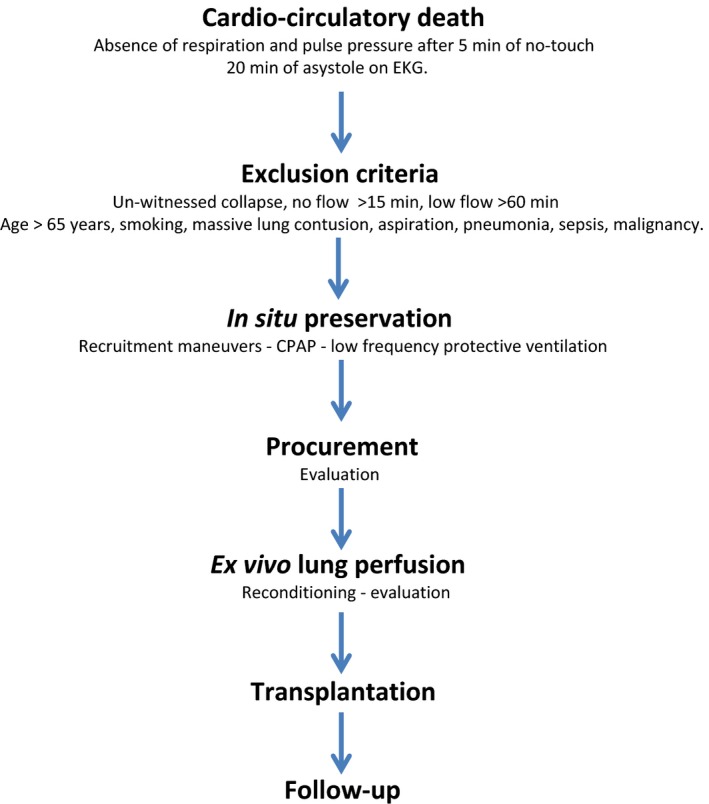
Lung‐ DCDD protocol flow. DCDD, circulatory determination of death.
Results
A pilot phase of potential donor's recruitment active from 8 am to 4 pm, 7 days a week started on May 12, 2014. Recruitment was interrupted for logistical reasons during the summer (from August 1, 2014 to September 15). During this first period, five subjects aged <65 years were treated because of cardiocirculatory arrest and transferred to the emergency room of the San Gerardo Hospital. However, all of them arrived beyond recruitment time and were not considered potential lung donors. On November 1, 2014 recruitment was activated on a 24‐h‐a‐day, 7‐days‐a‐week basis. Since then, the potential donor recruitment system has been activated for 10 subjects. Details are shown in Table 1.
Table 1.
Potential lung donors
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | M | M | M | M | M | M | M | M | M |
| Birth | September 01, 1968 | June 07, 1981 | November 30, 1969 | October 24, 1957 | July 29, 1965 | February 20, 1965 | October 11, 1957 | October 16, 1949 | April 15, 1966 | March 15, 1983 |
| Age | 46 | 33 | 45 | 57 | 49 | 49 | 57 | 65 | 48 | 32 |
| Clinical events | ||||||||||
| Date | November 1, 2014 | November 18, 2014 | December 11, 2014 | December 12, 2014 | December 25, 2014 | January 15, 2015 | January 31, 2015 | February 19, 2015 | March 22, 2015 | May 14, 2015 |
| CCA | 10:15 | – | 15:20 | 17:50 | 15:53 | 9:13 | 11:38 | 18:45 | 17:48 | 22:10 |
| CPR | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| BLS | 10:15 | 16:19 | 15:32 | 18:15 | 16:00 | 9:23 | 11:53 | 18:56 | 17:58 | 22:25 |
| Rhythm | PEA | Asystole | Asystole | PEA | PEA | VF | Asystole | VF | Asystole | Asystole |
| ALS | 10:15 | – | 15:35 | 18:17 | 16:05 | 9:29 | 11:53 | 18:56 | 18:04 | 22:32 |
| ER | 10:50 | 16:56 | 16:06 | 19:07 | 17:06 | 10:18 | 12:48 | 19:47 | 18:48 | 23:15 |
| Exitus | 11:00 | 17:07 | 16:23 | 19:08 | 17:30 | 10:38 | 13:29 | 19:55 | 19:00 | 23:24 |
| Exclusion criteria | ||||||||||
| Witness | Y | N | N | Y | Y | Y | Y | Y | Y | Y |
| No Flow | 0:00 | – | 0:12 | 0:25 | 0:07 | 0:10 | 0:15 | 0:11 | 0:10 | 0:15 |
| Low Flow | 0:45 | 0:48 | 0:51 | 0:53 | 1:30 | 1:15 | 01:36 | 00:59 | 01:02 | 00:59 |
| Other | – | – | Smoking | LMA | Smoking | Aspiration | – | Smoking | Aspiration | – |
| Consent | Y | – | Y | – | Y | N | – | – | – | N |
CCA, cardiocirculatory arrest; CPR, cardiopulmonary resuscitation; BLS, basic life support; PEA, pulseless electrical activity; VF, ventricular fibrillation; ALS, advanced life support; ER, emergency room; Smoking, active smoking of >20 cigarettes/day or history of >20 packs/year; LMA, laryngeal mask airway.
Subject number 1 in Table 1 was the only one with lungs procured that had EVLP. These lungs were transplanted. He was a 46‐year‐old male who had thoracic pain and, soon after arrival of the emergency team, collapsed. CPR was started immediately (0 min no flow); a first return of spontaneous circulation revealed ST‐elevated myocardial infarction, but pulseless electrical activity developed soon after. The subject was transferred to the emergency room while automated chest compression (LUCAS™, Jolife AB/Physio‐Control, Lund, Sweden) was ongoing. After the diagnosis of aortic dissection, the possibility of receiving extracorporeal life support (VA‐ECMO) was excluded and the medical team decided to withdraw further treatment. The subject was declared dead after a total low‐flow time of 45 min. Recruitment maneuvers and CPAP were applied and death was confirmed. Chest radiograph showed reduced lung volumes and a wide mediastinum consistent with the diagnosis of dissection (Figure 2). Consent for donation was obtained 2 h after death. At that time, heparin was given and ventilation started. The donor was then transferred to the operating room where lungs were procured (4 h and 48 min after death) and cold stored on ice. Upon arrival to the Fondazione Ca’ Granda, EVLP was run for a total of 6 h, after which lungs were deemed suitable for transplantation (Table 2) and cooled down. Time flow of the donation process is shown in Table 3. Lungs were offered to a rapidly deteriorating recipient with cystic fibrosis (LAS 46) who had been hospitalized for 4 months. The patient was on noninvasive ventilation 24 h a day and consented to the transplant in this experimental setting. Surgery was complicated by cardiogenic shock and need of VA‐ECMO support with massive bleeding. Intensive care unit stay (19 days) was initially characterized by distributive‐hypovolemic shock. Primary graft dysfunction at 72 h was grade 2; lung function was proper throughout the following days. Weaning from mechanical ventilation was difficult because of muscle fatigue due to preoperative deconditioning. Hospital length of stay was 39 days. Six months after transplantation, the recipient is at home, in good condition (forced expiratory volume in 1 s 85%). Three‐ and 6‐month surveillance lung biopsy were both negative.
Figure 2.
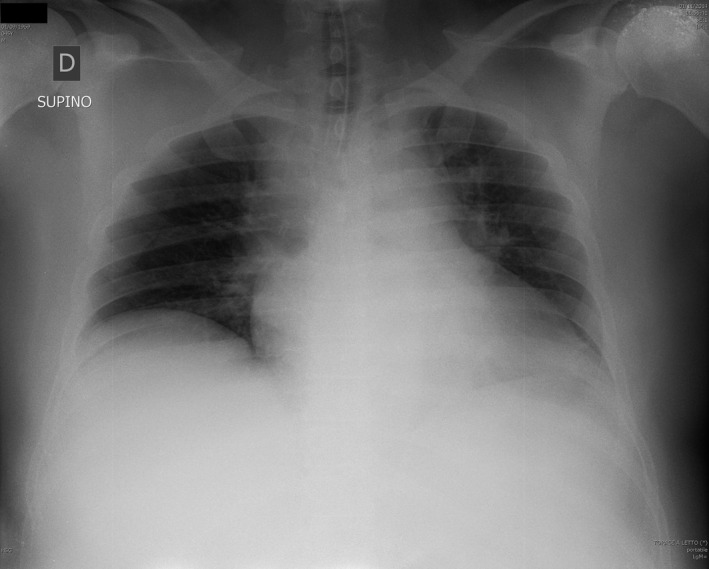
Chest radiograph of the donor.
Table 2.
Functional data during ex vivo lung perfusion (EVLP)
| 60 min | 120 min | 180 min | 240 min | Evaluation | |
|---|---|---|---|---|---|
| LA temp (°C) | 37.1 | 36.2 | 36.4 | 36.4 | 36.2 |
| Perfusate flow (L/min) | 2.45 | 2.42 | 2.43 | 2.4 | 2.4 |
| PAPm (cmH2O) | 17 | 18 | 19 | 18 | 19 |
| PVR (dine*s/cm5) | 489 | 528 | 559 | 533 | 566 |
| Vt (mL/kg) | 5.6 | 5.4 | 5.6 | 5.6 | 5.6 |
| Pawm (cmH2O) | 6 | 6 | 6 | 6 | 6 |
| Pawpeak (cmH2O) | 13 | 11 | 11 | 13 | 12 |
| Cpldyn (mL/cmH2O) | 70 | 90 | 93 | 70 | 80 |
| Gas mix | CO2/air | CO2/air | CO2/air | CO2/air | CO2/N2 |
| FiO2 ventilator | 0.21 | 0.4 | 0.4 | 0.4 | 1 |
| PCO2 IN (mmHg) | 39 | 36 | 31 | 28 | – |
| PCO2 OUT (mmHg) | 31 | 31 | 26 | – | – |
| PCO2 OUTleft (mmHg) | – | – | – | 28 | 32 |
| PCO2 OUTright (mmHg) | – | – | – | 22 | 28 |
| PO2 IN (mmHg) | 146 | 154 | 154 | 161 | 75 |
| PO2 OUT (mmHg) | 146 | 249 | 243 | – | – |
| PO2 OUTleft (mmHg) | – | – | – | 239 | 490 |
| PO2 OUTright (mmHg) | – | – | – | 221 | 436 |
EVLP was run as previously described 20. Briefly, during the first 40 min of the procedure blood flow was gradually increased up to a target perfusate flow of 40% of the estimated cardiac output, and temperature of the perfusate gradually increased from 25°C to a left atrium target temperature of 37°C. Once the lung outflow temperature exceeded 32°C, a gas mix of air and 5% CO2 was connected to the circuit oxygenator and mechanical ventilation was started. After 4 h from the start of the procedure, ventilator FiO2 was set at 1 and circuit oxygenator gas mix changed to N2/CO2. Twenty minutes later, measures were taken to evaluate lung suitability. At this time the decision was made to offer the lung to the recipient; EVLP continued during this time so that normothermic perfusion lasted a total of 320 min. Data are presented as mean ± standard deviation. LA temp, left atrium temperature (°C).
Vt, tidal volume (mL/kg donor weight); PAPm, mean pulmonary arterial pressure (mmHg); PVR, pulmonary vascular resistance (dine*s/cm5); PVR was calculated considering wedge pressure 2 mmHg, as measured at the end of the procedure in the pulmonary veins with a pressure probe; Pawm, mean airways pressure (cmH2O); Pawpeak, peak airways pressure (cmH2O); Cpldyn, dynamic lung compliance (mL/cmH2O); FiO2, fraction of inspired oxygen; PCO2 and PO2 IN, partial pressure of CO2 and O2 measured on a sample of perfusate taken from the pulmonary artery cannula (mmHg); PCO2 and PO2 OUT, partial pressure of CO2 and of O2 measured on a sample of perfusate taken from lung outflow (mmHg); PCO2 and PO2 OUTright/left, partial pressure of CO2 and of O2 measured on samples of perfusate taken from right/left pulmonary vein, respectively.
Table 3.
Timing of lung procurement
| Clinical events | ||
| CCA | 10:15 am | No flow, 0 h:0 min |
| ROSC | 10:38 | |
| CCA | 10:43 | |
| Diagnosis of death (hands off) | 11:00 | Low flow, 0 h:45 min |
| In situ preservation | ||
| RM + CPAP | 11:05 | |
| Confirmation of death (ECG, 20 min) | 11:25 | |
| Consent to donation | 01:25 pm | |
| Heparin + CPR | 01:33 | |
| RM + ventilation | 01:40 | |
| Surgery for procurement | 02:36 | |
| rTPA + 1st cooling | 03:48 | In situ preservation, 4 h:48 min |
| Ex vivo lung perfusion | ||
| Start of EVLP procedure | 08:16 | |
| 2nd Cooling | 02:26 am | EVLP, 6 h:10 min |
| Transplantation | ||
| Reperfusion 1st lung | 09:18 | Death to reperfusion, 22 h:18 min |
| Reperfusion 2nd lung | 12:34 | Death to reperfusion, 25 h:34 min |
CCA, cardiocirculatory arrest; ROSC, return of spontaneous circulation; RM, recruitment maneuver; CPAP, continuous positive end‐expiratory pressure; ECG, electrocardiogram; CPR, cardiopulmonary resuscitation, rTPA, recombinant tissue plasminogen activator; EVLP, ex vivo lung perfusion.
Discussion
The present case report confirms that lung procurement from uDCDD is feasible. The protocol implemented allowed procurement of lungs even after an extended period of warm ischemia.
Preclinical investigations show that lungs may be preserved in the non‐heart‐beating donor with lung inflation and ventilation 13, 15. Lungs are anatomically open to air and can receive oxygen through diffusion. Consequently, they better tolerate the absence of blood. Moreover, as many as 60 min of total warm ischemia time is considered clinically safe according to UK criteria for DCDD organ procurement 7. Taking advantage of this background, and after preclinical investigations, we developed an in situ preservation strategy to procure lungs from uDCDD. The procedure consisted of lung recruitment maneuvers, CPAP, and protective mechanical ventilation.
Lung recruitment maneuvers are of crucial importance to fully open up the lung at the beginning of in situ preservation in order to facilitate oxygen diffusion to distal alveoli. Recruitment maneuvers, together with chest radiograph (Figure 2), can also provide important information on lung function at early stages of the donation process. As keeping the lung open over time is imperative, in our protocol CPAP is applied at the outset. This maintains the lung fully open during the 20‐min ECG recordings required by Italian legislation, time possibly needed in other countries to procure organs. Thereafter, low tidal volume–high PEEP ventilation is applied with a low respiratory rate to avoid the harm of hypocapnia 21. Using this strategy, in our donor respiratory mechanics were stable over 4 h (Figure 3).
Figure 3.
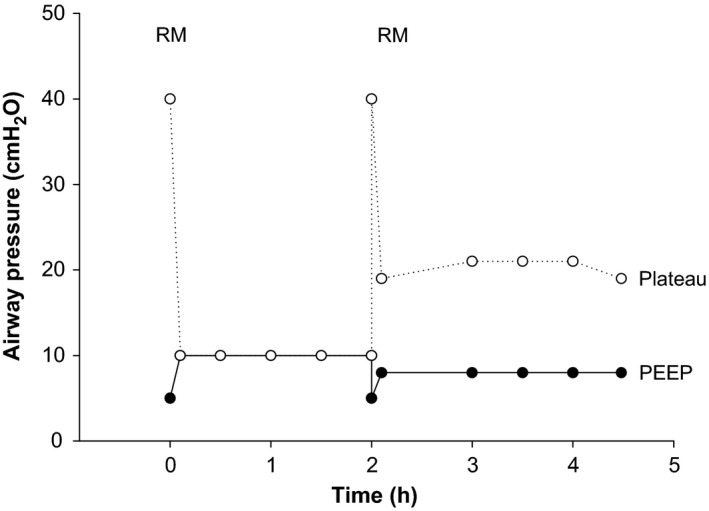
The figure shows airway pressure measured during the open‐lung preservation strategy. A first recruitment maneuver (RM, PEEP 5, I:E 1:1, RR 10, Pressure controlled + 25 ×2, PC + 30 ×2, PC + 35 ×4), was followed by continuous positive airway pressure (CPAP 10 cmH2O, 100% FiO2). Consent to donation was obtained 2 h later. Thereafter, a new recruitment maneuver was performed, and low frequency–low tidal volume–high PEEP ventilation started (respiratory rate: 4/min, tidal volume: 6 mL/kg, PEEP: 8 cm H2O, FiO2: 100%, inspiratory/expiratory ratio: 1:1). PEEP, positive end‐expiratory pressure.
The preservation strategy we adopted differs from that described by Steen et al. 22. Indeed, whereas they applied a technique of topical lung cooling via chest tubes, we used in situ preservation with lung recruitment maneuvers, CPAP, and mechanical ventilation between declaration of death and cold flush and storage. It is possible that topical cooling allows longer in situ preservation time 23, 24, relative to our strategy, aimed at gaining time for procurement. However, this case report indicates that lungs recovered from uDCDDs can be suitable for transplant after >4 h of total warm ischemic time, in line with results obtained from preclinical investigations 15. The recipient's postoperative course, likely caused by massive blood loss that required intraoperative ECMO, might have also been related to the use of uDCDD lungs. However, the 6‐month clinical outcome proves the feasibility of this preservation strategy.
The validity of machine perfusion when solid organs are procured from uDCDD donors has been suggested 25, 26. Because of the impossibility to obtain PaO2/FiO2 for lung evaluation after cardiac arrest, we decided to include EVLP in our protocol as Steen mentions in his seminal article 22.
During EVLP, pulmonary vascular resistances were higher than in DBD donors, as previously shown 27. Assessment of vascular resistance is of great relevance when dealing with DCDD lungs to exclude clot formation after circulation has stopped. In our protocol, heparin was added only after consent to donation was obtained, but lungs were treated with rTPA before flushing with the preservation solution. In fact, fibrinolytic treatment improves the quality of DCDD when applied during EVLP 27. Importantly, at this time of the process, response of the lung to vasculature flushing is used to decide whether to proceed with EVLP or not (see Figure 1, procurement). Indeed, in a rat model the time to flush the lungs with a constant volume of preservation solution correlates with the development of lung edema 28.
This first “successful case” supports the validity of our protocol. However, while it allows the assumption that lung procurement is feasible even after an extended warm ischemia time, efficacy and safety remain to be more extensively proven. Nevertheless, our case may add to the discussion on the relevance of lungs procurement from uDCDD. In fact, the present protocol has a number of potential advantages. There are virtually no costs; indeed, most of the subjects are intubated at the time of death after CPR withdrawal, and only a ventilator is needed to preserve the lungs. Procedures are not invasive: at the time of donor's death, relatives face an intact body, apart from endotracheal intubation. Clearly, impossibility to procure organs other than the lungs is a major weakness of the present protocol. However, tissues may be procured. Moreover, as the lung preservation strategy is simple, if proven safe and efficacious, many emergency rooms that do not have the possibility of setting up ECMO technology might be actively involved in lung and tissue procurement in a hub‐and‐spoke model as the one we have described.
As recently pointed out by Egan and Reqaurd, a number of ethical issues come with uDCDD programs 19. A clear separation of treatment from procurement is one of these. For this reason, the regional (AREU 118) and local (San Gerardo Hospital) emergency teams treated the subject until death diagnosis. Thereafter, a separate team of neuro‐intensivists (San Gerardo Hospital), eager in the process of donation, took the responsibility upon the arrival of the procurement team (Fondazione Ca’ Granda). As in the protocol of Egan et al 19, we are committed to build a multidisciplinary team. Moreover, a continuous program of medical and paramedical staff education is active.
During the first 6 months of activity, there were two denials of consent out of five requests. In a situation such as sudden death, this can be expected, particularly if, as in Egan's protocol 19, witness to the cardiac arrest is not necessarily the next of kin. In this regard, the possibility to extend the time from death to organ consent offered by the in situ preservation strategy is of great interest.
Lung preservation procedures, including heparin, ventilation, and bronchoscopy, were all applied postmortem. Only blood was withdrawn just before hands‐off. This decision might be considered a weakness of our protocol, particularly in countries where, unlike Italy, there is a general education about this kind of donation. We elected to use this strategy to make this novel donation process easier to accept.
In conclusion, we have confirmed previous findings on the feasibility of lung donation from uDCDD. We also provided evidence that in situ lung preservation with recruitment maneuvers, CPAP, and protective ventilation followed by EVLP after procurement allows lung transplantation after extended periods of warm ischemia.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Acknowledgments
Authors are in debt to Angelica Perazzoli for her logistical support to the implementation of the EVLP program. Authors are also indebted to all surgeons, anesthesiologists, critical care physicians, pneumologists, nurses, and personnel involved at all levels in the lung transplantation program of the Fondazione Ca’ Granda. This study was funded by Fondazione IRCCS Ca’ Granda–Ospedale Maggiore Policlinico, Milano, Italy, and by Regione Lombardia.
Valenza F, Citerio G, Palleschi A, Vargiolu A, Safaee Fakhr B, Confalonieri A, Nosotti M, Gatti S, Ravasi S, Vesconi S, Pesenti A, Blasi F, Santambrogio L & Gattinoni L. >Successful Transplantation of Lungs From an Uncontrolled Donor After Circulatory Death Preserved In Situ by Alveolar Recruitment Maneuvers and Assessed by Ex Vivo Lung Perfusion. Am J Transplant 2016; 16: 1312–1318
References
- 1. Bellingham JM, Santhanakrishnan C, Neidlinger N, et al. Donation after cardiac death: A 29‐year experience. Surgery 2011; 150: 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudge C, Matesanz R, Delmonico FL, Chapman J. International practices of organ donation. Br J Anaesth 2012; 108(Suppl 1): i48–i55. [DOI] [PubMed] [Google Scholar]
- 3. Morrissey PE, Monaco AP. Donation after circulatory death: Current practices, ongoing challenges, and potential improvements. Transplantation 2014; 97: 258–264. [DOI] [PubMed] [Google Scholar]
- 4. Kootstra G. Statement on non‐heart‐beating donor programs. Transpl Proc 1995; 27: 2965. [PubMed] [Google Scholar]
- 5. Detry O, Le Dinh H, Noterdaeme T, et al. Categories of donation after cardiocirculatory death. Transpl Proc 2012; 44: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 6. Report of the Madrid Consultation: Part 1: European and universal challenges in organ donation and transplantation, searching for global solutions. Transplantation 2011; 91(Suppl 11): S39–S66. [DOI] [PubMed] [Google Scholar]
- 7. Manara AR, Murphy PG, O'Callaghan G. Donation after circulatory death. Br J Anaesth 2012; 108(Suppl 1): i108–i121. [DOI] [PubMed] [Google Scholar]
- 8. Bendorf A, Kelly PJ, Kerridge IH, et al. An international comparison of the effect of policy shifts to organ donation following cardiocirculatory death (DCD) on donation rates after brain death (DBD) and transplantation rates. PLoS One 2013; 8: e62010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wall SP, Plunkett C, Caplan A. A potential solution to the shortage of solid organs for transplantation. JAMA 2015; 313: 2321–2322. [DOI] [PubMed] [Google Scholar]
- 10. Bernat JL, Bleck TP, Blosser SA, et al. Circulatory death determination in uncontrolled organ donors: A panel viewpoint. Ann Emerg Med 2014; 63: 384–390. [DOI] [PubMed] [Google Scholar]
- 11. Dominguez‐Gil B, Haase‐Kromwijk B, Van Leiden H, et al. Current situation of donation after circulatory death in European countries. Transpl Int 2011; 24: 676–686. [DOI] [PubMed] [Google Scholar]
- 12. Ulicny KS Jr, Egan TM, Lambert CJ Jr, Reddick RL, Wilcox BR. Cadaver lung donors: Effect of preharvest ventilation on graft function. Ann Thorac Surg 1993; 55: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 13. Van Raemdonck DE, Jannis NC, Rega FR, De Leyn PR, Flameng WJ, Lerut TE. Extended preservation of ischemic pulmonary graft by postmortem alveolar expansion. Ann Thorac Surg 1997; 64: 801–808. [DOI] [PubMed] [Google Scholar]
- 14. Greco R, Cordovilla G, Sanz E, et al. Warm ischemic time tolerance after ventilated non‐heart‐beating lung donation in piglets. Eur J Cardiothorac Surg 1998; 14: 319–325. [DOI] [PubMed] [Google Scholar]
- 15. Sakamoto J, Chen F, Yamada T, et al. Effect of preprocurement ventilation on lungs donated after cardiac death in a canine lung transplantation model. Transplantation 2011; 92: 864–870. [DOI] [PubMed] [Google Scholar]
- 16. Egan TM, Lambert CJ Jr, Reddick R, Ulicny KS Jr, Keagy BA, Wilcox BR. A strategy to increase the donor pool: Use of cadaver lungs for transplantation. Ann Thorac Surg 1991; 52: 1113–1120; discussion 1120–1111. [DOI] [PubMed] [Google Scholar]
- 17. Gomez‐de‐Antonio D, Campo‐Canaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non‐heart‐beating donors revisited. J Heart Lung Transplant 2012; 31: 349–353. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki Y, Tiwari JL, Lee J, et al. Should we reconsider lung transplantation through uncontrolled donation after circulatory death? Am J Transplant 2014; 14: 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egan TM, Requard JJ 3rd. Uncontrolled donation after circulatory determination of death donors (uDCDDs) as a source of lungs for transplant. Am J Transplant 2015; 15: 2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valenza F, Rosso L, Coppola S, et al. Ex vivo lung perfusion to improve donor lung function and increase the number of organs available for transplantation. Transpl Int 2014; 27: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laffey JG, Tanaka M, Engelberts D, et al. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med 2000; 162: 2287–2294. [DOI] [PubMed] [Google Scholar]
- 22. Steen S, Sjoberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non‐heart‐beating donor. Lancet 2001; 357: 825–829. [DOI] [PubMed] [Google Scholar]
- 23. Steen S, Sjoberg T, Ingemansson R, Lindberg L. Efficacy of topical cooling in lung preservation: Is a reappraisal due? Ann Thorac Surg 1994; 58: 1657–1663. [DOI] [PubMed] [Google Scholar]
- 24. Rega FR, Jannis NC, Verleden GM, Flameng WJ, Lerut TE, Van Raemdonck DE. Should we ventilate or cool the pulmonary graft inside the non‐heart‐beating donor? J Heart Lung Transplant 2003; 22: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 25. Cypel M, Yeung JC, Keshavjee S. Novel approaches to expanding the lung donor pool: Donation after cardiac death and ex vivo conditioning. Clin Chest Med 2011; 32: 233–244. [DOI] [PubMed] [Google Scholar]
- 26. Nakajima D, Chen F, Yamada T, et al. Reconditioning of lungs donated after circulatory death with normothermic ex vivo lung perfusion. J Heart Lung Transplant 2012; 31: 187–193. [DOI] [PubMed] [Google Scholar]
- 27. Inci I, Yamada Y, Hillinger S, Jungraithmayr W, Trinkwitz M, Weder W. Successful lung transplantation after donor lung reconditioning with urokinase in ex vivo lung perfusion system. Ann Thorac Surg 2014; 98: 1837–1838. [DOI] [PubMed] [Google Scholar]
- 28. Valenza F, Ruggieri GM, Froio S, Coppola S, Gattinoni L. Old and new strategies to preserve the lung before transplantation. Organs Tissues Cells 2012; 15: 109–114. [Google Scholar]


