Abstract
Redox‐regulated reversible phosphorylation of the light‐harvesting complex II (LHCII) controls the excitation energy distribution between photosystem (PS) II and PSI. The PsaL and PsaH subunits of PSI enable the association of pLHCII to PSI. Here, we show that the failure of the psal mutant to dock pLHCII to PSI induces excessive phosphorylation of LHCII, primarily due to a marked downregulation of the TAP38/PPH1 phosphatase occurring at post‐transcriptional level. TAP38/PPH1 is shown to be associated with megacomplex that contains both photosystems in a light‐ and LHCII‐PSII core‐phosphorylation‐dependent manner. It is suggested that proper megacomplex‐related association of TAP38/PPH1 protects it against degradation.
Keywords: light‐harvesting complex II, phosphorylation, photosystem I, TAP38/PPH1
Abbreviations
LHCII, light‐harvesting complex II
PS, photosystem
PQ, plastoquinone
pLHCII, phosphorylated LHCII
PVDF, polyvinylidene difluoride
CBB, coomassie brilliant blue
β‐DM, β‐dodecyl maltoside
ETC, electron transfer chain
In higher plants, the chloroplast‐located pigment‐pro tein complexes photosystem (PS) I and PSII with their light‐harvesting complex (LHC) antennas work in series to convert solar energy into chemical energy. In photosynthetic light reactions, PSII uses the harvested solar energy to reduce the intersystem electron transfer chain (ETC), which is composed of plastoquinone (PQ), cytochrome b6f, and plastocyanin. ETC becomes oxidized by the PSI complex that eventually reduces NADP+ to NADPH. The two PSs are energetically connected via the LHCII trimers 1, 2, 3 that host the majority of the light‐absorbing chlorophyll pigments.
Distribution of excitation energy between PSII and PSI is regulated through a reversible phosphorylation of the major LHCII proteins LHCB1 and LHCB2. The phosphorylation of LHCII is regulated by the STN7 kinase 4 and the counteracting TAP38/PPH1 phosphatase (hereafter TAP38) 5, 6. The activity and abundance of STN7 is dependent on the redox‐state of ETC. 4, 7, whereas TAP38 phosphatase has been suggested to be constitutively expressed and active, and thus redox‐independent 5. Phosphorylated LHCII (pLHCII) interacts with PSI from the site opposite to PSI antenna, where at least the PsaH and PsaL subunits are crucial for energy transfer from pLHCII to PSI reaction center 8, 9. The position of PsaL in the PSI complex is almost identical in plants and cyanobacteria, however, in plants the membrane‐exposed parts of the protein are almost completely covered by PsaH 9, 10, 11. This orientation of the subunits likely explains the reported 90% secondary loss of the PsaH subunit in the psal mutant 8 and thus the psal mutant can be considered to represent a complete LHCII docking site mutant. Deficiency in PsaL and PsaH results in disability to dock pLHCII to the PSI complex, despite the excessive phosphorylation of LHCII 8, 12, 13. Here, we addressed the regulation of the (de)phosphorylation of LHCII in the psal mutant that lacks the pLHCII docking site.
Materials and methods
Plant material and growth
Arabidopsis thaliana ecotype Columbia wild‐type (WT) and the stn7 4, tap38 5, 6, and psal 8 mutants were grown under a photon flux density of 120 μmol·m−2·s−1 in an 8 h light/16 h dark regime at 23 °C and in a relative humidity of 70%. OSRAM PowerStar HQIT 400/D Metal Halide Lamps served as a light source.
Light treatments, isolation of thylakoid membrane, and total leaf extract
Five‐week‐old plants were kept in darkness for 16 h and subsequently shifted to low light of 20 μmol photons·m−2·s−1 for 2.5 h and finally to high light of 1000 μmol·m−2·s−1 for another 2.5 h. Thylakoid membranes were isolated immediately after each light treatment from fresh leaves with buffers supplemented with 10 mm sodium fluoride 14. For isolation of the total leaf extract, leaves were homogenized in liquid nitrogen with extraction buffer (100 mm Tris–HCl pH 8.0; 50 mm EDTA pH 8.0; 0.25 m NaCl; 0.75% SDS; 1 mm DTT). The extract was incubated for 10 min at +68 °C, followed by centrifugation at 12 000 g for 10 min. Chlorophyll concentration was determined from the supernatant according to 15.
Protein separation with SDS/PAGE
Thylakoid membrane proteins were solubilized with Laemmli buffer supplemented with 10% β‐mercaptoethanol and run over night under constant current of 5.5 mA with SDS/PAGE containing 12% (w/v) polyacrylamide and 6 m urea. The gels were loaded on equal chlorophyll basis. The proteins were electroblotted to polyvinylidene difluoride (PVDF) membranes and immunoprobed with antiphosphothreonine (P‐Thr) (New England Biolabs), anti‐STN7 (Agrisera, catalog number AS10 1611), and anti‐TAP38 (gift from Prof. Roberto Barbato) antibodies. Horseradish peroxidase‐linked secondary antibody and enhanced chemiluminescence reagents (Amersham, GE Healthcare) were used for detection. The membranes were finally stained with Coomassie Brilliant Blue (CBB). Three biological replicates were used in all experiments. The changes of the pLHCII content within each genotype under different light conditions were quantified from three biological replicates with GeneTools software (PerkinElmer).
Native‐PAGE
Solubilization of the thylakoid membranes with 1% digitonin and 1% β‐dodecyl maltoside (β‐DM) was performed as described in 16. The protein complexes were separated with large pore blue native PAGE (lpBN) and the distinct subunits were resolved with two‐dimensional (2D) SDS/PAGE according to 14.
Real‐Time PCR
Total RNA extraction (Plant RNA Isolation Kit; Agilent Technologies, Santa Clara, CA, USA), DNase treatment (Turbo DNA‐free kit™; Ambion, Applied Biosystems, Austin, TX, USA), cDNA synthesis (iScript; Bio‐Rad Laboratories, Hercules, CA, USA), and RT‐PCR reactions (IQ SYBR Green Supermix; Bio‐Rad, Hercules, CA, USA) were performed as described in 17. One microgram of total RNA was converted to cDNA. For TAP38 amplification, TAP38_for primer (5′CCG CAT CTT CGC TTT CA‐3′), and TAP38_rev primer (5′GTG TAA CCC CAA CGA ATC GG‐3′) were employed. For internal controls UBIQUITIN 3_for primer (5′‐TGGTTCGTGTCTCATGCACT‐3′ and rev_5′‐TACAAAGGCCCGTTACAAGC‐3′) and PP2AA3 (for_5′‐GCGGTTGTGGAGAACATGATACG‐3′ and rev_5′‐GAACCAAACACAATTCGTTGCTG‐3′) primer pairs were used. Three technical replicates from each of the four biological replicates were applied on each reaction. Data were analyzed by using qbase plus software (Biogazelle NV, http://www.biogazelle.com).
Results and Discussion
LHCII hyperphosphorylation in the psal mutant is primarily maintained via downregulation of the TAP38 phosphatase
It has been previously shown that LHCII is hyperphosphorylated in the psal mutant that lacks the subunits required for pLHCII docking to PSI 12, 13. Comparison of the LHCII phosphorylation between WT, stn7, tap38, and psal under growth light conditions indeed revealed hyperphosphorylation of LHCII in psal, the amount of pLHCII being more than two times higher as compared to the level observed in tap38 (Fig. 1A). Next, the mechanism behind the LHCII hyperphosphorylation in psal was addressed by determining the amounts of the STN7 kinase and the TAP38 phosphatase from the same samples used above for analysis of thylakoid protein phosphorylation. In addition, the total leaf extracts were analyzed due to the fact that TAP38 has been shown to reside both in thylakoids and in soluble fractions 6. The most intriguing result was that the TAP38 phosphatase was practically missing from psal, both from the thylakoids (Fig. 1B) and the total leaf extract (Fig. S1), whereas in stn7 thylakoids the level was similar to that in WT (Fig. 1B).
Figure 1.
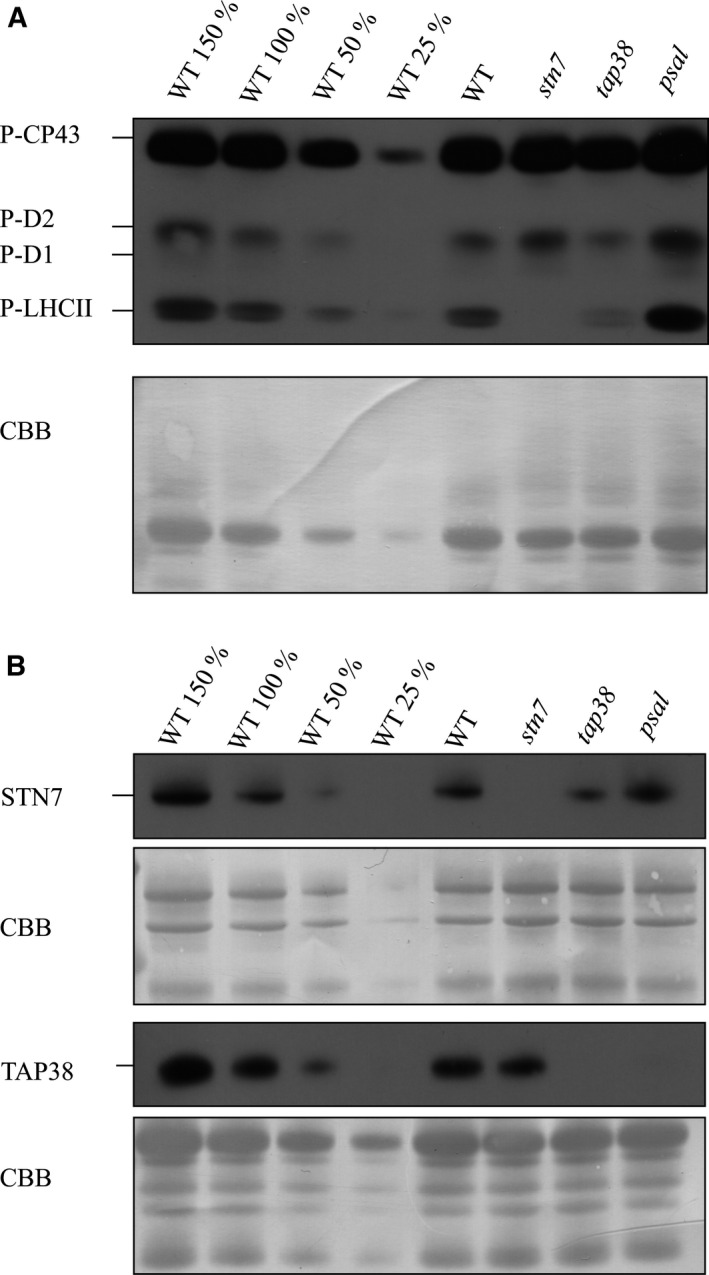
Immunoblots demonstrating the phosphorylation state of LHCII and the accumulation of the STN7 kinase and TAP38 phosphatase in different genetic backgrounds under growth light conditions. The thylakoid proteins from WT and stn7, tap38, and psal mutants were isolated and subsequently fractionated with SDS PAGE. (A) The abundance of pLHCII was elucidated with P‐Thr‐specific antibody. (B) The abundance of the STN7 kinase as well as that of the TAP38 phosphatase was determined with specific antibodies. All the membranes were stained with Coomassie (CBB) to demonstrate equal loading.
The psal mutant not only downregulated the TAP38 phosphatase but concomitantly upregulated the STN7 kinase which, in turn, was downregulated in the tap38 mutant as compared to WT (Fig. 1B). Increase in the content of STN7 in the psal mutant has been observed earlier 7, 8 and was suggested to result from a more reduced PQ pool as compared to WT. In WT a hyperphosphorylation of LHCII is not observed, apparently due to a stable presence of the TAP38 phosphatase. Therefore, despite the modestly upregulated STN7 in psal, we conclude that the downregulation of the TAP38 phosphatase primarily ensures the maintenance of LHCII hyperphosphorylation in the psal mutant. Indeed, TAP38 seems to be regulated solely at a dose‐dependent manner while the regulation of STN7 activity involves a number of different mechanisms, for example, the N‐terminal cysteine motif reduction 18, the stromal thioredoxin‐f 19, and phosphorylation of the kinase itself 20. The questions remain of why and how the psal mutant downregulates the amount of the TAP38 phosphatase.
Strong downregulation of the TAP38 protein and consequent hyperphosphorylation of LHCII in psal most conceivably represent an appropriate compensatory mechanism for deficient formation of the PSI‐LHCII complexes. Indeed, even though psal is not capable of binding pLHCII to PSI, the capacity for ‘state transitions’ has yet been reported to be 30–40% of that in WT 8. It was recently suggested that besides the PSI‐LHCII complex, an additional route for excitation energy transfer between LHCII and PSI exists, presumably involving LHCI 3. High level of pLHCII, typical to the psal mutant, possibly maintains this LHCI‐mediated route, thus explaining the remaining capacity for ‘state transitions’.
TAP38 deficiency in the psal mutant is regulated mainly at post‐transcriptional level
To elucidate the mechanism(s) for TAP38 downregulation in psal, the relative TAP38 transcript abundancy from the WT, psal, tap38, and stn7 plants, grown under moderate light conditions, were evaluated with quantitative real‐time PCR (qRT‐PCR) (Fig. 2). As expected, the TAP38 transcripts showed a 40‐fold downregulation in the tap38 mutant. In the psal mutant, however, the TAP38 transcript level was only marginally lower as compared to WT and stn7. Thus, the regulation at transcript level is not involved in the nearly complete absence of the TAP38 protein in the psal mutant (Figs 1B and 2). This implies that the downregulation of TAP38 in the psal mutant virtually takes place at the post‐transcriptional level involving, for example, inefficient translation of the mRNA or high degradation rate of the protein.
Figure 2.
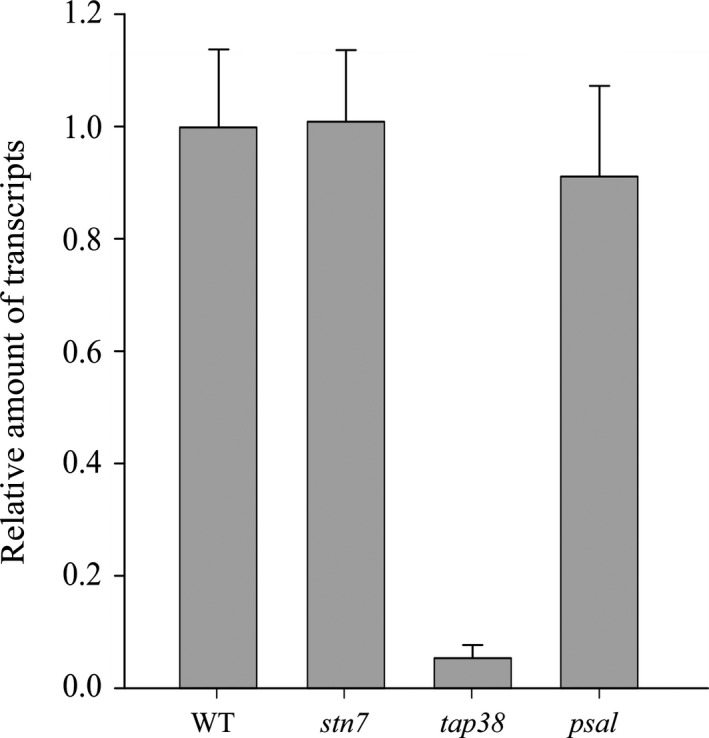
The TAP38 transcript level in the stn7, tap38, and psal mutants as compared to WT. The columns denote the relative amount of the TAP38 transcript under growth light conditions. Data are normalized to the expression of reference genes PP2A3 and UBQ3 and studied by quantitative RT‐PCR. 1 denotes no change in transcript level, < 1 denotes decreased level of transcripts and > 1 denotes increased levels of transcripts as compared to WT.
Despite the lack of TAP38, the psal mutant is capable of LHCII dephosphorylation under high light
Dramatic downregulation of the TAP38 phosphatase in the psal mutant under growth light conditions (Fig. 1B) raised a question whether the psal mutant is capable of expressing the TAP38 phosphatase under conditions that in WT induce LHCII dephosphorylation, that is, under darkness and upon exposure to high intensity of white light 16. To this end, WT and the stn7, tap38, and psal mutants were first acclimated to darkness for 16 h, then transferred to low light for 2.5 h and subsequently to high light for another 2.5 h. The thylakoids were isolated after each light treatment, followed by determination of the pLHCII, STN7, and TAP38 quantities.
Changes in the accumulation of pLHCII upon short‐term light shifts were determined first (Fig. 3A). In WT, LHCII was almost completely dephosphorylated after 16 h of darkness, whereas a strong phosphorylation was induced upon shift to low light. Finally, shift to high light resulted in an almost complete dephosphorylation of LHCII (Fig. 3A and Table S1). As expected, LHCII remained dephosphorylated in stn7 under all light conditions. In contrast, tap38 showed unexpected variation in LHCII phosphorylation during the light treatments: in comparison to darkness, its LHCII was found to become dephosphorylated upon shifts to low and subsequently to high light (Fig. 3A and Table S1). The psal mutant showed higher LHCII phosphorylation as compared to tap38 under all light conditions. However, similar yet more extensively to tap38, the phosphorylation of LHCII in psal markedly decreased upon shift from low light to high light (Fig. 3A and Table S1). Subsequent analysis of the abundances of STN7 and TAP38 (Fig. 3B) demonstrated that the amount of the STN7 kinase remained stable within each genotype regardless of the light condition, with STN7 being again slightly more abundant in psal and less abundant in tap38 as compared to WT. Most importantly, it was shown that even though the psal mutant was unable to accumulate the TAP38 phosphatase under any light conditions, a strong LHCII dephosphorylation was evident upon shift from low to high light (Fig. 3A,B, Table S1).
Figure 3.
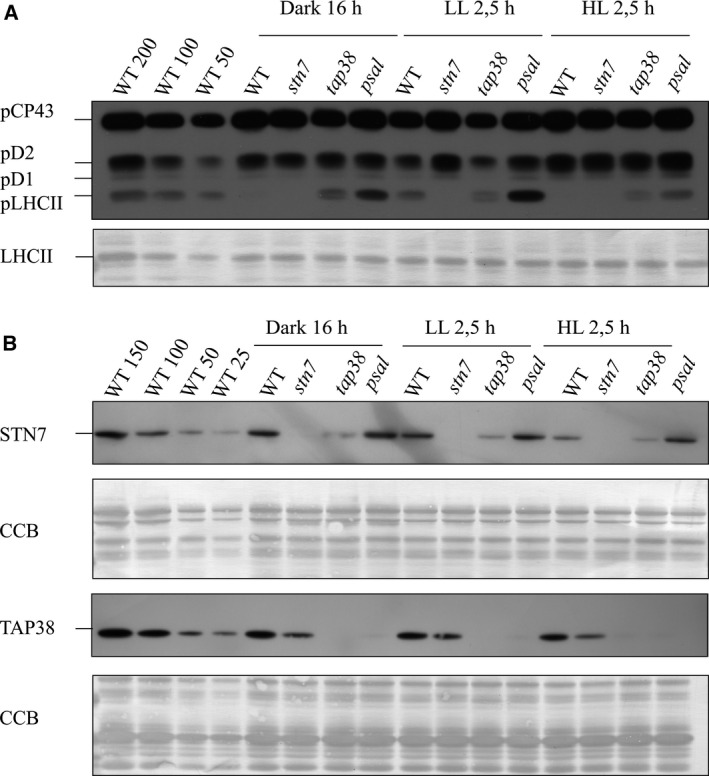
Immunoblots demonstrating the phosphorylation state of LHCII and the accumulation of the STN7 kinase and the TAP38 phosphatase in different genetic backgrounds upon changes in light intensity. WT and the stn7, tap38 and psal mutants were adapted to darkness for 16 h and subsequently exposed to low light for 2.5 h and eventually to high light for another 2.5 h. The thylakoids were isolated after each light treatment, and fractionated with SDS/PAGE. (A) The phosphorylated thylakoid proteins after each light treatment were detected with P‐Thr‐specific antibody. The membrane was subsequently stained with Coomassie (CBB) to demonstrate the unaltered amount of LHCII. (B) The abundances of the STN7 kinase and the TAP38 phosphatase were detected with specific antibodies. All the membranes were stained with CBB to demonstrate equal loading.
Dephosphorylation of pLHCII in psal, virtually devoid of TAP38 phosphatase, implies that the phosphorylation level of LHCII, shown to be essential for optimal growth and fitness of plants under natural light conditions 21, 22, 23, 24, is regulated in a more complex manner than previously anticipated. Even though the roles of the STN7 kinase and TAP38 phosphatase cannot be overlooked, it is evident that the final LHCII phosphorylation status is dependent also on additional factors/mechanisms, some of which remain currently uncharacterized. Particularly the dephosphorylation of LHCII upon a shift of plants to high light, not only in WT but also in psal and to a lesser extent in tap38 both lacking the only LHCII phosphatase known so far, strongly indicates that (an)other currently unidentified phosphatase(s) participate/s in LHCII dephosphorylation under high light intensities, as was suggested also previously 25, 26. It has been shown that the PHOTOSYSTEM II CORE PHOSPHATASE (PBCP), responsible for dephosphorylation of the PSII core phosphoproteins, partially loses its substrate specificity, and thus has an overlapping activity with TAP38 when overexpressed 27. WT and the psal mutant, however, did not reveal any significant difference in the phosphorylation level of the PSII core proteins, PBCP likely is not overexpressed in psal and thus cannot explain the dephosphorylation of LHCII.
TAP38 association with PSI‐PSII megacomplex – possible implications on protein stability
Next we addressed the possible association of TAP38 with thylakoid membrane protein complexes. Previous localization studies have shown that aside from small soluble fraction the TAP38 phosphatase, it is mainly present in the thylakoid membrane with the membrane‐spanning region near to the C‐terminus 5, 28. Nonetheless, the possible association of TAP38 with any specific thylakoid membrane protein complex(es) has remained obscure. In particular, since psal is not capable of forming the ‘state‐transition’‐specific protein complex PSI‐LHCII 24, it can be speculated that the deficiency of TAP38 in psal (Fig. 1B) stems from a high degradation rate of TAP38 due to the disability to accumulate the PSI‐LHCII‐complex, a logical associate partner for TAP38.
To monitor the association of TAP38 with thylakoid membrane complexes, thylakoids were isolated from WT plants exposed to low and subsequently to high light intensity. Thylakoids were solubilized with mild anionic detergents, β‐DM and digitonin, separated with lpBN‐PAGE, and finally resolved with 2D‐SDS/PAGE. Immunodetection of TAP38 in the 2‐SDS/PAGE gel from β‐DM solubilized thylakoids, using the TAP38 protein‐specific antibody, revealed a major part of TAP38 in the free‐protein fraction (Fig. 4A), which contradicts the location of STN7 shown to be almost exclusively present in high molecular weight (HMW) complexes 18, 19. Interestingly, when the thylakoids were solubilized with digitonin, which is superior to β‐DM in preservation of the weak protein–protein interactions 14, 16, a fraction of TAP38 was found to be associated with the largest thylakoid membrane protein megacomplexes. (Fig. 4B). In this region, particularly the largest protein megacomplex (Mc1) has been shown to be composed of both PSII and PSI 14, 16 and to allow energy transfer between PSII and PSI 1.
Figure 4.
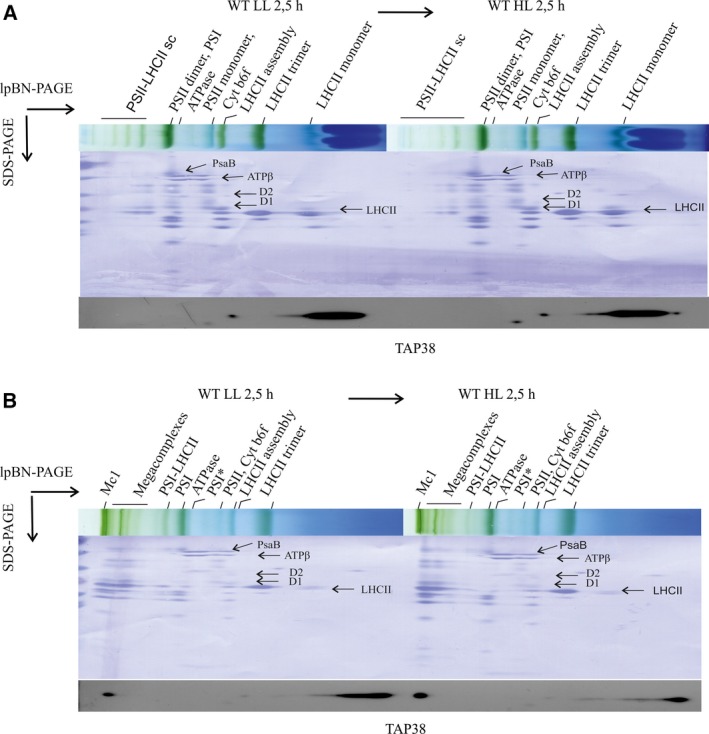
Localization of the TAP38 phosphatase within the thylakoid membrane after exposure of WT plants to short‐time light treatments. Thylakoid membrane protein complexes were isolated from fresh leaves after 2.5 h treatment with low light and after following 2.5 h exposure to high light. The protein complexes were solubilized with either (A) 1% β‐DM or (B) 1% digitonin, and subsequently separated with lpBN‐PAGE. The distinct subunits of each complex were fractionated with SDS/PAGE. TAP38‐specific antibody was used to localize the protein. Finally, the membranes were stained with Coomassie (CBB) to allow localization of the protein complexes. Protein subunit identification was based on 16, 29.
The association of TAP38 with the megacomplexes, analyzed from low light‐treated plants and from plants subsequently shifted to high light, was found to be distinctively dependent on the light condition (Fig. 4B). The relative amount of TAP38 associated with the megacomplexes significantly increased upon a shift of plants from low to high light, while the relative amount of TAP38 in the free‐protein fraction concomitantly decreased (Fig. 4B). Thus, even though TAP38 has been shown to be constitutively expressed in WT under all light conditions 5, it seems conceivable that regulation of LHCII dephosphorylation involves dynamic localization of the TAP38 phosphatase to the megacomplexes. Particularly the Mc1, that is, the complex harboring the physical and energetic connection between PSs, is likely to represent the prevailing in vivo situation in grana margins 1, 2, 3, 16 and probably changes in the amount according to environmental and metabolic cues. Considering that Mc1 stands for the megacomplex that regulates energy transfer between PSII and PSI, the amount of pLHCII in Mc1 must be under fast and efficient control. Thus, association of TAP38 with Mc1, particularly under high light that induces LHCII dephosphorylation (Figs 3A, 4), ensures optimal energy transfer and ‘spillover’ in thylakoids. Furthermore, it is proposed that the association of TAP38 with the megacomplex Mc1 protects the phosphatase from degradation. Since the amount of Mc1 in psal is markedly reduced as compared to WT 13, it can be speculated that the deficiency of TAP38 results from a low amount of the assembly partner, the situation in psal which could make TAP38 prone to degradation.
Conclusion
Taken together, it is concluded that the hyperphosphorylation of LHCII in the psal mutant that lacks the LHCII docking site in PSI, is essentially a consequence of a strong downregulation of the TAP38 phosphatase at post‐transcriptional level. Thylakoid fractionation showed a light‐dependent association of TAP38 with a megacomplex Mc1 that consists of both photosystems with their light‐harvesting antennas. Comparison of the WT and psal mutant revealed the scarcity of thylakoid Mc1 megacomplex together with the TAP38 phosphatase in psal. It is suggested that the light‐dependent modifications in the association of TAP38 with the Mc1 complex are involved in the variety of mechanisms that control the maintenance of balanced energy distribution between the two PSs.
Author Contributions
MR and MS designed the experiments, MR and NL performed the experiments, and MR, E‐MA and MS wrote the manuscript.
Supporting information
Fig. S1. Immunoblot demonstrating the accumulation of the TAP38 protein in the total leaf extract from WT and the psal mutant.
Table S1. The changes in the level of LHCII phosphorylation within WT, tap38, and psal upon short‐term light shifts. The pLHCII level is normalized to the pLHCII level observed in LL in each genotype.
Acknowledgements
Research was financially supported by the Academy of Finland (project numbers 272424, 271832 and 273870), TEKES LIF 40128/14, DPMLS, the Initial Marie Curie Training Networks (ITN) CALIPSO (607607), and PHOTOCOMM (317184). We thank Professor Dario Leister for providing the tap38 mutant seeds and Professor Jean‐David Rochaix for the stn7 mutant seeds. Professor Roberto Barbato is thanked for the TAP38 antibody and Dr Mikko Tikkanen for scientific discussions. We also thank Virpi Paakkarinen, Kurt Ståhle, Saara Mikola, and Ville Käpylä for their excellent technical assistance.
Edited by Ulf‐Ingo Flüugge
References
- 1. Yokono M, Takabayashi A, Akimoto S and Tanaka A (2015) A megacomplex composed of both photosystem reaction centres in higher plants. Nat Commun 6, 6675. [DOI] [PubMed] [Google Scholar]
- 2. Grieco M, Suorsa M, Jajoo A, Tikkanen M and Aro EM (2015) Light‐harvesting II antenna trimers connect energetically the entire photosynthetic machinery ‐ including both photosystems II and I. Biochim Biophys Acta 1847, 607–619. [DOI] [PubMed] [Google Scholar]
- 3. Benson SL, Maheswaran P, Ware MA, Hunter CN, Horton P, Jansson S, Ruban AV and Johnson MP (2015) An intact light harvesting complex I antenna system is required for complete state transitions in Arabidopsis. Nat Plants 1, 15176. [DOI] [PubMed] [Google Scholar]
- 4. Bellafiore S, Barneche F, Peltier G and Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895. [DOI] [PubMed] [Google Scholar]
- 5. Pribil M, Pesaresi P, Hertle A, Barbato R and Leister D (2010) Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol 8, e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shapiguzov A, Ingelsson B, Samol I, Andres C, Kessler F, Rochaix JD, Vener AV and Goldschmidt‐Clermont M (2010) The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc Natl Acad Sci USA 107, 4782–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wunder T, Liu Q, Aseeva E, Bonardi V, Leister D and Pribil M (2013) Control of STN7 transcript abundance and transient STN7 dimerisation are involved in the regulation of STN7 activity. Planta 237, 541–558. [DOI] [PubMed] [Google Scholar]
- 8. Lunde C, Jensen PE, Haldrup A, Knoetzel J and Scheller HV (2000) The PSI‐H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408, 613–615. [DOI] [PubMed] [Google Scholar]
- 9. Mazor Y, Borovikova A and Nelson N (2015) The structure of plant photosystem I super‐complex at 2.8 A resolution. Elife 4, e07433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ben‐Shem A, Frolow F and Nelson N (2004) Evolution of photosystem I ‐ from symmetry through pseudo‐symmetry to asymmetry. FEBS Lett 564, 274–280. [DOI] [PubMed] [Google Scholar]
- 11. Jordan P, Fromme P, Witt HT, Klukas O, Saenger W and Krauss N (2001) Three‐dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 411, 909–917. [DOI] [PubMed] [Google Scholar]
- 12. Haldrup A, Jensen PE, Lunde C and Scheller HV (2001) Balance of power: a view of the mechanism of photosynthetic state transitions. Trends Plant Sci 6, 301–305. [DOI] [PubMed] [Google Scholar]
- 13. Leoni C, Pietrzykowska M, Kiss AZ, Suorsa M, Ceci LR, Aro EM and Jansson S (2013) Very rapid phosphorylation kinetics suggest a unique role for Lhcb2 during state transitions in Arabidopsis. Plant J 76, 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarvi S, Suorsa M, Paakkarinen V and Aro EM (2011) Optimized native gel systems for separation of thylakoid protein complexes: novel super‐ and mega‐complexes. Biochem J 439, 207–214. [DOI] [PubMed] [Google Scholar]
- 15. Porra RJ, Thompson WA and Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975, 384–394. [Google Scholar]
- 16. Suorsa M, Rantala M, Mamedov F, Lespinasse M, Trotta A, Grieco M, Vuorio E, Tikkanen M, Jarvi S and Aro EM (2015) Light acclimation involves dynamic re‐organization of the pigment‐protein megacomplexes in non‐appressed thylakoid domains. Plant J 84, 360–373. [DOI] [PubMed] [Google Scholar]
- 17. Lintala M, Allahverdiyeva Y, Kangasjarvi S, Lehtimaki N, Keranen M, Rintamaki E, Aro EM and Mulo P (2009) Comparative analysis of leaf‐type ferredoxin‐NADP oxidoreductase isoforms in Arabidopsis thaliana . Plant J 57, 1103–1115. [DOI] [PubMed] [Google Scholar]
- 18. Lemeille S, Willig A, Depege‐Fargeix N, Delessert C, Bassi R and Rochaix JD (2009) Analysis of the chloroplast protein kinase Stt7 during state transitions. PLoS Biol 7, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wunder T, Xu W, Liu Q, Wanner G, Leister D and Pribil M (2013) The major thylakoid protein kinases STN7 and STN8 revisited: effects of altered STN8 levels and regulatory specificities of the STN kinases. Front Plant Sci 4, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willig A, Shapiguzov A, Goldschmidt‐Clermont M and Rochaix JD (2011) The phosphorylation status of the chloroplast protein kinase STN7 of Arabidopsis affects its turnover. Plant Physiol 157, 2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grieco M, Tikkanen M, Paakkarinen V, Kangasjarvi S and Aro EM (2012) Steady‐state phosphorylation of light‐harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol 160, 1896–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frenkel M, Bellafiore S, Rochaix J and Jansson S (2007) Hierarchy amongst photosynthetic acclimation responses for plant fitness. Physiol Plant 129, 455–459. [Google Scholar]
- 23. Tikkanen M, Grieco M and Aro EM (2011) Novel insights into plant light‐harvesting complex II phosphorylation and ‘state transitions’. Trends Plant Sci 16, 126–131. [DOI] [PubMed] [Google Scholar]
- 24. Pesaresi P, Hertle A, Pribil M, Kleine T, Wagner R, Strissel H, Ihnatowicz A, Bonardi V, Scharfenberg M, Schneider A et al (2009) Arabidopsis STN7 kinase provides a link between short‐ and long‐term photosynthetic acclimation. Plant Cell 21, 2402–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Puthiyaveetil S, Woodiwiss T, Knoerdel R, Zia A, Wood M, Hoehner R and Kirchhoff H (2014) Significance of the photosystem II core phosphatase PBCP for plant viability and protein repair in thylakoid membranes. Plant Cell Physiol 55, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mekala NR, Suorsa M, Rantala M, Aro EM and Tikkanen M (2015) Plants actively avoid state transitions upon changes in light intensity: role of light‐harvesting complex II protein dephosphorylation in high light. Plant Physiol 168, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samol I, Shapiguzov A, Ingelsson B, Fucile G, Crevecoeur M, Vener AV, Rochaix JD and Goldschmidt‐Clermont M (2012) Identification of a photosystem II phosphatase involved in light acclimation in Arabidopsis. Plant Cell 24, 2596–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei X, Guo J, Li M and Liu Z (2015) Structural mechanism underlying the specific recognition between the arabidopsis state‐transition phosphatase TAP38/PPH1 and phosphorylated light‐harvesting complex protein Lhcb1. Plant Cell 27, 1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aro EM, Suorsa M, Rokka A, Allahverdiyeva Y, Paakkarinen V, Saleem A, Battchikova N and Rintamaki E (2005) Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes. J Exp Bot 56, 347–356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Immunoblot demonstrating the accumulation of the TAP38 protein in the total leaf extract from WT and the psal mutant.
Table S1. The changes in the level of LHCII phosphorylation within WT, tap38, and psal upon short‐term light shifts. The pLHCII level is normalized to the pLHCII level observed in LL in each genotype.


