Summary
More than 50 interventions have been used to treat hidradenitis suppurativa (HS), and so therapy decisions can be challenging. Our objective was to summarize and appraise randomized controlled trial (RCT) evidence for HS interventions in adults. Searches were conducted in Medline, Embase, CENTRAL, LILACS, five trials registers and abstracts from eight dermatology conferences until 13 August 2015. Two review authors independently assessed study eligibility, extracted data and assessed methodological quality. Primary outcomes were quality of life and adverse effects of the interventions. Twelve trials, from 1983 to 2015, investigating 15 different interventions met our inclusion criteria. The median trial duration was 16 weeks and the median number of participants was 27. Adalimumab 40 mg weekly improved the Dermatology Life Quality Index (DLQI) by 4·0 points, which equates to the minimal clinically important difference for the scale, compared with placebo (95% confidence interval −6·5 to −1·5 points). Evidence quality was reduced to ‘moderate’ because the results are based on only a single study. Adalimumab 40 mg every other week was ineffective in a meta‐analysis of two studies comprising 124 participants. Infliximab 5 mg kg−1 improved the DLQI score by 8·4 points after 8 weeks in a moderate‐quality study completed by 33 of 38 participants. Etanercept 50 mg twice weekly was ineffective. Inclusion of a gentamicin sponge prior to primary closure did not improve outcomes. Other interventions, including topical and oral antibiotics, were investigated by relatively small studies, preventing treatment recommendations due to imprecision. More, larger RCTs are required to investigate most HS interventions, particularly oral treatments and surgical therapy. Moderate‐quality evidence suggests that adalimumab given weekly and infliximab are effective, whereas adalimumab every other week is ineffective.
Short abstract
What's already known about this topic?
Many interventions have been tried for hidradenitis suppurativa (HS).
Evidence supporting the choice of intervention for HS is often limited.
What does this study add?
Moderate‐quality evidence suggests that adalimumab given weekly and infliximab are effective, whereas adalimumab every other week is ineffective.
There are very limited or no randomized controlled trial data in HS for antibiotic therapy, retinoids, oral immunomodulators or the timing and type of surgery to perform.
Linked Comment: Blok. Br J Dermatol 2016; 174: 953–954
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic inflammatory skin disease characterized by painful nodules, sinuses and scarring in flexural locations.1 Treatment of HS can be challenging, and more than 50 interventions have been reported in the literature, often supported by only low‐quality evidence. As a result, it can be difficult for clinicians to make evidence‐based decisions in partnership with patients.
In 2011 a Cochrane review team was assembled to undertake a review of all medical and surgical interventions for HS, restricting the systematic search for trial data to only the highest‐quality evidence, in the form of randomized controlled trials (RCTs). The quality of the RCTs was also assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, incorporated into summary of findings tables.2 The GRADE system evaluates the trial data for a particular intervention in terms of each key outcome predetermined by the review team, and can be used to convert evidence quality and effect sizes into strengths of recommendation. The full review was recently published in the Cochrane Library and is summarized here.3
Methods
The protocol for our Cochrane review was prepublished in the Cochrane Library, and any deviations from the protocol are highlighted in the final published review.3 Our inclusion criteria to select relevant studies were RCTs of any HS intervention involving male and female adults of any age and ethnicity. Only the first phase of crossover trials was included to avoid carry‐over effects of interventions with a long duration of action. Primary and secondary outcomes are given in Table 1 and were selected following discussion between the clinicians and consumer author on the review team. In keeping with Cochrane guidelines,4 one primary outcome was selected to assess treatment benefit, namely quality of life, and one to assess potential harm, in this case the adverse effects of interventions. Adverse effects were defined as serious if they resulted in death, hospital admission or a longer hospital stay.
Table 1.
Primary and secondary outcomes of the review
| Primary outcomes | Secondary outcomes |
|---|---|
| 1. Quality of life measured on a validated dermatology‐specific scale | 1. Participant global self‐assessment |
| 2. Adverse effects | 2. Pain score |
| 3. Physician‐assessed lesion scoring system specific to hidradenitis suppurativa | |
| 4. Physician's Global Assessment | |
| 5. Duration of remission (number of days until the first new lesion or flare) |
Search strategies
Using the terms ‘acne inversa’, ‘hidradenitis suppurativa’, ‘velpeau’ or ‘verneuil’, we searched for RCTs in the following databases from inception until 13 August 2015: Cochrane Skin Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), Medline, Embase and LILACS. We also searched five trials registers, namely the metaRegister of Controlled Trials (http://www.isrctn.com/page/mrct), the US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov), the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au), the World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch) and the EU Clinical Trials Register (https://www.clinicaltrialsregister.eu). In addition, hand searching of the abstracts from eight international dermatology conferences was undertaken, and bibliographies from both included and excluded studies were examined. Two authors (J.R.I. and A.C.K.) independently undertook study selection and no language restrictions were applied.
Data extraction and analysis
Following piloting of our data extraction form, two pairs of authors (J.R.I. and either P.N.W., S.L.C. or A.D.O.) independently extracted data from the included studies and made an assessment of methodological study quality using a Cochrane ‘risk of bias’ tool.4 The GRADE profiler (GRADEpro) was then used to assess evidence quality for each review outcome.2 Using GRADE, evidence quality is downgraded from ‘high quality’ by one level for each serious issue identified in the domains of risk of bias, imprecision, indirectness, inconsistency and publication bias.
Dichotomous outcome measures were expressed as risk ratios and continuous outcomes were reported as mean differences. Side‐to‐side, within‐participant trials of topical therapies were permitted provided that a systemic effect was unlikely and that the left and right sides of the same anatomical site were compared, because different sites may respond differently to particular interventions. Statistical heterogeneity was assessed using the I 2 statistic. We used a fixed‐effects model for I 2 statistic values < 40% and a random‐effects model for values between 40% and 75% (there were no I 2 statistic values > 75%).
Results
Description of the included studies
Our searches identified 12 trials for inclusion in the review (Fig. 1), in which a total of 615 adults with HS participated.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 The 12 trials investigated 15 different interventions and most were relatively small, with a median number of participants of 27. In terms of trial design, eight were parallel‐group studies,5, 6, 7, 8, 10, 12, 13, 14 three were within‐participant trials9, 11, 16 and one was a crossover study for which we included only the first‐phase data because carry‐over effects were likely.15 The median trial duration was 16 weeks.
Figure 1.

Flow diagram of study selection.
We divided the interventions into topical therapy, systemic therapy, surgical treatment and other therapies. Antitumour necrosis factor‐α therapies were investigated in four studies5, 10, 13, 14 and are classified as a subgroup of systemic therapy.
Risk of bias in the included studies
The risk of bias for each domain across all included studies is given in Figure 2. There was a high risk of performance bias for within‐participant laser and light studies that did not employ a sham intervention on the untreated side.9, 11, 16 Detection bias was avoided for investigator‐reported outcomes in these studies by using assessors who were blinded to treatment allocation; however, participant‐reported outcomes remained at high risk of bias. Two studies were at high risk of attrition bias due to an attrition rate > 20% and lack of an intention‐to‐treat analysis.12, 16
Figure 2.
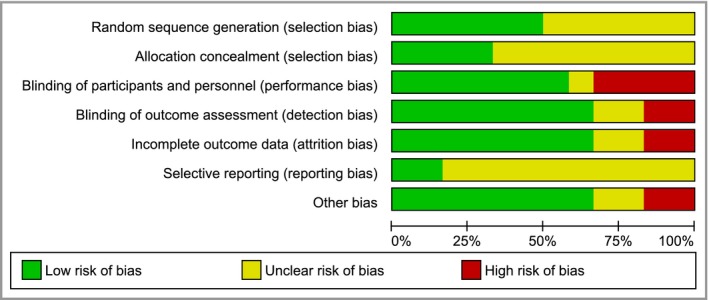
‘Risk of bias’ graph: review authors’ judgements about each ‘risk of bias’ item presented as percentages across all included studies.
Effects of interventions
Data for the effects of interventions and the evidence quality assessed using GRADE methodology are presented in the summary of findings tables (Tables 2, 3, 4 and Tables S1–9).
Table 2.
Summary of findings: adalimumab weekly compared with placebo for hidradenitis suppurativa. Patient or population: participants with hidradenitis suppurativa. Setting: hospital based. Intervention: adalimumab weekly. Comparison: placebo
| Outcomes | Illustrative comparative risksa (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Placebo | Adalimumab weekly | ||||
| Change in DLQI score (imputation). Follow‐up: 16 weeks | – | The mean change in DLQI score (imputation) in the intervention groups was 4 lower (6·49–1·51 lower) | – | 102 (1 study) | ⊕⊕⊕⊝ moderateb |
| Change in DLQI score (LOCF). Follow‐up: 16 weeks | – | The mean change in DLQI score (LOCF) in the intervention groups was 4·1 lower (6·59–1·61 lower) | – | 102 (1 study) | ⊕⊕⊕⊝ moderateb |
| Frequency of serious adverse effects. Follow‐up: 16 weeks | 39 per 1000 | 78 per 1000 (15–409) | RR 2·00 (0·38–10·44) | 102 (1 study) | ⊕⊕⊕⊝ moderateb |
| Frequency of treatment discontinuation. Follow‐up: 16 weeks | 0 per 1000 | 39 per 1000c | RR 5 (0·25–101·63) | 102 (1 study) | ⊕⊕⊕⊝ moderateb |
| Proportion of participants with infectious adverse effects. Follow‐up: 16 weeks | 353 per 1000 | 332 per 1000 (194–572) | RR 0·94 (0·55–1·62) | 102 (1 study) | ⊕⊕⊕⊝ moderateb |
| Proportion with improvement in pain VAS. Follow‐up: 16 weeks | 271 per 1000 | 479 per 1000 (276–831) | RR 1·77 (1·02–3·07) | 96 (1 study) | ⊕⊕⊕⊝ moderateb |
| Change in modified Sartorius scale score (imputation). Follow‐up: 16 weeks | – | The mean change in modified Sartorius scale score (imputation) in the intervention groups was 23 lower (50·16 lower to 4·16 higher) | – | 102 (1 study) | ⊕⊕⊕⊝ moderateb |
CI, confidence interval; DLQI, Dermatology Life Quality Index; LOCF, last‐observation‐carried‐forward; RR, risk ratio; VAS, visual analogue scale. GRADE Working Group grades of evidence: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very low quality, we are very uncertain about the estimate. aThe assumed risk is the risk in the placebo group of the study population. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bDowngraded one level for imprecision because the evidence is based on the results of a single study and subsequent studies are likely to have an important impact on our confidence in the estimate of effect and may change the estimate.17 cDue to the low frequency of events (0) in the control group, the corresponding risk reflects the observed events in the intervention group.
Table 3.
Summary of findings: adalimumab every other week compared with placebo for hidradenitis suppurativa. Patient or population: participants with hidradenitis suppurativa. Setting: hospital based. Intervention: adalimumab every other week. Comparison: placebo
| Outcomes | Illustrative comparative risksa (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Adalimumab every other week | |||||
| Change in DLQI score (LOCF). Follow‐up: 16 weeksb | – | The mean change in DLQI score (LOCF) in the intervention groups was 1·61 lower (3·86 lower to 0·64 higher) | – | 124 (2 studies) | ⊕⊕⊕⊕ high | – |
| Frequency of serious adverse effects. Follow‐up: 16 weeksb | 35 per 1000 | 52 per 1000 (9–296) | RR 1·47 (0·26–8·44) | 124 (2 studies) | ⊕⊕⊕⊕ high | – |
| Frequency of treatment discontinuation. Follow‐up: 16 weeksb | 0 per 1000 | 0 per 1000 (0–0) | RR 4·91 (0·24–99·74) | 124 (2 studies) | ⊕⊕⊕⊕ high | – |
| Proportion of participants with infectious adverse effects. Follow‐up: 16 weeksb | 333 per 1000 | 533 per 1000 (190–1000) | RR 1·60 (0·57–4·53) | 124 (2 studies) | ⊕⊕⊕⊕ high | – |
| Change in pain VAS. Follow‐up: 12 weeks | – | The mean change in pain VAS in the intervention groups was 16·57 lower (55·28 lower to 22·14 higher) | – | 21 (1 study) | ⊕⊕⊝⊝ lowc , d | – |
| Proportion with improvement in pain. Follow‐up: 16 weeks | 271 per 1000 | 363 per 1000 (198–658) | RR 1·34 (0·73–2·43) | 95 (1 study) | ⊕⊕⊕⊝ moderated | – |
| Change in Sartorius scale score (LOCF). Follow‐up: 16 weeksb | – | The mean change in Sartorius scale score (LOCF) in the intervention groups was 0·42 SD lower (1·22 lower to 0·37 higher) | – | 124 (2 studies) | ⊕⊕⊕⊝ moderatee | SMD −0·42 (−1·22 to 0·37) |
CI, confidence interval; DLQI, Dermatology Life Quality Index; LOCF, last‐observation‐carried‐forward; RR, risk ratio; SMD, standardized mean difference; VAS, visual analogue scale. GRADE Working Group grades of evidence: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very low quality, we are very uncertain about the estimate. aThe basis for the assumed risk is the mean risk in the placebo groups of the study populations. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bFollow‐up 12 weeks for 21 participants.14 cImbalance in baseline disease severity between the two groups: downgraded due to indirectness as the results may not be of relevance to the wider population. dDowngraded one level for imprecision because the evidence is based on the results of a single study (for each of these outcomes) and subsequent studies are likely to have an important impact on our confidence in the estimate of effect and may change the estimate.17 eDowngraded one level for inconsistency as the I 2 statistic of 59% demonstrates substantial study heterogeneity for this outcome.
Table 4.
Summary of findings: infliximab compared with placebo for hidradenitis suppurativa (HS). Patient or population: participants with HS. Setting: hospital based. Intervention: infliximab. Comparison: placebo
| Outcomes | Illustrative comparative risksa (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Placebo | Infliximab | ||||
| At least 50% decrease in HS Severity Index. Follow‐up: 8 weeks | 56 per 1000 | 267 per 1000 (33–1000) | RR 4·80 (0·6–38·48) | 33 (1 study) | ⊕⊕⊕⊝ moderateb |
| Physician's Global Assessment. Follow‐up: 8 weeks | 167 per 1000 | 800 per 1000 (277–1000) | RR 4·80 (1·66–13·9) | 33 (1 study) | ⊕⊕⊕⊝ moderateb |
CI, confidence interval; RR, risk ratio. GRADE Working Group grades of evidence: high quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very low quality, we are very uncertain about the estimate. aThe assumed risk is the risk in the placebo group of the study population. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bDowngraded one level for imprecision due to a small number of events in only a single study.
Topical therapies
Topical clindamycin vs. placebo
A single trial of 30 participants compared clindamycin 1% solution with vehicle solution for 12 weeks (frequency of application unstated).8 There was no difference in adverse effects between the two groups, which were reported to be mild (Table S1). SDs for the study efficacy data were unavailable, preventing inclusion in the summary of findings table, and a quality‐of‐life outcome was not measured. Regarding the available efficacy outcomes, there was no difference in participant self‐assessment scores between the two groups. However, there was a significant improvement in the HS score, a composite scale incorporating the Participant's Global Assessment and the number of inflammatory nodules, abscesses and pustules, in favour of topical clindamycin.
Systemic therapies
Oral tetracycline vs. topical clindamycin
Forty‐six participants with mild‐to‐moderate HS were randomized to receive either oral tetracycline 500 mg twice daily and vehicle solution or oral placebo and clindamycin 1% solution for 16 weeks.12 Quality of life was not measured, but oral tetracycline did produce a statistically significant improvement in Participant's Global Assessment compared with topical clindamycin (Table S2). There was no difference in pain, number of HS lesions or Physician's Global Assessment. Three adverse events occurred in the tetracycline group and five events in the topical clindamycin group, but the type and severity of events were not recorded. Quality of evidence was downgraded to ‘low’ due to attrition bias, because 12 participants did not complete the study and were unaccounted for, and because of imprecision from a single small study.
Ethinylestradiol and cyproterone acetate vs. ethinylestradiol and norgestrel
A 12‐month crossover study of 24 female participants with moderate‐to‐severe HS compared ethinylestradiol 50 μg and norgestrel 500 μg daily on days 5–25 of each menstrual cycle with ethinylestradiol 50 μg and cyproterone acetate 50 mg on days 5–14 of each cycle.15 We included efficacy data up to the 6‐month crossover point and there was no significant difference in Participant's Global Assessment (Table S3). Only 18 participants completed the study, and thus the evidence quality was reduced to ‘moderate’ due to imprecision.
Systemic therapies: antitumour necrosis factor‐α therapies
Etanercept vs. placebo
Twenty participants with active HS were randomized to subcutaneous etanercept 50 mg twice weekly or placebo injections for 12 weeks.5 The study reported P‐values without original trial data, and from the P‐values there were no differences between the two groups at 12 weeks in Dermatology Life Quality Index (DLQI) scores (Table S4), Participant's Global Assessment, pain or Physician's Global Assessment. There were no serious adverse effects reported, only mild injection‐site reactions.
Adalimumab weekly vs. placebo
One of the three arms of a placebo‐controlled trial of adalimumab therapy investigated a subcutaneous dose of 40 mg weekly from weeks 4 to 15, following loading doses of 160 mg at week 0 and 80 mg at week 2.13 Fifty‐one participants received weekly adalimumab and 51 were given placebo injections. The results were presented using both last‐observation‐carried‐forward (LOCF) and imputation methods for handling missing data. At 16 weeks, adalimumab weekly improved the DLQI score by 4·0 points compared with placebo [95% confidence interval (CI) −6·49 to −1·51, imputation method] (Table 2). Comparing the two groups, there was no significant difference in serious adverse events [relative risk (RR) 2·00, 95% CI 0·38–10·44] or infectious adverse events (RR 0·94, 95% CI 0·55–1·62) (Table 2). Adalimumab weekly was superior to placebo for nearly all of our other secondary outcomes, as well as the economic outcome Total Work Productivity Impairment score [mean difference (MD) −19·50, 95% CI −30·07 to −8·93, imputation method]. The study was assessed to be at low risk of bias for all domains, but the evidence quality was reduced to ‘moderate’ because it is based on only a single study and subsequent studies are likely to impact on our confidence in the effect estimate and may change the estimate.17
Adalimumab every other week vs. placebo
A meta‐analysis of two studies was possible for several outcomes in this comparison. Another of the three arms of the RCT described above compared 52 participants given subcutaneous adalimumab 40 mg every other week (EOW), following loading doses of 80 mg at week 0 and 40 mg at week 1, with 51 participants given placebo injections, reporting primary outcomes at week 16.13 A smaller study compared 15 participants given adalimumab 40 mg EOW with six participants who received placebo injections, with primary outcomes measured after 12 weeks.14 From the meta‐analysis (Table 3) there was no statistically significant difference between adalimumab EOW and placebo for change in DLQI score (MD −1·61, 95% CI −3·86 to 0·64). There was also no difference in the secondary outcomes of pain, HS lesion score, Physician's Global Assessment and Total Work Productivity Impairment.
Infliximab vs. placebo
One RCT of 38 participants, of whom 33 completed the trial, compared intravenous infliximab 5 mg kg−1 with intravenous placebo, reporting primary outcomes at week 8.10 Infliximab was given in the standard dosing regimen, at weeks 0, 2 and 6. Infliximab improved the DLQI score relative to placebo, with an effect size of 8·4 points (P = 0·003, Wilcoxon rank‐sum test) (Table 4). There were two serious adverse events in the infliximab group, a pregnancy (outcome not reported) and hospitalization for hypertension, compared with none for those given placebo. Infliximab improved pain and Physician's Global Assessment relative to placebo, but there was no significant difference in the proportion of participants in the two groups achieving ≥ 50% improvement in an unvalidated ‘HS Severity Index’ score. The evidence quality was downgraded to ‘moderate’ because of the imprecision resulting from a single, relatively small study.
Surgical interventions
Gentamicin sponge vs. primary closure alone
Two hundred participants with HS undergoing excision of symptomatic lesions were randomized to insertion of a gentamicin–collagen sponge prior to closure or primary closure alone.7 Assessment of surgical complications found no difference between the groups at week 1 (RR 0·78, 95% CI 0·58–1·05) or after 3 months (RR 0·90, 95% CI 0·50–1·62) (Table S5). The duration of remission, measured by the recurrence rate at 3 months, was not significantly altered by addition of the gentamicin sponge (RR 0·96, 95% CI 0·68–1·34). The evidence quality was downgraded to ‘moderate’ due to an unclear risk of bias in most domains, including an imbalance in randomization due to early study cessation.
Other interventions
Intense pulsed light vs. no treatment
A within‐participant trial randomized 17 participants to intense pulsed light treatment of one side of a bilaterally affected region, compared with no treatment of the other side.11 Twelve participants underwent treatment of the axilla, four had groin involvement and one had inframammary disease. Treatment‐related pain caused one participant to withdraw (treatment site unknown). Participant treatment satisfaction was measured with an unvalidated Likert scale and we defined treatment success as ratings of good, excellent or clear compared with baseline. Overall, intense pulsed light provided better participant satisfaction than no treatment (RR 9·67, 95% CI 2·10–46·43) (Table S6); however, the evidence quality was downgraded to ‘low’ due to performance bias and imprecision.
Neodymium‐doped yttrium aluminium garnet laser vs. topical control
A trial of neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser therapy included 34 bilaterally affected anatomical sites in 22 participants.16 One side of each site was randomized to receive four laser treatments at monthly intervals, as well as topical antimicrobials, and the other side received topical antimicrobial therapy alone. At 3 months, results were available for 25 anatomical sites (11 groin, 10 axillary, four inframammary) in 17 participants. Forty per cent of participants reported laser‐treatment‐related pain, but none withdrew from the trial as a consequence. Using the modified HS Lesion, Area and Severity Index (HS‐LASI) severity score, there was significant benefit from Nd:YAG laser therapy for all treated sites combined at 3 months (MD −14·03, 95% CI −18·84 to −9·22 points), and after a further 2 months of observation (Table S7).18 However, the evidence quality was downgraded to ‘very low’ due to the high risk of performance bias and attrition bias, and also imprecision.
Niosomal methylene blue gel photodynamic therapy vs. free methylene blue gel photodynamic therapy
A within‐participant trial compared niosomal methylene blue gel photodynamic therapy with free methylene blue gel photodynamic therapy given once every 2 weeks for up to 6 months.9 In the 10 participants who received treatment, niosomal methylene blue gel produced a significantly larger improvement in HS‐LASI score than free methylene blue gel (MD −4·30, 95% CI −8·36 to −0·24) (Table S8). The evidence quality was downgraded to ‘low’ due to a high risk of performance bias and imprecision.
Staphage lysate vs. placebo broth
Thirty‐one participants were randomized to receive staphage lysate both subcutaneously and as an inhaled aerosol, or vehicle broth via the same administration routes, once weekly for 20 weeks.6 Staphage lysate is designed to induce an immunological response and was obtained by bacteriophage lysis of Staphylococcus aureus. No serious adverse events occurred in either group. Based on a Physician's Global Assessment grading of ‘improved’, staphage lysate was of greater benefit than placebo broth (RR 6·25, 95% CI 1·68–23·27) (Table S9). The evidence quality was downgraded to ‘moderate’ due to imprecision.
Discussion
Our review has highlighted a relative lack of high‐quality evidence to guide treatment decisions in HS. Only 12 RCTs with a total of 615 participants met our inclusion criteria, whereas the recent Cochrane review update for vitiligo, a condition with a similar prevalence, contained 96 trials and 4512 participants.19 Many of the RCTs included in our HS review are small, with a median of 27 participants, and most interventions were investigated by only a single RCT. As a result, evidence quality had to be downgraded using GRADE methodology for most comparisons due to imprecision, limiting our clinical practice recommendations. For example, we did not find sufficient high‐quality evidence to determine the effects of topical clindamycin or oral tetracycline, which are standard therapies for mild‐to‐moderate HS.20, 21
Four relatively recent RCTs of antitumour necrosis factor‐α therapies included our primary outcome of quality of life. The results suggest that adalimumab 40 mg weekly improves quality of life compared with placebo, with a reduction in DLQI score of 4·0 points, which is equivalent to the minimal clinically important difference for the scale.22 However, the 95% CI includes an effect size of only 1·5 points, which may represent an insufficient clinical response. There was no significant difference in serious or infectious adverse effects compared with placebo, but any rare or delayed adverse effects of weekly adalimumab are currently unknown because psoriasis biological registers provide data only for EOW dosing.23 Another issue is the higher cost of weekly treatment compared with EOW therapy. The available evidence suggests that adalimumab EOW and etanercept 50 mg twice weekly are ineffective for HS. A single trial of infliximab providing efficacy data for 33 participants reported a DLQI reduction of 8·4 points relative to placebo, which is likely to be clinically relevant, but imprecision is a limiting factor due to the small number of participants.
Our review demonstrates a need for more surgical trials to improve HS care; in particular there are no RCTs investigating the timing of surgery or type of surgical procedure. We identified one trial of insertion of a gentamicin sponge wound‐healing adjuvant prior to primary closure, but this showed no benefit compared with primary closure alone.
Based on the RCT evidence available, laser and light therapies cannot be recommended for HS because the within‐participant trial designs did not incorporate a sham intervention, which, combined with imprecision, led to downgrading of GRADE evidence quality. It is difficult to draw conclusions from the crossover trial investigating two endocrine interventions because of the small study size and lack of placebo control. Regarding staphage lysate, there have been no further trials since one small RCT was performed in 1987, resulting in insufficient evidence to recommend a change in practice.
The recent HS Priority Setting Partnership (PSP)24 ranked trials of oral therapies as the most important research priority, and our review has highlighted a lack of RCT evidence in this area, including an absence of RCTs investigating oral immunomodulators and retinoids, and only one RCT of oral antibiotic therapy. In line with HS PSP priorities, we found no trials investigating treatment of an acute flare or HS‐associated pain management, and only one surgical trial. It will be important for the design of future trials to improve validation of HS outcome measures because many of the instruments employed by our included studies remain unvalidated and there is a lack of consensus regarding which outcomes to use.
There is hope for the future because our review identified eight ongoing HS RCTs in trial registers that have not yet been published in full. Interventions currently under investigation include topical antiseptics, the Nd:YAG and CO2 lasers, anakinra, novel biological therapies, and the PIONEER I and II studies of adalimumab therapy. Results from these studies will be incorporated into the planned update to our review.
Supporting information
Table S1. Topical clindamycin compared with placebo for hidradenitis suppurativa.
Table S2. Oral tetracycline compared with topical clindamycin for hidradenitis suppurativa.
Table S3. Ethinylestradiol and cyproterone acetate compared with ethinylestradiol and norgestrel for hidradenitis suppurativa.
Table S4. Etanercept compared with placebo for hidradenitis suppurativa.
Table S5. Gentamicin sponge compared with primary closure alone for hidradenitis suppurativa.
Table S6. Intense pulsed light compared with no treatment for hidradenitis suppurativa.
Table S7. Neodymium‐doped yttrium aluminium garnet laser compared with topical control for hidradenitis suppurativa.
Table S8. Niosomal methylene blue gel photodynamic therapy compared with free methylene blue gel photodynamic therapy for hidradenitis suppurativa.
Table S9. Staphage lysate compared with placebo broth for hidradenitis suppurativa.
Acknowledgments
We gratefully acknowledge the support of the Cochrane Skin Group editorial base.
Funding sources No external funding was obtained by the individual authors. Funding for the Cochrane Skin Group is mainly from the National Institute for Health Research (NIHR), U.K.
Conflicts of interest P.N.W., S.L.C., A.O.D., A.C.K., K.H. and S.E.G. declare no conflicts of interest. J.R.I. is a local principal investigator for an observational study sponsored by AbbVie, manufacturers of adalimumab. He has not acted as a consultant for the manufacturer or taken part in paid advisory boards. N.D. has received consulting fees for advisory board work for AbbVie, which produces adalimumab. T.B. received honoraria for speaking at AbbVie meetings in July 2014 and January 2015 about her personal patient journey. In June 2015 the Hidradenitis Suppurativa Trust received a £5000 core funding grant from AbbVie, which produces adalimumab. F.K. received honoraria for research or speaking agreements from Janssen Biotech, Inc., which produces Remicade® (infliximab); AbbVie, formally Abbott, which produces Humira® (adalimumab); and Amgen/Pfizer, which produces Enbrel® (etanercept). V.P. undertakes personal advisory work with Pfizer, AbbVie, Janssen, Novartis and Almirall. He has received departmental support from AbbVie, Almirall, Alliance, Beiersdorf U.K. Ltd, Biotest, Celgene, Galderma, Genus Pharma, Janssen, LEO Pharma, Meda, MSD, Novartis, Pfizer, Sinclair Pharma, Spirit Pharmaceuticals, Stiefel, Sumed and TyPharm. His department also receives financial support from the Dermatology Life Quality Index copyright. Please note that adalimumab is produced by AbbVie, ustekinumab is produced by Janssen, infliximab is produced by MSD, and etanercept is produced by Pfizer.
This manuscript is adapted from a Cochrane review published in issue 10, October 2015 of the Cochrane Library (see http://www.cochranelibrary.com for further information). Cochrane reviews are regularly updated and the Cochrane Library should be checked for the most recent version.
References
- 1. Jemec GB. Clinical practice Hidradenitis suppurativa. N Engl J Med 2012; 366:158–64. [DOI] [PubMed] [Google Scholar]
- 2. Schunemann H, Brozek J, Guyatt G, Oxman A. GRADE Handbook. Available at: http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html (last accessed 2 February 2016).
- 3. Ingram JR, Woo PN, Chua SL et al Interventions for hidradenitis suppurativa. Cochrane Database Syst Rev 2015; 10:CD010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Available at: www.cochrane-handbook.org (last accessed 2 February 2016).
- 5. Adams DR, Yankura JA, Fogelberg AC, Anderson BE. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol 2010; 146:501–4. [DOI] [PubMed] [Google Scholar]
- 6. Angel MF, Ramasastry SS, Manders EK et al Beneficial effects of staphage lysate in the treatment of chronic recurrent hidradenitis suppurativa. Surgical Forum 1987; 38:111–12. [Google Scholar]
- 7. Buimer MG, Ankersmit MF, Wobbes T, Klinkenbijl JH. Surgical treatment of hidradenitis suppurativa with gentamicin sulfate: a prospective randomized study. Dermatol Surg 2008; 34:224–7. [DOI] [PubMed] [Google Scholar]
- 8. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol 1983; 22:325–8. [DOI] [PubMed] [Google Scholar]
- 9. Fadel MA, Tawfik AA. New topical photodynamic therapy for treatment of hidradenitis suppurativa using methylene blue niosomal gel: a single‐blind, randomized, comparative study. Clin Exp Dermatol 2015; 40:116–22. [DOI] [PubMed] [Google Scholar]
- 10. Grant A, Gonzalez T, Montgomery MO et al Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double‐blind, placebo‐controlled crossover trial. J Am Acad Dermatol 2010; 62:205–17. [DOI] [PubMed] [Google Scholar]
- 11. Highton L, Chan WY, Khwaja N, Laitung JK. Treatment of hidradenitis suppurativa with intense pulsed light: a prospective study. Plast Reconstr Surg 2011; 128:459–65. [DOI] [PubMed] [Google Scholar]
- 12. Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol 1998; 39:971–4. [DOI] [PubMed] [Google Scholar]
- 13. Kimball AB, Kerdel F, Adams D et al Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 2012; 157:846–55. [DOI] [PubMed] [Google Scholar]
- 14. Miller I, Lynggaard CD, Lophaven S et al A double‐blind placebo‐controlled randomized trial of adalimumab in the treatment of hidradenitis suppurativa. Br J Dermatol 2011; 165:391–8. [DOI] [PubMed] [Google Scholar]
- 15. Mortimer PS, Dawber RP, Gales MA, Moore RA. A double‐blind controlled cross‐over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol 1986; 115:263–8. [DOI] [PubMed] [Google Scholar]
- 16. Tierney E, Mahmoud BH, Hexsel C et al Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium‐doped yttrium aluminium garnet laser. Dermatol Surg 2009; 35:1188–98. [DOI] [PubMed] [Google Scholar]
- 17. Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA 2005; 294:218–28. [DOI] [PubMed] [Google Scholar]
- 18. Mahmoud BH, Tierney E, Hexsel CL et al Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long‐pulsed neodymium:yttrium‐aluminium‐garnet laser. J Am Acad Dermatol 2010; 62:637–45. [DOI] [PubMed] [Google Scholar]
- 19. Whitton ME, Pinart M, Batchelor J et al Interventions for vitiligo. Cochrane Database Syst Rev 2015; 4:CD003263. [DOI] [PubMed] [Google Scholar]
- 20. Zouboulis CC, Desai N, Emtestam L et al European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29:619–44. [DOI] [PubMed] [Google Scholar]
- 21. Ingram JR, McPhee M. Management of hidradenitis suppurativa: a U.K. survey of current practice. Br J Dermatol 2015; 173:1070–2. [DOI] [PubMed] [Google Scholar]
- 22. Basra MK, Salek MS, Camilleri L et al Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015; 230:27–33. [DOI] [PubMed] [Google Scholar]
- 23. Iskandar IY, Ashcroft DM, Warren RB et al Demographics and disease characteristics of patients with psoriasis enrolled in the British Association of Dermatologists Biologic Interventions Register. Br J Dermatol 2015; 173:510–18. [DOI] [PubMed] [Google Scholar]
- 24. Ingram JR, Abbott R, Ghazavi M et al The Hidradenitis Suppurativa Priority Setting Partnership. Br J Dermatol 2014; 171:1422–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Topical clindamycin compared with placebo for hidradenitis suppurativa.
Table S2. Oral tetracycline compared with topical clindamycin for hidradenitis suppurativa.
Table S3. Ethinylestradiol and cyproterone acetate compared with ethinylestradiol and norgestrel for hidradenitis suppurativa.
Table S4. Etanercept compared with placebo for hidradenitis suppurativa.
Table S5. Gentamicin sponge compared with primary closure alone for hidradenitis suppurativa.
Table S6. Intense pulsed light compared with no treatment for hidradenitis suppurativa.
Table S7. Neodymium‐doped yttrium aluminium garnet laser compared with topical control for hidradenitis suppurativa.
Table S8. Niosomal methylene blue gel photodynamic therapy compared with free methylene blue gel photodynamic therapy for hidradenitis suppurativa.
Table S9. Staphage lysate compared with placebo broth for hidradenitis suppurativa.


