Abstract
β-cell dysfunction in type 1 and type 2 diabetes is accompanied by a progressive loss of β-cells, and an understanding of the cellular mechanism(s) that regulate β-cell mass will enable approaches to enhance hormone secretion. It is becoming increasingly recognized that enhancement of human β-cell proliferation is one potential approach to restore β-cell mass to prevent and/or cure type 1 and type 2 diabetes. While several reports describe the factor(s) that enhance β-cell replication in animal models or cell lines, promoting effective human β-cell proliferation continues to be a challenge in the field. In this review, we discuss recent studies reporting successful human β-cell proliferation including WS6, an IκB kinase and EBP1 inhibitor; harmine and 5-IT, both DYRK1A inhibitors; GNF7156 and GNF4877, GSK-3β and DYRK1A inhibitors; osteoprotegrin and Denosmab, RANK inhibitors; and SerpinB1, a protease inhibitor. These studies provide important examples of proteins and pathways that may prove useful for designing therapeutic strategies to counter the different forms of diabetes.
Keywords: human β-cell proliferation, harmine, GNF4877, osteoprotegrin, SerpinB1, WS6
Introduction
Progressive loss of functional β-cell mass, which can contribute to inadequate insulin secretion, is a central feature of both type 1 and type 2 diabetes, and provides a sound rationale for research efforts to maintain and/or enhance human β-cell expansion. Among the strategies to achieve this goal include, promoting β-cell proliferation, prevention of β-cell apoptosis and/or enhancement of β-cell reprogramming/differentiation from progenitor cells, pancreatic acinar cells, pancreatic ductal cells, or other cell types such as circulating stem cells. Other approaches include an induction of trans-differentiation to β-cells from non-β islet cells, such as α, δ, or PP cells, or from other tissues such as intestinal cells or hepatocytes, prevention of de-differentiation and transplantation of pancreatic islets from donors or β-cells derived from induced pluripotent stem (iPS) cells.
From a practical viewpoint it is conceivable that both academia and industry are focused on efforts to identify an orally administered small molecule that can safely and selectively expand human β-cell mass to potentially treat the enormous number of patients with diabetes (estimated to be 9% of adults in the world). However, several limitations need to be overcome to achieve this desirable goal.
A major challenge continues to be the limited understanding of signaling pathways relevant for human β-cell growth highlighted by the large number of documented differences in signaling mechanisms that operate to modulate the function and growth of human and rodent islets [1–4]. A further challenge is the extremely low levels of proliferation capacity of adult human β-cells even after mitogenic stimulation (~<0.5%). Notwithstanding, recent reports discussed in this review indicate a slow but steady advance in identifying molecules that have the potential to increase proliferation of human β-cells. Part of this observation is secondary to the large variability in the behavior of human islet samples that are received by investigators to study their function and/or growth [5] and the differences in ethnicity, gender and genetic background of the individual donors [6]. Nevertheless, sustained efforts have unraveled new candidate factors with a significant ability to enhance human β-cell proliferation and are reviewed in this article.
Callout “Human and rodent islets differ in structure, composition, function, and gene expression. Adult human β-cells are mostly quiescent and exhibit extremely low levels of proliferation capacity even after mitogenic stimulation.”
WS6: an IκB Kinase and EBP1 Inhibitor
The factors driving β-cell proliferation in vitro are generally expected to facilitate identical actions in vivo. This concept prompted researchers to conduct in vitro screening to identify factors that are able to stimulate β-cell proliferation [7–12]. Shen et al. conducted a high-throughput screen for the identification of proliferative small molecules using R7T1 cells, a growth-arrested rat β-cell line [10]. Their screen led to a diarylurea WS1, a chemotype which can induce cell proliferation. Subsequently, they synthesized its analogue, diarylamide WS6, which promoted R7T1 cell proliferation. Interestingly, WS6 induced human β-cell proliferation both in a dispersed islet proliferation assay using 5-ethynyl-2′-deoxyuridine (EdU) incorporation as well as in intact human islet cultures using Ki67 staining [10]. IκB kinase ε (IKKε) and Erb3 binding protein-1 (EBP1) were identified as binding partners of WS-6 by affinity purification and tandem mass spectrometry [10]. IκB kinase plays a role in the upstream NF-κB signal transduction cascade by inactivating the NF-κB transcription factor [13]. Previous studies have demonstrated that cytokines or chemokines released from CD4+ and CD8+ T cells enhance β-cell proliferation in mouse islets [14]. Thus, it is possible that modulation of IκB kinase activity by WS6 contributes to a similar pathway to promote proliferation.
Overexpression of EBP1 reduced the ability of WS6 to induce R7T1 cell proliferation [10]. EBP1 encodes a cell-cycle regulator which plays a role in cell survival, cell cycle arrest and differentiation [15]. EBP1 inhibits transcription of E2F1-regulated promoters by recruiting histone acetylase activity and suppresses cell replication [16]. E2F1 knockout mouse exhibited reduced β-cell mass and impaired β-cell function that was associated with dysfunctional PDX-1 activity [17]. Therefore, the inhibition of EBP1 by WS6 likely contributed to an upregulation of PDX-1 activity. An independent group confirmed that WS6 not only stimulated human β-cell proliferation, but also human α cell proliferation, using Ki67 immunostaining as a marker of proliferation [18]. However, WS6 has also been reported to have little effect on β-cell proliferation in dispersed human islets [11]. Thus, these studies suggest that evaluation of human β-cell proliferation is variable and depends upon the assay system (e.g., intact islets, dispersed cells, proliferation markers, etc), culture media (glucose, growth factors, etc), and/or the type of cell (donor background, viability, cell-to-cell contact, etc).
Harmine and 5-IT: DYRK1A Inhibitors
In a different approach Wang et al. exploited the property of MYC as a major driver of proliferation. Specifically, they used the human hepatocyte cell line, HepG2, stably expressing a luciferase reporter induced under the human MYC promoter to isolate candidate molecules of β-cell mitogen using chemical libraries [11]. Following the screening by induction of bromodeoxyuridine (BrdU) incorporation into rat β-cells, the authors identified a compound, harmine, as a potential candidate inducer of cell replication [11]. Importantly, harmine was able to induce human β-cell proliferation in both in vitro and in vivo models using NOD-SCID mice transplanted with human islets [11].
Harmine inhibits kinase activities of dual-specificity tyrosine-regulated kinase-1a (DYRK1A), DYRK1B, DYRK2, DYRK3, monoamine oxidases (MAOs), and cdc-like kinases (CLKs). The authors also showed that inhibition of DYRK1A contributes to hamine-mediated β-cell proliferation through the attenuation of the phosphorylation of nuclear factors of activated T cells (NFAT) (Fig. 1). Recently, using a high-throughput system to culture dissociated human islet cells themselves and measuring proliferation by EdU incorporation, we identified 5-iodotubercidin (5-IT), an adenosine kinase inhibitor also promoted human β-cell proliferation in vitro and in vivo [19]. 5-IT also inhibited DYRK1A and CLKs and enhanced an identical pathway as harmine to promote human β-cell replication.
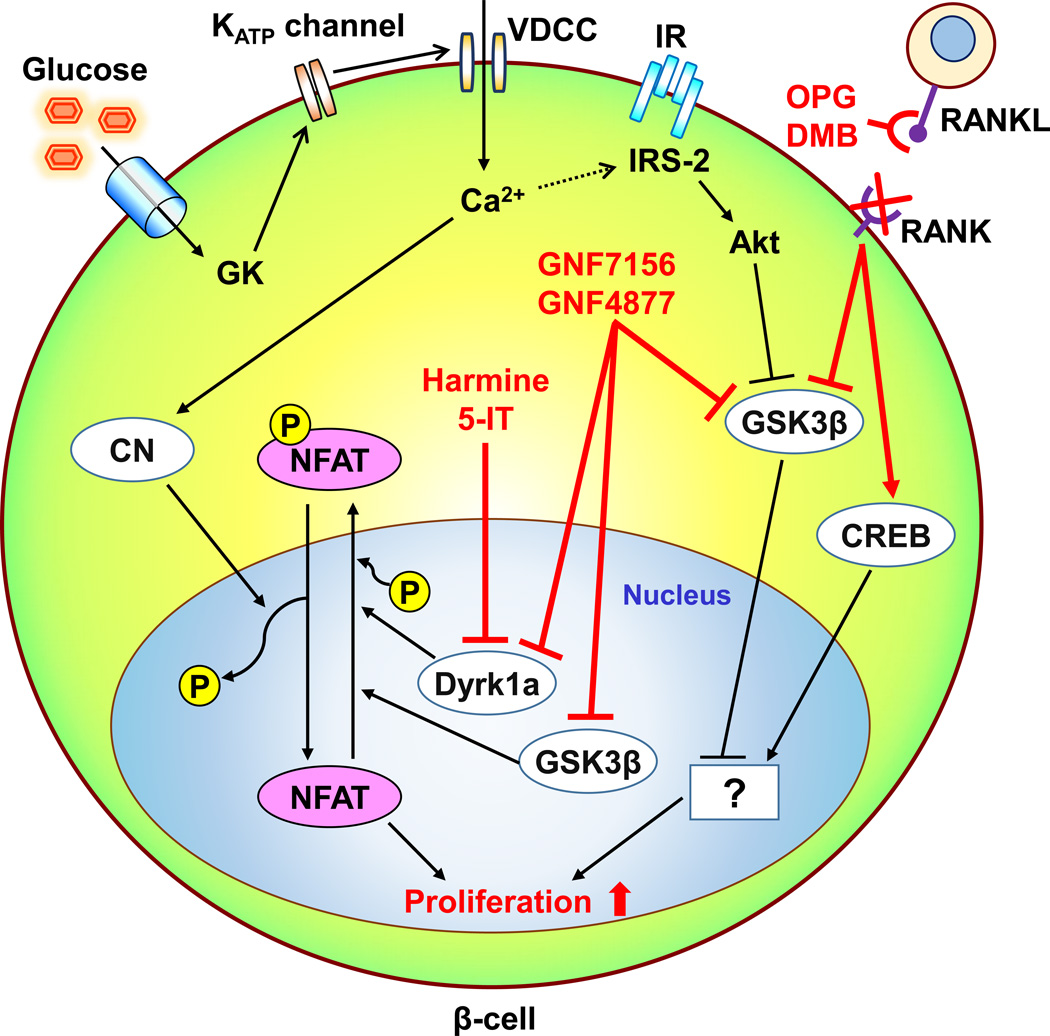
Fig. 1.
Schematic of the mechanism(s) by which recently identified factors modulate human β-cell proliferation. Glucokinase (GK)-mediated glucose signals activate human β-cell proliferation by the upregulation of intracellular calcium levels and IRS-2 mediated insulin signals. The calcium influx also activates a protein phosphatase calcineurin (CN). NFAT is translocated to the nucleus and activated by dephosphorylation with CN, while being inactivated by Dyrk1 and possibly GSK3β via its phosphorylation which results in nuclear export of NFAT. Harmine and 5-HT inhibit Dyrk1a, and GNF7156 and GNF4877 inhibit both Dyrk1 and GSK3β. These actions potentiate nuclear NFAT activation and β-cell proliferation. The phosphorylation-dependent GSK3β inactivation is also observed in insulin signal-induced Akt activation in β-cells, but the precise role of GSK3β in β-cell proliferation is still unclear. Osteoprotegrin (OPG) and Denosmab (DMG) activate CREB and inactivate GSK3β through the inhibition of an interaction between RANKL and RANK and stimulate human β-cell proliferation. However, the downstream signals of those proteins in human β-cells are unknown. CREB, cAMP response element-binding protein; DMG, Denosmab; Dyrk1A, dual-specificity tyrosine-regulated kinase-1a, GSK3β, glycogen synthase kinase-3β, IR, insulin receptor; KATP channel, ATP-sensitive potassium channel; NFAT, nuclear factor of activated T-cells, OPG, osteoprotegrin; VDCC, voltage-dependent calcium channel.
Phosphorylated NFATs are localized in the cytosol and their transcriptional activity is inactive. After dephosphorylation by a Ca2+/calmodulin-dependent protein phosphatase calcineurin, NFATs translocate to the nucleus and drive gene expression. Nuclear NFATs are then phosphorylated by DYRK1A, glycogen synthase kinase-3 (GSK3), and casein kinase 1 (CKI) and translocate back into the cytosol [20,21] (Fig. 1).
β-cell-specific calcineurin phosphatase regulatory subunit knockout mice showed age-dependent diabetes secondary to decreased β-cell proliferation and mass with a reduced expression of known regulators of β-cell function and proliferation including INS1, PDX1, BETA2, and CCND2 which are modulated by NFAT [22]. A link between growth factor signaling and the calcineurin/NFAT pathway is mediated by insulin receptor substrate-2 (IRS-2), an essential factor in β-cell proliferation [23], which has been suggested to be up-regulated by glucose [24]. Calcineurin/NFAT also binds promoters and regulates expression of β-cell dense core granule components CHGB, IAPP, and IA2, and cell proliferative mediators CCNA2, CCND2, and FOXM in human and mouse islets [25]. Furthermore, patients treated with calcineurin inhibitors, such as tacrolimus (FK-506) or cyclosporin A as immunosuppressive drugs, frequently develop diabetes partly due NFAT inactivation in β-cells [26]. Collectively, these observations indicate that NFAT is a reasonable target for human β-cell proliferation.
Callout “Harmine and 5-IT unmask human β-cell proliferation by inhibiting DYRK1A and activating calcineurin. Calcineurin dephosphorylates and activates a key transcription factor, NFAT, for β-cell development and function.”
However, studies in rodent models suggest paradoxical effects of Dyrk1a in β-cell proliferation. For example, Dyrk1a-haploinsufficient mice showed reduced β-cell mass and decreased β-cell proliferation [27], while mice with overexpression of Dyrk1A, driven by endogenous regulatory sequences, exhibited expansion of β-cell mass through increased proliferation [28]. Additional investigation is necessary to examine whether these models represent potential side effects of modulation of Dyrk1A or highlight the variable effects of administration of DYRK1A inhibitors. It is also worth noting that the ability of harmine to inhibit monoamine oxidase (MAO)-A, its use as a therapeutic drug may be limited [29]. Whether selective Dyrk1A inhibitors such as β-carboline derivative which highly selectively inhibits DYRK1A against MAO-A, DYRK2, DYRK3, DYRK4 and CLK2 is synthesized according to a model of a structure-based protein/ligand docking [30]. An additional concern for the application of harmine, 5-IT or related-products to clinical work is their off-target effects. Harmine has been reported to have psychoactive effects and induces excitation, anxiety, tremor, convulsion and ataxia [31]. Harmine is also identified by screening for compounds that promote adipogenesis [32]. In that study, harmine activates PPARγ and promotes adipogenesis via inhibition of Wnt signaling [32]. Harmine also has effects on hepatocyte survival, immune cell fuctions, and circadian period [33–35]. Furthermore, harmine also stimulates proliferation of islet α cells and pancreatic ductal cells [11]. Thus, it will be important to develop β-cell-specific targeted drug delivery to maintain specificity of action of harmine and 5-IT compounds.
GNF7156 and GNF4877: GSK-3β and DYRK1A Inhibitors
Shen et al. used information from a previously developed high-throughput screen for β-cell proliferation to synthesize compounds from an aminopyrazine scaffold [10,12]. Among them, GNF7156 and GNF4877 were identified as candidate compounds for further studies on β-cell proliferation [12]. Both compounds significantly increased β-cell proliferation in assays utilizing dissociated adult human islets, intact human islets, or transplantation into diabetic mice in vivo. A series of pyrazine analogues are reported as potent GSK-3β inhibitors [36]. Since GNF7156 and GNF4877 were synthesized from a pyrazine scaffold, these 2 compounds were evaluated and confirmed as inhibitors of GSK-3β. The authors also observed that the 2 compound inhibited dual-specificity tyrosine-regulated kinase-1a (DYRK1A) activity in a human KINOMEscan kinase screening. They confirmed that three other DYRK1A inhibitors (harmine, 5-IT, and TG003) also induced human β-cell proliferation in dispersed islet assays. Consistent with the actions of harmine [11] and 5-IT [19], DYRK1A inhibition by GNF7156 and GNF 4877 induced nuclear retention of NFAT in β-cells (Fig. 1). Furthermore they demonstrated that the dual inhibition of DYRK1A and GSK-3β had a stronger effect on enhancing β-cell proliferation, compared with harmine or 5-IT alone.
GSK-3β has been considered to be a negative regulator of β-cell proliferation and β-cell mass expansion in rodent models [37,38]. For example, inhibition of GSK-3β has been reported to ameliorate β-cell apoptosis induced by high glucose, fatty acids, or endoplasmic reticulum (ER) stress [39,40]. Haploinsufficiency for GSK-3β significantly increased β-cell proliferation and mass even in IRS-2 deficient mice which are known to develop significant β-cell loss [41]. GSK-3 inhibition also enhanced BrdU incorporation and Ki-67 expression in human β-cells in combination with activation of mechanistic target of rapamycin (mTOR) activation [42]. Furthermore, while GSK-3β negatively regulates NFAT by its nuclear export similar to DYRK1A [43] (Fig. 2), GSK-3β inhibition itself was not sufficient to induce human β-cell proliferation [12]. Thus, further studies are required to clarify the mechanisms underlying the enhanced human β-cell proliferation induced by GSK-3β inhibition.
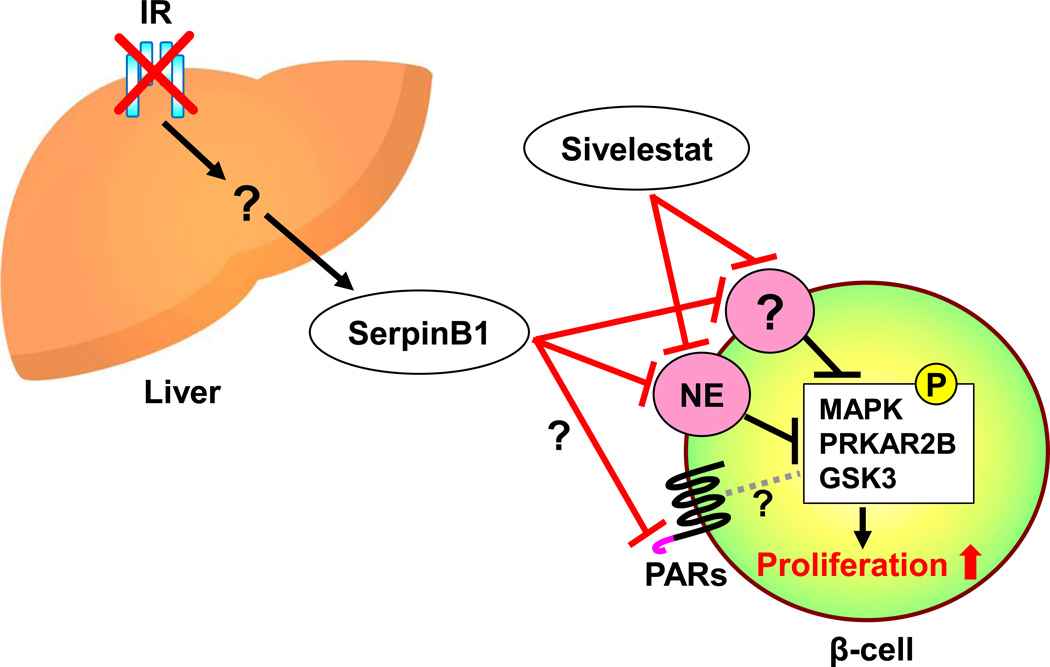
Fig. 2.
Schematic of the proteins that interact with SerpinB1, a protease inhibitor, in promoting human β-cell proliferation. Hepatic insulin resistance increases the expression and secretion of SerpinB1. SerpinB1, or Sivelestat which also exhibits neutrophil (or pancreatic) elastase (NE) inhibitor activity, potentiates human β-cell proliferation. Although inhibition of PE is associated with β-cell proliferation, the effects of SerpinB1 on other proteases (e.g. proteinase 3 or cathepsinG) which also could contribute to the proliferation effects are not fully explored. Upon stimulation with SerpinB1, the phosphorylation levels of MAPK, PRKAR2B, and GSK are elevated in β-cells. Other pathways that may mediate the effects of SerpinB1 include action via the protease-activated receptors (PARs). GSK3, glycogen synthase kinase-3; IR, insulin receptor; PRKAR2B, protein kinase cAMP-dependent type II regulatory subunit beta; MAPK, mitogen-activated protein kinases.
Osteoprotegrin and Denosumab: RANK Inhibitors
Pregnancy is a widely studied physiological model of β-cell mass expansion and the β-cell proliferation has been attributed to prolactin (PRL) and placental lactogen (PL) in rodent models [44]. PRL and PL have been reported to mediate their effects via FoxM1, HGF, menin, and serotonin pathways [45–48]. However, studies to date do not provide a clear consensus on human β-cell proliferation during pregnancy. Since the direct stimulation of human β-cells with PRL is not adequate to promote proliferation in vitro [49], the precise signaling pathways and proteins that are activated by lactogens to enhance human β cell replication in the pregnant state requires further investigation. Osteoprotegerin (OPG), one of the upregulated molecules in the islets of pregnant mice [50], has also been reported to be expressed and released in human islets in response to inflammatory cytokine treatment; and OPG has been shown to protect β-cells from cytokine-induced cell death through inhibition of the p38 MAPK phosphorylation [51]. A direct role for OPG in stimulating human β-cell proliferation was reported recently by Kondegowda and colleagues [52].
Osteoprotegerin (OPG), is a soluble decoy receptor for receptor activator of nuclear factor-κB ligand (RANKL), and TNF-related apoptosis-inducing ligand (TRAIL) [53]. OPG binds to RANKL and TRAIL, and inhibits association with their receptors, which have been termed the receptor activator of NF-kB (RANK) and the death receptor (DR) respectively [53]. Since OPG was required for PRL-induced mouse β-cell proliferation, the expression of OPG was thought to be regulated downstream of the PRL receptor [52]. Treatment of human β-cells with OPG showed a 3-fold increase in cell proliferation assessed by both BrdU incorporation and Ki67 staining in dispersed islets. Denosumab (DMB) is a monoclonal antibody used in the clinic for the treatment of osteoporosis and bone metastases of tumor [54]. DMB which also binds to RANKL and inhibits RANK receptor interaction also stimulated human β-cell proliferation [52]. OPG stimulated the phosphorylation of GSK-3β and cAMP response element-binding protein (CREB) in both mouse and human islets (Fig. 1). However, the mechanisms underlying the phosphorylation of GSK-3β and CREB by inhibition of RANK/RANKL signals is unclear. The inhibition of GSK-3β by its phosphorylation is in agreement with the effects described above for GNF7156 and GNF 4877. While CREB is involved in the β-cell proliferation induced by glucose or incretins in mouse β-cells [55,56], its role in human β-cell proliferation is not fully understood. Since DMB is widely used in clinical practice, it is hoped that future studies will unravel its precise mechanism of action to repurpose its use in humans with diabetes in vivo.
Callout “RANKL inhibition by osteoprotegerin or a FDA approved osteoporosis drug, Denosumab, promotes human β-cell proliferation. RANKL/RANK signal is a potential negative regulator of β-cell proliferation.”
SerpinB1: a Protease Inhibitor
The ability of mammals to mount a compensatory increase in β-cell mass to produce enough insulin to overcome insulin resistance and maintain glycemic control has been recognized for decades in both rodents and humans [57,58]. Rodent models of diabetes or insulin resistance, such as high-fat diet-fed mice, the ob/ob mice, the insulin receptor substrate-1 (IRS-1) knockout mice, and the Zucker fatty rat exhibit islet hyperplasia by enhancing β-cell proliferation [59,60]. While several groups have undertaken studies to characterize the factor(s) that promote a compensatory increase in β-cells, the identity of the putative factor(s) has been elusive. We used an islet transplantation approach in insulin receptor and IRS-1 double heterozygous mice or ob/ob mice, to establish the existence of circulating β-cell growth factors [61]. Using, the liver-specific insulin receptor knockout (LIRKO) mouse which exhibits dramatic β-cell hyperplasia in response to hepatic insulin resistance [62], El Ouaamari et al. used parabiosis, islet transplantation, and in vitro islet culture experiments to demonstrated the liver as a source of circulating β-cell growth factor(s) [63]. A similar parabiosis approach has been used to report that systemic factor(s) in the circulation of young mice increased cell proliferation in β-cell of old mice [64].
El Ouaamari et al. used differential proteomics analyses of liver, liver explant-conditioned media, hepatocyte culture conditioned media, and plasma from LIRKO and control mice to identify a liver-derived secretory protein that promotes β proliferation in mouse, zebrafish, and human [65]. SerpinB1, also known as leukocyte-neutrophil elastase inhibitor, enhanced β proliferation in mouse and human cultured islets (Fig. 2). Sivelestat, an inhibitor of human neutrophil elastase, which has been reported to treat acute lung injury (ALI) associated with systemic inflammation [66], was able to increase human β-cell proliferation both in the islet culture experiment in vitro and in the islet transplantation experiment in vivo (Fig. 2). Overexpression of SerpinB1 in zebrafish enhanced β-cell proliferation and SerpinB1 knockout mouse manifested impaired β-cell proliferation under high-fat diet-induced insulin resistant states. The cross-species reactivity of SerpinB1 on β-cells from zebrafish, mice and humans underscores its conserved effects. A phospho-proteomics approach revealed that SerpinB1 upregulated the phosphorylation of the mitogen-activated protein kinases (MAPK), the protein kinase cAMP-dependent type II regulatory subunit beta (PRKAR2B), and GSK3 in mouse islets. In human subjects, circulating SerpinB1 levels were correlated with insulin resistance [65], and a variant in SerpinB1 was observed to segregate with diabetes [65]. Among the questions to be addressed in future studies include whether SerpinB1 also acts via other proteases such as cathepsin G or proteinase 3 and/or via protease activated receptors (PARs) and the factor(s) that regulate expression and secretion of SerpinB1 from hepatocytes. Identifying molecules that have actions similar to SerpinB1 will be useful to explore their potential for the development of therapeutic strategies to counter diabetes.
Callout “Circulating factors derived from hepatocytes possess properties to promote proliferation of β-cells. SerpinB1 is a conserved protease inhibitor that can enhance β-cell proliferation in zebrafish, mice, and humans.”
Future Perspectives
In this review, we highlight recent findings on studies focused on human β-cell replication. We were unable to include several related reports due to space limitations. Some other factors have been also reported to be able to induce human β-cell proliferation. For example, Nodal a TGF-β superfamily stimulated β-cell proliferation and inhibited α cell stimulation in cultured human islets possibly through the phosphorylation of SMAD-2 [67]. Whereas we reported that the inhibition of TGF-β promoted human β-cell proliferation via prevention of SMAD-2-mediated Ink4a expression [68]. A neurotransmitter γ-aminobutyric acid (GABA) is also reportedly accelerate human β-cell proliferation via autocrine actions [69].. Besides, we need to investigate the long-term effects. For instance, glucokinase activation by ambient glucose, glucokinase activators (GKAs), or gain-of-function mutation could facilitate β-cell proliferation in both mouse and human through, at least in part, IRS-2 [70,71]. On the contrary, sustained continuous activation of glucokinase caused human and mouse β-cell apoptosis [72]. Interestingly co-transplantation of human islets with neural crest stem cells increased β-cell proliferation and enhances neural and vascular densities [73]. Vascularized organ buds including pancreatic bud by mesenchyme-driven condensation generated functional tissues via in vivo self-organization [74]. Thus, vascularization might be an important factor to drive β-cell replication in vivo.
It is also notable that most factors have predictable and/or unpredictable effects on non-β-cells in the islet or other tissues with implications for therapeutics. In this context it can be argued that intrinsic factors such as SerpinB1 or clinically approved drug such as Denosumab are considered more translatable. One limitation of expanding approaches to the humans in vivo is a need for suitable imaging techniques which can evaluate β-cell mass and proliferation in humans in vivo. An emerging area of interest is the concept of reprogramming, differentiation, or dedifferentiation to/from β-cells. Although several studies have highlighted evidence from rodent models [75], β-cell dedifferentiation is also proposed to be a feature in the pathophysiology of human type 2 diabetes [76]. Redifferentiation of functional β-cells, from other tissue such as stomach or gut tissues, is an emerging concept of how functional β-cell mass can overcome diabetes [77,78]. It is very likely that the number of candidate factors to induce human β-cell proliferation will noticeably increase. Going forward it will be important to examine the ability of each or a combination of factors that can safely, selectively and reversibly enhance human β-cell proliferation.
Acknowledgments
This work was supported by NIH RO1 DK67536, RO1 DK103215 and UC4 104167 (to R.N.K.). J.S. is supported by a Post-doctoral Fellowship for Research Abroad, the Japan Society for the Promotion of Science (JSPS).
Footnotes
Conflict of Interests
The authors have no conflict of interest
References
- 1.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human beta-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocana A. Human beta-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes. 2014;63:819–831. doi: 10.2337/db13-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart AF, Hussain MA, Garcia-Ocana A, et al. Human beta-cell proliferation and intracellular signaling: part 3. Diabetes. 2015;64:1872–1885. doi: 10.2337/db14-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang P, Fiaschi-Taesch NM, Vasavada RC, Scott DK, Garcia-Ocana A, Stewart AF. Diabetes mellitus--advances and challenges in human beta-cell proliferation. Nat Rev Endocrinol. 2015;11:201–212. doi: 10.1038/nrendo.2015.9. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni RN, Stewart AF. Summary of the Keystone islet workshop (April 2014): the increasing demand for human islet availability in diabetes research. Diabetes. 2014;63:3979–3981. doi: 10.2337/db14-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakradhar S. Diabetes researchers fear worsening access to human islets. Nat Med. 2014;20:567. doi: 10.1038/nm0614-567. [DOI] [PubMed] [Google Scholar]
- 7.Walpita D, Hasaka T, Spoonamore J, et al. A human islet cell culture system for high-throughput screening. J Biomol Screen. 2012;17:509–518. doi: 10.1177/1087057111430253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Walker JR, Wang X, et al. Identification of small-molecule inducers of pancreatic beta-cell expansion. Proc Natl Acad Sci U S A. 2009;106:1427–1432. doi: 10.1073/pnas.0811848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annes JP, Ryu JH, Lam K, et al. Adenosine kinase inhibition selectively promotes rodent and porcine islet beta-cell replication. Proc Natl Acad Sci U S A. 2012;109:3915–3920. doi: 10.1073/pnas.1201149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen W, Tremblay MS, Deshmukh VA, et al. Small-molecule inducer of beta cell proliferation identified by high-throughput screening. J Am Chem Soc. 2013;135:1669–1672. doi: 10.1021/ja309304m. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Alvarez-Perez JC, Felsenfeld DP, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med. 2015;21:383–388. doi: 10.1038/nm.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen W, Taylor B, Jin Q, et al. Inhibition of DYRK1A and GSK3B induces human beta-cell proliferation. Nat Commun. 2015;6:8372. doi: 10.1038/ncomms9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Identification and characterization of an IkappaB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 14.Dirice E, Kahraman S, Jiang W, et al. Soluble factors secreted by T cells promote beta-cell proliferation. Diabetes. 2014;63:188–202. doi: 10.2337/db13-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radomski N, Jost E. Molecular cloning of a murine cDNA encoding a novel protein, p38-2G4, which varies with the cell cycle. Exp Cell Res. 1995;220:434–445. doi: 10.1006/excr.1995.1335. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Woodford N, Xia X, Hamburger AW. Repression of E2F1-mediated transcription by the ErbB3 binding protein Ebp1 involves histone deacetylases. Nucleic Acids Res. 2003;31:2168–2177. doi: 10.1093/nar/gkg318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fajas L, Annicotte JS, Miard S, Sarruf D, Watanabe M, Auwerx J. Impaired pancreatic growth, beta cell mass, and beta cell function in E2F1 (−/−)mice. J Clin Invest. 2004;113:1288–1295. doi: 10.1172/JCI18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boerner BP, George NM, Mir SU, Sarvetnick NE. WS6 induces both alpha and beta cell proliferation without affecting differentiation or viability. Endocr J. 2015;62:379–386. doi: 10.1507/endocrj.EJ14-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirice E, Walpita D, Vetere A, et al. Inhibition of DYRK1A stimulates human beta-cell proliferation. Diabetes. 2016;65:1660–1671. doi: 10.2337/db15-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arron JR, Winslow MM, Polleri A, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 21.Gwack Y, Sharma S, Nardone J, et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 22.Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 23.Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 24.Demozay D, Tsunekawa S, Briaud I, Shah R, Rhodes CJ. Specific glucose-induced control of insulin receptor substrate-2 expression is mediated via Ca2+-dependent calcineurin/NFAT signaling in primary pancreatic islet beta-cells. Diabetes. 2011;60:2892–2902. doi: 10.2337/db11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodyer WR, Gu X, Liu Y, Bottino R, Crabtree GR, Kim SK. Neonatal beta cell development in mice and humans is regulated by calcineurin/NFAT. Dev Cell. 2012;23:21–34. doi: 10.1016/j.devcel.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penfornis A, Kury-Paulin S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006;32:539–546. doi: 10.1016/s1262-3636(06)72809-9. [DOI] [PubMed] [Google Scholar]
- 27.Rachdi L, Kariyawasam D, Guez F, et al. Dyrk1a haploinsufficiency induces diabetes in mice through decreased pancreatic beta cell mass. Diabetologia. 2014;57:960–969. doi: 10.1007/s00125-014-3174-3. [DOI] [PubMed] [Google Scholar]
- 28.Rachdi L, Kariyawasam D, Aiello V, et al. Dyrk1A induces pancreatic beta cell mass expansion and improves glucose tolerance. Cell Cycle. 2014;13:2221–2229. doi: 10.4161/cc.29250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reniers J, Robert S, Frederick R, Masereel B, Vincent S, Wouters J. Synthesis and evaluation of beta-carboline derivatives as potential monoamine oxidase inhibitors. Bioorg Med Chem. 2011;19:134–144. doi: 10.1016/j.bmc.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 30.Drung B, Scholz C, Barbosa VA, Nazari A, Sarragiotto MH, Schmidt B. Computational & experimental evaluation of the structure/activity relationship of beta-carbolines as DYRK1A inhibitors. Bioorg Med Chem Lett. 2014;24:4854–4860. doi: 10.1016/j.bmcl.2014.08.054. [DOI] [PubMed] [Google Scholar]
- 31.Abbassi R, Johns TG, Kassiou M, Munoz L. DYRK1A in neurodegeneration and cancer: Molecular basis and clinical implications. Pharmacol Ther. 2015;151:87–98. doi: 10.1016/j.pharmthera.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Waki H, Park KW, Mitro N, et al. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab. 2007;5:357–370. doi: 10.1016/j.cmet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa Y, Suzuki T, Ishii H, Ogata A, Nakae D. Mitochondrial dysfunction and biotransformation of beta-carboline alkaloids, harmine and harmaline, on isolated rat hepatocytes. Chem Biol Interact. 2010;188:393–403. doi: 10.1016/j.cbi.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Khor B, Gagnon JD, Goel G, et al. The kinase DYRK1A reciprocally regulates the differentiation of Th17 and regulatory T cells. eLife. doi: 10.7554/eLife.05920. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondoh D, Yamamoto S, Tomita T, et al. Harmine lengthens circadian period of the mammalian molecular clock in the suprachiasmatic nucleus. Biol Pharm Bull. 2014;37:1422–1427. doi: 10.1248/bpb.b14-00229. [DOI] [PubMed] [Google Scholar]
- 36.Berg S, Bergh M, Hellberg S, et al. Discovery of novel potent and highly selective glycogen synthase kinase-3beta (GSK3beta) inhibitors for Alzheimer's disease: design, synthesis, and characterization of pyrazines. J Med Chem. 2012;55:9107–9119. doi: 10.1021/jm201724m. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Tanabe K, Baronnier D, et al. Conditional ablation of Gsk-3beta in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia. 2010;53:2600–2610. doi: 10.1007/s00125-010-1882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Tanabe K, Bernal-Mizrachi E, Permutt MA. Mice with beta cell overexpression of glycogen synthase kinase-3beta have reduced beta cell mass and proliferation. Diabetologia. 2008;51:623–631. doi: 10.1007/s00125-007-0914-7. [DOI] [PubMed] [Google Scholar]
- 39.Shirakawa J, Togashi Y, Sakamoto E, et al. Glucokinase activation ameliorates ER stress-induced apoptosis in pancreatic beta-cells. Diabetes. 2013;62:3448–3458. doi: 10.2337/db13-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasan S, Ohsugi M, Liu Z, Fatrai S, Bernal-Mizrachi E, Permutt MA. Endoplasmic reticulum stress-induced apoptosis is partly mediated by reduced insulin signaling through phosphatidylinositol 3-kinase/Akt and increased glycogen synthase kinase-3beta in mouse insulinoma cells. Diabetes. 2005;54:968–975. doi: 10.2337/diabetes.54.4.968. [DOI] [PubMed] [Google Scholar]
- 41.Tanabe K, Liu Z, Patel S, et al. Genetic deficiency of glycogen synthase kinase-3beta corrects diabetes in mouse models of insulin resistance. PLoS Biol. 2008;6:e37. doi: 10.1371/journal.pbio.0060037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Remedi MS, Pappan KL, et al. Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets. Diabetes. 2009;58:663–672. doi: 10.2337/db07-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 44.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;21:151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Zhang J, Pope CF, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2010;59:143–152. doi: 10.2337/db09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demirci C, Ernst S, Alvarez-Perez JC, et al. Loss of HGF/c-Met signaling in pancreatic beta-cells leads to incomplete maternal beta-cell adaptation and gestational diabetes mellitus. Diabetes. 2012;61:1143–1152. doi: 10.2337/db11-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 48.Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H, Kleinberger JW, Takane KK, et al. Augmented Stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human beta-cells. Diabetes. 2015;64:3784–3797. doi: 10.2337/db15-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rieck S, White P, Schug J, et al. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol. 2009;23:1702–1712. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schrader J, Rennekamp W, Niebergall U, et al. Cytokine-induced osteoprotegerin expression protects pancreatic beta cells through p38 mitogen-activated protein kinase signalling against cell death. Diabetologia. 2007;50:1243–1247. doi: 10.1007/s00125-007-0672-6. [DOI] [PubMed] [Google Scholar]
- 52.Kondegowda NG, Fenutria R, Pollack IR, et al. Osteoprotegerin and denosumab stimulate human beta cell proliferation through inhibition of the receptor activator of NF-kappaB ligand pathway. Cell Metab. 2015;22:77–85. doi: 10.1016/j.cmet.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014;5:511. doi: 10.3389/fimmu.2014.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 55.Terauchi Y, Takamoto I, Kubota N, et al. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007;117:246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song WJ, Schreiber WE, Zhong E, et al. Exendin-4 stimulation of cyclin A2 in beta-cell proliferation. Diabetes. 2008;57:2371–2381. doi: 10.2337/db07-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mezza T, Muscogiuri G, Sorice GP, et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes. 2014;63:994–1007. doi: 10.2337/db13-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montanya E. Insulin resistance compensation: not just a matter of beta-cells? Diabetes. 2014;63:832–834. doi: 10.2337/db13-1843. [DOI] [PubMed] [Google Scholar]
- 59.El Ouaamari A, Zhou JY, Liew CW, et al. Compensatory islet response to insulin resistance revealed by quantitative proteomics. J Proteome Res. 2015;14:3111–3122. doi: 10.1021/acs.jproteome.5b00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Araki E, Lipes MA, Patti ME, et al. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 61.Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci U S A. 2001;98:7475–7480. doi: 10.1073/pnas.131192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michael MD, Kulkarni RN, Postic C, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 63.El Ouaamari A, Kawamori D, Dirice E, et al. Liver-derived systemic factors drive beta cell hyperplasia in insulin-resistant states. Cell Rep. 2013;3:401–410. doi: 10.1016/j.celrep.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y. Systemic regulation of the age-related decline of pancreatic beta-cell replication. Diabetes. 2013;62:2843–2848. doi: 10.2337/db13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Ouaamari A, Dirice E, Gedeon N, et al. SerpinB1 promotes pancreatic beta cell proliferation. Cell Metab. 2016;23:194–205. doi: 10.1016/j.cmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aikawa N, Kawasaki Y. Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther Clin Risk Manag. 2014;10:621–629. doi: 10.2147/TCRM.S65066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boerner BP, George NM, Targy NM, Sarvetnick NE. TGF-beta superfamily member Nodal stimulates human beta-cell proliferation while maintaining cellular viability. Endocrinology. 2013;154:4099–4112. doi: 10.1210/en.2013-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dhawan S, Dirice E, Kulkarni RN, Bhushan A. Inhibition of TGF-beta signaling promotes human pancreatic beta cell replication. Diabetes. 2016;65:1208–1218. doi: 10.2337/db15-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Purwana I, Zheng J, Li X, et al. GABA promotes human beta-cell proliferation and modulates glucose homeostasis. Diabetes. 2014;63:4197–4205. doi: 10.2337/db14-0153. [DOI] [PubMed] [Google Scholar]
- 70.Kassem S, Bhandari S, Rodriguez-Bada P, et al. Large islets, beta-cell proliferation, and a glucokinase mutation. N Engl J Med. 2010;362:1348–1350. doi: 10.1056/NEJMc0909845. [DOI] [PubMed] [Google Scholar]
- 71.Stamateris RE, Sharma RB, Kong Y, et al. Glucose induces mouse beta cell proliferation via IRS2, mTOR and cyclin D2 but not the insulin receptor. Diabetes. 2016;65:981–995. doi: 10.2337/db15-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tornovsky-Babeay S, Dadon D, Ziv O, et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in beta cells. Cell Metab. 2014;19:109–121. doi: 10.1016/j.cmet.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Grapensparr L, Vasylovska S, Li Z, et al. Co-transplantation of human pancreatic islets with post-migratory neural crest stem cells increases beta-cell proliferation and vascular and neural regrowth. J Clin Endocrinol Metab. 2015;100:E583–E590. doi: 10.1210/jc.2014-4070. [DOI] [PubMed] [Google Scholar]
- 74.Takebe T, Enomura M, Yoshizawa E, et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 2015;16:556–565. doi: 10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cinti F, Bouchi R, Kim-Muller JY, et al. Evidence of beta-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab. 2016;101:1044–1054. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet. 2012;44:406–412. S401. doi: 10.1038/ng.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ariyachet C, Tovaglieri A, Xiang G, et al. Reprogrammed stomach tissue as a renewable source of functional beta cells for blood glucose regulation. Cell Stem Cell. 2016;18:410–421. doi: 10.1016/j.stem.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]