Diabetes is caused by a combination of impaired responsiveness to insulin and reduced production of insulin by the pancreas. Until recently, the decline of insulin production had been ascribed to β-cell death. But recent research has shown that β-cells don’t die in diabetes, but undergo a silencing process, termed “dedifferentiation”. The main implication of this discovery is that β-cells can be revived by appropriate treatments. We have shown that mitochondrial abnormalities are a key step in the progression of β-cell dysfunction toward dedifferentiation. In normal β-cells, mitochondria generate energy required to sustain insulin production and its finely timed release in response to the body’s nutritional status. A normal β-cell can adapt its mitochondrial fuel source based on substrate availability, a concept known as “metabolic flexibility”. This capability is the first casualty in the progress of β-cell failure. β-cells lose the ability to select the right fuel for mitochondrial energy production. Mitochondria become overloaded, and accumulate byproducts derived from incomplete fuel utilization. Energy production stalls, and insulin production drops, setting the stage for dedifferentiation. The ultimate goal of these investigations is to explore novel treatment paradigms that will benefit people with diabetes.
Diabetes and the failure of insulin-producing β-cells
Diabetes arises as a consequence of combined abnormalities of insulin production and function [1]. Although alterations of only one arm of this biological network can result in full-blown disease, in most individuals the two abnormalities coexist. While target organs show an impaired response to insulin–so-called insulin resistance, β-cells of diabetics show a blunted and mistimed response to nutrients. Moreover, unlike insulin resistance, which appears to remain relatively constant during diabetes progression, β-cell function steeply deteriorates with time in a manner that is impervious to, and possibly worsened by, existing treatments [2]. In fact, an intrinsic susceptibility of the β-cell to functional exhaustion–what for want of a better term has been referred to as “β-cell failure”, sets apart those individuals who go on to develop diabetes from those who, at the same level of insulin resistance, don’t [2]. There are at least 3 abnormalities in islet cell function in diabetes: an impaired insulin response to stimulus, a reduced number of β-cells, and an inappropriate glucagon response [3]. This occurs despite the fact that reversal of hyperglycemia can partly restore β-cell function, even in patients with advanced disease [4]; hence the clinical conundrum of what is to be done to treat β-cell dysfunction. Treatments range from preserving β-cell function by reducing the metabolic demand on β-cells, to increasing β-cell performance and mass to meet the increased metabolic demand [4]. Despite research efforts, it is unclear whether the two primary components of β-cell failure, impaired insulin secretion and reduced β-cell mass, are mechanistically linked. In our studies of Foxo function in β-cells, we have discovered a seamless mechanism that leads from impaired insulin secretion to decreased β-cell mass by way of dedifferentiation. Thus, we suggested that abnormalities of Foxo function can explain the link between impaired insulin secretion and β-cell dedifferentiation. The challenge is to translate this exciting biology into new approaches to intervene on diabetes progression.
An intrinsic susceptibility of the β–cell to functional exhaustion sets apart individuals who go on to develop diabetes from those that don’t
Foxo in insulin action and β-cell function
Foxo 1, 3a, and 4 are three genes encoding forkhead-type transcription factors. There are over one hundred forkhead domain-containing genes in the human genome [5], but there are compelling differences that account for the selective involvement of the “O” subfamily in hormone action, a concept first discovered in C.elegans [6,7].
Unique among the forkhead domain-containing proteins, Foxo change their subcellular localization and hence their activity in response to Akt-dependent phosphorylation as well as NAD+-dependent acetylation [8]. The latter is thought to reflect the intracellular ratio of reduced NAD equivalents. Thus, Foxo can be viewed as a relay of metabolic signals to the nucleus. The overarching physiologic role of Foxo is to enable metabolic flexibility, i.e., the ability to switch from glucose to lipid utilization depending on nutrient availability [9,10]. However, at a more granular level, this general property morphs into a more nuanced mode of action. Thus, in the central nervous system Foxo integrates energy intake with energy expenditure through its actions on neuropeptide production, processing, and signaling [11–13]. In liver, Foxo regulates hepatic glucose and lipid production, and in adipocytes, free fatty acid turnover [9,14,15]. In the vasculature, it regulates nitric oxide production and inflammatory responses [16–18]. In addition, Foxo has seemingly distinct functions in tissue differentiation and lineage determination that are best illustrated by its role to maintain stability of insulin-producing β-cells and prevent their dedifferentiation during diabetes progression [19,20].
The overarching physiologic role of Foxo is to enable metabolic flexibility, i.e., the ability to switch from glucose to lipid utilization depending on nutrient availability
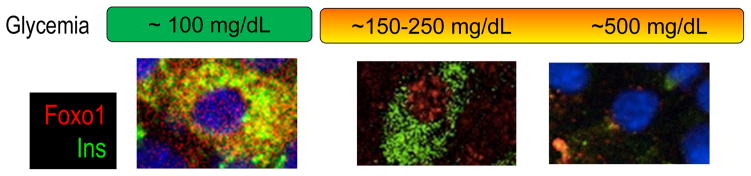
Our interest in this area was driven by the observation that Foxo subcellular localization changes in β-cells, depending on their pathophysiologic state (Fig. 1) [21,22]. In the “resting” β-cell, under physiological conditions, Foxo is seemingly inactive. When β-cells are exposed to increased glucose and/or fatty acid levels [21,23], or to cytokines and other inflammatory agents [24], Foxo undergoes nuclear translocation. This is due to different post-translational modifications that include phosphorylation and acetylation [8,25]. The residence of Foxo in the nucleus leads to activation of certain pathways and inhibition of others. The net outcome of this response is described below. However, it’s equally important to note that Foxo activation is limited in time, as the deacetylated nuclear protein has decreased stability [26]. As Foxo levels decrease, the stage is set for dedifferentiation through the loss of gene expression networks necessary to the maintenance of β-cell characteristics [27].
Figure 1. Changes to Foxo sub-cellular localization during diabetes progression.
Transcription factor Foxo1 translocates from the cytoplasm to the nucleus of the β-cell in response to changes in glucose, lipid, and cytokine levels in the environment. Nuclear translocation is associated with the activation of a stress response that aims to maintain mitochondrial function and β-cell identity. Nuclear Foxo1 is more rapidly degraded; thus, if hyperglycemia is not reversed, Foxo1 gradually fall, paving the way for β-cell dysfunction.
Insulin secretion and β-cell function
Our understanding of the regulation of β-cell function has been shaped by the metabolic paradigm, according to which insulin secretion responds to metabolic cues [28]. More controversial is the role of insulin itself as a regulator of β-cell function. The concept that insulin controls its own secretion remains controversial, but the thrust of our work is that Foxo integrates insulin/hormone-dependent pathways with glucose/nutrient-dependent pathways [21], thus superseding the debate on whether insulin or glucose are to blame for abnormal β-cell function.
In β-cells, Foxo integrates insulin-dependent with glucose-dependent pathways, thus superseding the debate on whether insulin or glucose are to blame for abnormal β-cell function
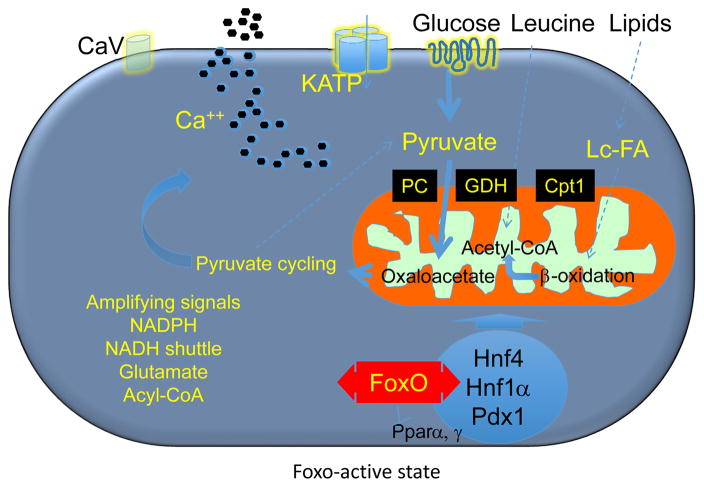
There are two main phases to insulin secretion: an ATP-dependent first phase [28], and a second–or amplifying–phase, variously assumed to be controlled by pyruvate cycling [29], NADH shuttle [30], long-chain acyl-CoAs [31], glutamate [32], or NADPH [33,34]. Substrate for mitochondrial oxidative phosphorylation can be derived from glucose, amino acids, and lipids. The balance between glucose and lipid oxidation is very important: during fasting, fatty acid oxidation allows β-cells to maintain a trickle of insulin secretion; after a meal, the rise in plasma glucose drives mitochondrial energy generation for ATP production and insulin release [3]. Experimental animal studies show that Foxo activation in the early phases of diabetes preserves the balance between glucose and lipids in the generation of acyl-CoA for mitochondrial oxidation. Foxo maintains the activation state of the maturity onset diabetes of youth (MODY) genes Hnf4, Hnf1, and Pdx1, and suppresses the fatty acid oxidation network supervised by nuclear receptor Pparα, to curtail generation of lipid-derived acyl-CoA (Figure 2) [10,27,35].
Figure 2. Model of Foxo (1, 3a, 4) role in physiologic β-cell function.
In the early phases of diabetes, Foxo nuclear translocation mediates the effects of glucose on gene expression through MODY gene networks, allowing glucose flux into mitochondria for ATP production (thick arrows), while limiting the contributions by lipids and amino acids (thin dotted arrows). This situation likely prevents the generation of toxic metabolic intermediates that can be detrimental to β-cell health.
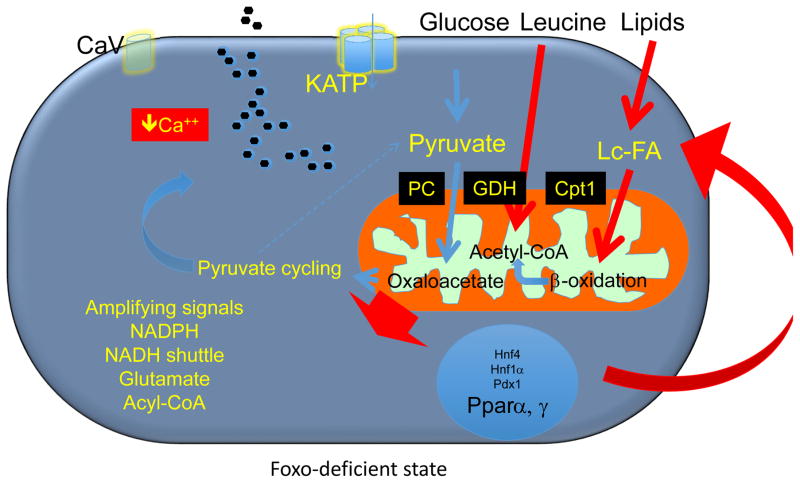
This stress response aims to preserve the physiologic balance of mitochondrial substrate, and keeps β-cells from “overheating” [10,35]; but it’s not unlimited. There is a penalty to be paid for Foxo activation: it becomes rapidly degraded, leading to its depletion if hyperglycemia does not resolve [10]. As Foxo expression wanes, β-cells switch from glucose oxidation-driven energy generation to lipid oxidation-driven energy generation for insulin secretion, becoming “blindsided” to glucose. Excessive lipid oxidation leads to generation of toxic products, primarily peroxides, and impairs ATP production, calcium mobilization, and insulin secretion (Figure 3). Interestingly, loss of Foxo is also associated with increased Pparγ and triglyceride synthesis [3]. We have suggested that this increase is compensatory in nature, and is meant to divert acyl-CoA toward lipid synthesis, to alleviate mitochondrial overload. The inability of the β-cell to adapt from lipid to glucose utilization is similar to what has been described in other tissues as metabolic inflexibility [36]. We have proposed that this inflexibility is a key step in β-cell failure [10].
Figure 3. Model of β-cell dysfunction and role of Foxo in pathophysiologic conditions.
As Foxo become functionally exhausted, β-cells are transcriptionally blindsided to the effects of glucose, increasing lipid and amino acid flux. MODY genes are suppressed, and Pparα increased, consistent with the role of Foxo to suppress Pparα in liver [67]. Interestingly, pathway analysis of RNA sequencing data indicates that the decrease in Foxo levels also leads to increased Pparγ. These data could be interpreted to suggest that Foxo promotes lipogenesis to prevent excessive mitochondrial fat oxidation [68]. PC: Pyruvate carboxylase; GDH: glutamate dehydrogenase; Cpt1: carnitine palmitoyltransferase-1.
What are the long-term consequences of metabolic inflexibility? Gradually β-cells lose, along with insulin secretion, their terminally differentiated features. This conclusion was arrived at using lineage-tracing studies to monitor the fate of β-cells during diabetes development [19]. The expectation of these experiments was that, if diabetic β-cells died of apoptosis, they would simply disappear over time. Instead, β-cells were still present, but lost their defining features and partly turned into glucagon-producing cells, providing a potential explanation for the hyperglucagonemia of diabetes [19]. This observation has now been reproduced [37–41], and significantly advanced by the demonstration that in rodents, non-human primates, and human islets dedifferentiation is reversible by insulin and other treatments [20,39,42–45]. Although insulin treatment of humans has not been found to result in significant restoration of β-cell dysfunction [46], the data raise the possibility that new agents, acting on different mechanisms, might prevent or reverse β–cell failure.
What might such new agents look like? To begin to address this question, we performed a simple gain-of-function experiment. The rationale of this experiment was that, if Foxo loss-of-function was detrimental to the islet by causing metabolic inflexibility and loss of differentiation, then a gain of Foxo function might suggest ways to protect the β-cell. We generated mutant mice homozygous for an allele encoding a constitutively deacetylated Foxo1 (6KR) [47]. This mutation results in prolonged nuclear residence of the protein, conferring an increase in Foxo activity [48]. We then investigated the hypothesis that Foxo1 deacetylation benefits β-cell function. Indeed, we found opposite changes to those identified when Foxo is ablated. Insulin secretion increased, albeit mildly, in vitro and in vivo. Glucose utilization by islets was unchanged, but lipid oxidation was decreased [27]. These findings are consistent with the expectation that Foxo nuclear translocation does indeed protect the β-cell from damage. When we analyzed gene expression in control islets transitioning from fasting to re-feeding, we found significant changes to ~4,000 genes. The key networks participating in the islet response to feeding were those involved with protein translation, degradation, mitochondrial complex I function, and Rho/Gef-dependent cytoskeletal remodeling associated with docking and fusion of secretory granules that occurs during second-phase insulin release [49].
All these changes occurred normally in β-cells carrying the mutant Foxo1. Remarkably, the gene expression signature of the mutant islets showed only ~80 differentially affected genes. This indicates that Foxo1 has a selective effect in β-cells, and is not a generic stress-reliever. More remarkably still, the genes specifically affected by Foxo1 can be divided into two sub-groups: one includes β-cell identity factors, such as Pdx1, MafA, Pax6, Hnf1α, Glut2, Gpr119, an orphan G-protein-coupled receptor of therapeutic interest [50], as well as the two insulin genes. The second group of genes regulated by Foxo1 controls the balance of mitochondrial glucose vs. lipid utilization [9], consistent with the finding of decrease of lipid utilization in isolated islets from Foxo1 mutant animals. Among the genes affected by Foxo1, mitochondrial carrier proteins of the Slc25 family stand out both quantitatively and qualitatively. These genes belong to the same family as uncoupling proteins, and regulate solute transport into the mitochondrial matrix [51]. For example, Foxo1 decreased expression of the carnitine/acylcarnitine carrier, consistent with the observation that levels of these carriers are increased in glucotoxicity and lipotoxicity [52], and that inhibition of carnitine translocase enhances insulin secretion [53]. Thus, the effect of Foxo1 to tune down expression of mitochondrial solute carriers might be part of a protective function aimed at limiting lipid availability within the mitochondria. In addition, several features of the gene expression patterns in Foxo1 mutant mice are expected to result in reduced lipid utilization, including the decreases of Pparγ, C/ebps, Cd36, and Cpt1α and -β; and the increase of the carbohydrate response element binding factor Chrebp, which would promote glucose oxidation. Thus, we can conclude that activation of Foxo inhibits lipid oxidation, primarily by inhibiting the Pparα program as well as the function of mitochondrial solute transporters [10]. The latter are an appealing target for investigations of new treatment modalities.
Dedifferentiation: a cellular hypothesis rooted in clinical observation
From the studies described above, it can be concluded that metabolic impairment predisposes to dedifferentiation. The key novel findings in this area are that, as β-cells lose their identity, they come to resemble endocrine progenitor cells [19,20,37,38]. The notion that β-cells might become dedifferentiated during diabetes progression is not rooted in some arcane cell biological fantasy, but in the daily clinical reality of treating diabetic patients. Beginning in the 1980’s, with the advent of glucose clamp techniques, the idea that type 2 diabetes could be subsumed under the paradigm of insulin resistance became commonplace. And certainly treating insulin resistance is a large unmet medical need [54]. But prior to that, diabetes treatment was primarily viewed as addressing the need to improve insulin secretion. Astute clinicians knew that insulin secretion becomes worse with each passing year, and early clinical studies showed the benefits of β-cell “rest” [55–57]. Beginning with the UKDPS [58], these findings became settled law, jumpstarting a search for treatments that would “protect” the β-cells and “modify” the course of the disease. Thus, the concept of dedifferentiation provides an underpinning for the reversibility of β-cell failure in the early phases of diabetes, and at the same time an explanation for the slow decline of β-cell function.
The notion that β-cells might become dedifferentiated is not rooted in some arcane cell biological fantasy, but in the daily clinical reality of treating diabetic patients
An important step in the process of determining the role of β-cell dedifferentiation was to test the human relevance of the mouse observations. While one cannot easily assess cellular plasticity of the endocrine pancreas in living humans, it’s however possible to use animal studies to formulate testable hypotheses on the expected features of dedifferentiated human β-cells [59,60]. With this goal in mind, we undertook a survey of human diabetic pancreata from organ donors to assess if β-cells become dedifferentiated. Our assumptions were that dedifferentiated β-cells would no longer contain insulin, or other pancreatic hormones, to exclude cells arising from converted β-cells. However, dedifferentiated cells would retain endocrine as well as progenitor cell features that could be detected by immunohistochemical techniques [19]. Under these assumptions, we were able to confirm the prediction that β-cells become dedifferentiated in patients with type 2 diabetes. In our studies, we found that all features of murine dedifferentiation occur in the human islets: ~40% of β-cells were dedifferentiated according to these criteria, and displayed patterns of transcription factor expression reminiscent of murine islets, with decreased Foxo1, Nkx6.1, and MafA. In addition, 4% of β-cells were degranulated, as assessed by electron microscopy. While this number accounts for only 10% of “dedifferentiated” cells, it should be emphasized that the distinction between a degranulated cell and a dedifferentiated cell is necessarily arbitrary. Is a cell containing 100 insulin granules a normal cell? Or is it a cell on its way to becoming dedifferentiated? Is a cell that no longer displays insulin immunoreactivity but still retains proinsulin immunoreactivity a dedifferentiated cell? We suggest that what’s important is that these cells can no longer be counted on to contribute to metabolic control and, regardless of how advanced the cellular pathology is, herald a disease process that leads to dedifferentiation.
Germane to this issue is the issue of whether human dedifferentiated or dedifferentiating β-cells do indeed have progenitor cell-like features. Demonstrating that human cells have Neurogenin3 immunoreactivity, the original finding of our murine studies, has been technically beyond reach. But we have been able to identify an easier-to-use marker that confirms the progenitor-like nature of these cells, aldehyde dehydrogenase 1A3, which will be invaluable going forward to understand the nature of dedifferentiating cells [61]. The number of ALDH1A3-positive/hormone-negative cells rose in direct proportion to the amount of dedifferentiated β-cells in human diabetics. As ALDH1A3 is a marker of cancer progenitor cells, these data are consistent with the possibility that endocrine cells in the diabetic pancreas become progenitor-like.
Autoptic surveys of human pancreata also allowed us to test the role of Foxo1 in human diabetes. The data show that human expression patterns mirror those found in rodents: Foxo1 is restricted to β-cells in the normal pancreas, and its levels decline with diabetes, a condition in which Foxo1 also appears in a small subset of α-cells. Are these trans-differentiated β-cells? With the advent of single-cell transcriptomics, we will soon be able to answer this question. Foxo1 represents a potential integration point for the effects of insulin sensitivity–or lack thereof–and glucose or lipid levels in the pathogenesis of β-cell dysfunction [21]. Thus, an overarching Foxo1-dependent mechanism regulating β-cell function can explain the interaction of insulin resistance with hyperglycemia as causes of β-cell failure, and offers a potential explanation for the benefits of glucose-lowering agents as well as insulin sensitizers on β-cell function [62].
The onset of type 2 diabetes is characterized by a steep decline of β-cell function, while insulin resistance remains relatively stable [2,62]. Counterintuitively, treating insulin resistance results in better outcomes than stimulating insulin secretion [63,64]. A possible explanation of these data is that insulin secretagogues somehow promote dedifferentiation by depleting β-cells of insulin, whereas treating insulin resistance reduces the requirement for insulin production and thus the β-cell afterload. Again, this explanation finds a potential clinical correlate in the longstanding concept of that β-cell “rest” is conducive to preservation of β-cell function [65]. If β-cells are not dead from apoptosis or marooned in a state of advanced cellular distress such as autophagy, endoplasmic reticulum stress or unfolded protein response, but rather lie quiescent as dedifferentiated cells and can be re-differentiated to produce insulin, there is a possibility to restore β-cell function and ameliorate insulin secretion even after the onset of hyperglycemia [55,57,66].
In the few years since the original report [19], we were gratified by the attention that this hypothesis has received and by the rapid progress that has been made by many laboratories. While there remain significant gaps in knowledge that will require further investigation to arrive at a consensus, we shouldn’t lose sight of the opportunity provided by these observations for a truly innovative approach to treating β-cell dysfunction in type 2 diabetes.
Acknowledgments
We acknowledge members of the Accili laboratory for reading the manuscript and for useful discussion. This work has been supported by NIH grants DK57539, DK63608, and DK64819.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict, financial or otherwise, with the content of this article.
References
- 1.Pajvani UB, Accili D. The new biology of diabetes. Diabetologia. 2015;58:2459–2468. doi: 10.1007/s00125-015-3722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Ferrannini E. The stunned beta cell: a brief history. Cell metabolism. 2010;11:349–352. doi: 10.1016/j.cmet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 6.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 7.Ogg S, Paradis S, Gottlieb S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 8.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 9.Haeusler RA, Hartil K, Vaitheesvaran B, et al. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nat Commun. 2014;5:5190. doi: 10.1038/ncomms6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim-Muller JY, Zhao S, Srivastava S, et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metab. 2014;20:593–602. doi: 10.1016/j.cmet.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura T, Feng Y, Kitamura YI, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 12.Plum L, Lin HV, Dutia R, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren H, Orozco IJ, Su Y, et al. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 2012;149:1314–1326. doi: 10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakrabarti P, Kandror KV. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J Biol Chem. 2009;284:13296–13300. doi: 10.1074/jbc.C800241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya K, Banks AS, Liang CP, Tabas I, Tall AR, Accili D. Homozygosity for an allele encoding deacetylated FoxO1 protects macrophages from cholesterol-induced inflammation without increasing apoptosis. Arterioscler Thromb Vasc Biol. 2011;31:2920–2928. doi: 10.1161/ATVBAHA.110.219477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya K, Tanaka J, Shuiqing Y, et al. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchiya K, Westerterp M, Murphy AJ, et al. Expanded granulocyte/monocyte compartment in myeloid-specific triple FoxO knockout increases oxidative stress and accelerates atherosclerosis in mice. Circ Res. 2013;112:992–1003. doi: 10.1161/CIRCRESAHA.112.300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 2014;19:872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura T, Nakae J, Kitamura Y, et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez SC, Cras-Meneur C, Bernal-Mizrachi E, Permutt MA. Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet beta-cell. Diabetes. 2006;55:1581–1591. doi: 10.2337/db05-0678. [DOI] [PubMed] [Google Scholar]
- 24.Kawamori D, Kaneto H, Nakatani Y, et al. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006;281:1091–1098. doi: 10.1074/jbc.M508510200. [DOI] [PubMed] [Google Scholar]
- 25.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiang L, Banks AS, Accili D. Uncoupling of acetylation from phosphorylation regulates FoxO1 function independent of its subcellular localization. J Biol Chem. 2010;285:27396–27401. doi: 10.1074/jbc.M110.140228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim-Muller JY, Kim YJ, Fan J, et al. FoxO1 deacetylation decreases fatty acid oxidation in beta-cells and sustains insulin secretion in diabetes. J Biol Chem. 2016;291:1062–1072. doi: 10.1074/jbc.M115.705608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 29.Odegaard ML, Joseph JW, Jensen MV, et al. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem. 2010;285:16530–16537. doi: 10.1074/jbc.M109.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eto K, Tsubamoto Y, Terauchi Y, et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- 31.Schuit F, De Vos A, Farfari S, et al. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 32.Gheni G, Ogura M, Iwasaki M, et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 2014;9:661–673. doi: 10.1016/j.celrep.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivarsson R, Quintens R, Dejonghe S, et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 35.Buteau J, Shlien A, Foisy S, Accili D. Metabolic diapause in pancreatic beta-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J Biol Chem. 2007;282:287–293. doi: 10.1074/jbc.M606118200. [DOI] [PubMed] [Google Scholar]
- 36.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 37.Puri S, Akiyama H, Hebrok M. VHL-mediated disruption of Sox9 activity compromises beta-cell identity and results in diabetes mellitus. Genes Dev. 2013;27:2563–2575. doi: 10.1101/gad.227785.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 2013;4:1262–1275. doi: 10.1016/j.celrep.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blum B, Roose AN, Barrandon O, et al. Reversal of beta cell de-differentiation by a small molecule inhibitor of the TGFbeta pathway. eLife. 2014;3:e02809. doi: 10.7554/eLife.02809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chera S, Baronnier D, Ghila L, et al. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casellas A, Mallol C, Salavert A, et al. Insulin-like growth factor 2 overexpression induces beta-cell dysfunction and increases beta-cell susceptibility to damage. J Biol Chem. 2015;290:16772–16785. doi: 10.1074/jbc.M115.642041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nathan G, Kredo-Russo S, Geiger T, et al. MiR-375 promotes redifferentiation of adult human beta cells expanded in vitro. PLoS One. 2015;10:e0122108. doi: 10.1371/journal.pone.0122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toren-Haritan G, Efrat S. TGFbeta pathway inhibition redifferentiates human pancreatic islet beta cells expanded in vitro. PLoS One. 2015;10:e0139168. doi: 10.1371/journal.pone.0139168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman-Mazursky O, Elkon R, Efrat S. Redifferentiation of expanded human islet beta cells by inhibition of ARX. Sci Rep. 2016;6:20698. doi: 10.1038/srep20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiori JL, Shin YK, Kim W, et al. Resveratrol prevents beta-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes. 2013;62:3500–3513. doi: 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 47.Banks AS, Kim-Muller JY, Mastracci TL, et al. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Cell Metab. 2011;14:587–597. doi: 10.1016/j.cmet.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiang L, Tsuchiya K, Kim-Muller JY, Lin HV, Welch C, Accili D. Increased atherosclerosis and endothelial dysfunction in mice bearing constitutively deacetylated alleles of Foxo1 gene. J Biol Chem. 2012;287:13944–13951. doi: 10.1074/jbc.M111.332767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev. 2010;31:52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu ZL, Jones RM, He H, et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148:2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- 51.Palmieri F. Mitochondrial transporters of the SLC25 family and associated diseases: a review. J Inherit Metab Dis. 2014;37:565–575. doi: 10.1007/s10545-014-9708-5. [DOI] [PubMed] [Google Scholar]
- 52.Brun T, Scarcia P, Li N, et al. Changes in mitochondrial carriers exhibit stress-specific signatures in INS-1Ebeta-cells exposed to glucose versus fatty acids. PLoS One. 2013;8:e82364. doi: 10.1371/journal.pone.0082364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soni MS, Rabaglia ME, Bhatnagar S, et al. Downregulation of carnitine acyl-carnitine translocase by miRNAs 132 and 212 amplifies glucose-stimulated insulin secretion. Diabetes. 2014;63:3805–3814. doi: 10.2337/db13-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim-Muller JY, Accili D. Cell biology. Selective insulin sensitizers. Science. 2011;331:1529–1531. doi: 10.1126/science.1204504. [DOI] [PubMed] [Google Scholar]
- 55.Savage PJ, Bennion LJ, Flock EV, et al. Diet-induced improvement of abnormalities in insulin and glucagon secretion and in insulin receptor binding in diabetes mellitus. J Clin Endocrinol Metab. 1979;48:999–1007. doi: 10.1210/jcem-48-6-999. [DOI] [PubMed] [Google Scholar]
- 56.Doar JW, Wilde CE, Thompson ME, Sewell PF. Influence of treatment with diet alone on oral glucose-tolerance test and plasma sugar and insulin levels in patients with maturity-onset diabetes mellitus. Lancet. 1975;305:1263–1266. doi: 10.1016/s0140-6736(75)92550-7. [DOI] [PubMed] [Google Scholar]
- 57.Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet. 1976;307:444–447. doi: 10.1016/s0140-6736(76)91473-2. [DOI] [PubMed] [Google Scholar]
- 58.U.K. Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 59.White MG, Marshall HL, Rigby R, et al. Expression of mesenchymal and alpha-cell phenotypic markers in islet beta-cells in recently diagnosed diabetes. Diabetes Care. 2013;36:3818–3820. doi: 10.2337/dc13-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marselli L, Suleiman M, Masini M, et al. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia. 2014;57:362–365. doi: 10.1007/s00125-013-3098-3. [DOI] [PubMed] [Google Scholar]
- 61.Cinti F, Bouchi R, Kim-Muller JY, et al. Evidence of beta-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab. 2016;101:1044–1054. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Defronzo RA, Tripathy D, Schwenke DC, et al. Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes. 2013;62:3920–3926. doi: 10.2337/db13-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 64.U.K. Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 65.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 66.Wajchenberg BL. beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 67.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]