Abstract
Recovery of functional β-cell mass continues to be an ongoing challenge in treating diabetes. Initial work studying β-cells suggested apoptotic β-cell death as a main contributor for the loss of β-cell mass in diabetes. Restoration of β-cells either by transplant or stimulating proliferation of remaining β-cells or precursors would then logically be a viable therapeutic option for diabetes. However, recent work has highlighted the inherent β-cell plasticity and the critical role of loss of β-cell identity in diabetes, and has suggested that β-cells fail to maintain a fully differentiated glucose-responsive and drug-responsive state, particularly in diabetic individuals with poorly-controlled, long-lasting periods of hyperglycemia. Understanding the underlying mechanisms of loss of β-cell identity and conversion in other cell types, as well as how to regain their mature differentiated functional state, is critical to develop novel therapeutic strategies to prevent or reverse these processes. In this review, we discuss the role of plasticity and loss of β-cell identity in diabetes, the current understanding of mechanisms involved in altering this mature functional β-cell state, and potential progresses to identify novel therapeutic targets providing better opportunities for slowing or preventing diabetes progression.
Keywords: glucotoxicity, β-cell, type 2, type 1, monogenic, diabetes, hormones, insulin, glucose, sulfonylureas, insulin, human, mice, therapy, apoptosis, KATP, progenitor, differentiation, dedifferentiation, transdifferentiation, regeneration, proliferation, re-differentiation, obesity, identity, factors, environmental, treatment, stem, fate, glibenclamide, glyburide
Reduction of functional pancreatic β-cell mass in diabetes
Diabetes mellitus is a metabolic disorder characterized by progressive loss or dysfunction of pancreatic insulin-producing β-cells, resulting in multiple long-term complications and organ damage (reviewed in [1]). In type 1 diabetes the deficit of insulin is caused by autoimmune destruction of β-cells, and the logical therapy is the replacement of β-cells by islet transplantation from cadaveric donors or from in vitro generated β-cells from stem cells, although these methods are not always effective or available (reviewed by [2]). In transplants, many of the islets decline progressively in a similar manner to that observed in type 2 diabetes [3], and several of the same stressors that are suggested to induce β-cell dysfunction in type 2 diabetes, such as hyperglycemia and increased secretory demand, inflammation, oxidative and endoplasmic reticulum stress, are also seen in islet grafts concurrently with decline [4].
In contrast to the destruction of β-cells typically seen in type 1 diabetes, type 2 diabetes generally results from high insulin demand due to peripheral insulin resistance with compensatory β-cell expansion and hyperinsulinemia [5-7]. However, this process gradually leads to ‘glucotoxic’ loss of β-cell mass, which has been frequently attributed to enhanced β-cell apoptosis [8-11]. Progressive deterioration in β-cell function, reduction of glucose-stimulated insulin secretion (GSIS), decreased β-cell mass and increased β-cell apoptosis have been found in type 2 diabetic human islets, regardless of the antidiabetic therapy [10,12-15] (Figure 1). Importantly however, the impairment of β-cell function and the decrease in β-cell mass in diabetes seems to be much greater than could be explained only by the observed increase in the rate of apoptosis [10], arguing that another alternative mechanism may also play a role in the progressive loss of β-cell mass in diabetes.
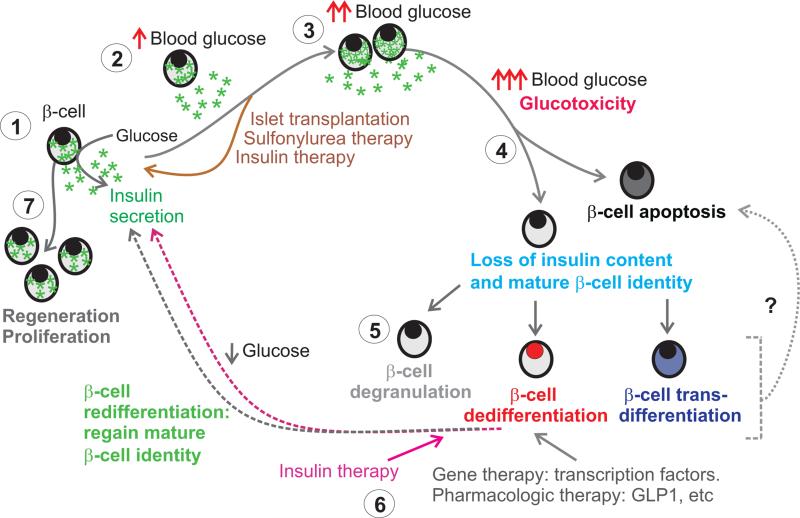
Figure 1. Metabolic state influences cell fate decisions in adult β-cells.
At rest (1) β-cells secrete insulin in response to glucose. In cases where insulin supply is insufficient to respond to metabolic demand (2), β-cells begin to prime themselves to both proliferate and relieve stress. At this point, the functionality of β-cells can be recovered completely with interventions (brown arrow). With sufficiently high blood glucose (3) however, the cells begin to undergo changes induced by glucotoxicity, at which point they may encounter a fate decision (4) between altering their terminally differentiated state and undergoing apoptosis. As changes in cell transcription factor expression occur (5), the β-cells can degranulate, undergo dedifferentiation to more progenitor-like cell fate, or transdifferentiate to an alternative, terminally-differentiated state. Whether this plays a role in further cell susceptibility to apoptosis is not well understood. With therapies (6) that alter cell fate such as intensive insulin therapy to relieve glucotoxicity (pink arrows), gene therapy to restore transcription factors, or treatment with other metabolic modulators (gray arrows), the cells undergo re-differentiation and regain markers of mature β-cell identity as well as insulin content. Under physiological conditions or in the presence of certain stimuli, β-cells can proliferate and grow (7).
β-Cell proliferation and regeneration in diabetes
For many years, it has been assumed that the endocrine pancreas belonged to a class of tissues that were terminally differentiated and irreplaceable in the adult. However, many reports support the view that the endocrine pancreas is a plastic organ, especially regarding the ability of the β-cell mass to change according to the metabolic demand of insulin in conditions such as pregnancy and obesity (reviewed in [16]). Studies have shown an underappreciated proliferative capacity of β-cells with self-replication being one of the major mechanisms regulating β-cell expansion in rodents [17-20] (Figure 1). Glucose and insulin are potent stimulators of β-cell growth and function both in vivo and in vitro (reviewed in [16]). However, the proliferative capacity of β-cells declines over time independently of the species, and human replication seems to be lower than in rodents [19,21-26], which poses a major hurdle to harnessing β-cell proliferation as a therapy for human diabetes.
Many studies of factors linked to replication of human islets have been done in vitro. Some in vitro studies have suggested that the various pathways that stimulate proliferation do so by suppressing the terminally differentiated phenotype of β-cells. Studying human islet replication in these conditions may therefore not reveal sufficient information about all of the metabolic stressors and modulators that affect β-cell function. By transplanting human islets into mice (human islet-to-mouse) it was demonstrated that metabolic factors that induce replication of rodent β-cells fail to have the same effect in human islets [27]. In a similar study using insulin-resistant rodents, betatrophin was found to increase rodent β-cell proliferation but not human transplanted islets [28]. Moreover, clinical data suggest that in contrast to rodents, humans with insulin deficiency or who are obese with poor glycemic control show increased levels of betatrophin [29]. Only a few exogenous factors/inhibitors were shown to have proliferative effects on human β-cells in the in vivo human-to-mouse transplant model. These results highlight the need to focus on human islet function as well as increased β-cell number and/or mass in deriving therapies for diabetes.
“Many efforts have been made to increase β-cell proliferation and regeneration in diabetes, however only a few factors have been identified to have positive effects”
Several studies indicate that β-cells regenerate upon injury-driven severe tissue loss [23,30]. Mice overexpressing c-Myc or treated with diphtheria-toxin, with ~90% and ~70% ablation of β-cells, respectively, showed β-cell regeneration presumably through β-cell neogenesis but not replication [23,31](reviewed by [32]). However, how and to what extent it happens and how much the other islet cells contribute to this phenomenon is currently not completely understood. Although replication appears to be the dominant method by which the pancreatic endocrine compartment maintains or recovers β-cell mass, under extreme circumstances, the pancreas can exploit cell plasticity (see below).
Glucotoxicity and exhaustion as causes of dysfunctional β-cells: are β-cells dead or hidden?
Studies of human pancreatic samples obtained from organ donors have shown that β-cell mass is significantly reduced in type 2 diabetic individuals [5,10,33], a decrease that is frequently attributed to apoptotic β-cell death [34,35]. Some have shown that the decline in β-cell replication in human pancreatic tissue with age is not associated with an increase in the frequency of apoptosis [36], whereas other reports showed that the low frequency of β-cell replication in cadaveric obese and type 2 diabetic organ donors was accompanied by a higher rate of apoptosis [8-10]. Initial in vitro and in vivo studies in mice demonstrated an increase in apoptosis as a mechanism of β-cell loss in islets that were exposed to chronic high glucose, which was attributed to increased inflammatory cytokine production, increased oxidative stress, and various other factors (Figure 1).
Although a marked decrease in β-cell insulin immunostaining has been shown in human pancreases from type 2 cadaveric organ donors, only a small number of apoptotic cells were found [37-41], despite the morphological variations reported in type 2 diabetic islets [42]; suggeting that apoptosis might not be the main contritutor to the decrease in β-cell mass in diabetes. Mouse models of type 2 diabetes demonstrated an over-time marked reduction of insulin-containing β-cells [12,30,43,44]; however, only a small increase in apoptosis was detected. Recently, Accili and colleagues have challenge this paradigm of pancreatic β-cell apoptosis as the main mechanism of β-cell failure in diabetes demonstrating only small increases in apoptosis in both FoxO1 deficient mouse model of diabetes under conditions of stress, and in mouse models of type 2 diabetes [45]; with such increases not sufficient to explain the marked decreases in insulin-producing β-cells in these models [45]. Additionally, in human islet-to-mouse studies, neither chronic hyperglycemia nor peripheral insulin resistance was sufficient to cause apoptosis in human islets, in contrast to initial findings in rodent islets [27].
Gain-of-function (GOF) mutations in the ATP-sensitive potassium (KATP) channel cause neonatal diabetes mellitus (a disease characterized by insulin deficiency without destruction of β-cells or preceding peripheral insulin resistance and overnutrition) and a KATP-GOF variant is highly associated with development of type 2 diabetes (reviewed in [46]). We and others have previously generated mouse models of human neonatal diabetes induced by a KATP-GOF mutation [47-49]. Not only these mice reiterate the human features of neonatal diabetes, but also, as the disease progresses these mice show a marked decrease in insulin-containing β-cells [47,49-51] (Figure 1). Syngeneic islet transplantation or early treatment with glibenclamide (an antidiabetic sulfonylurea widely used to treat diabetes) at disease onset prevented loss of insulin content, suggesting that tight glycemic control early in disease progression could prevent β-cell loss [47] (Figure 1). However, we and others have recently shown that mice with hyperglycemia-induced glucotoxicity have only a small increase in apoptotic cell death [27,45,50,51]. Together, these results do not explain the progressive and marked decrease in β-cell mass frequently observed in humans, and suggest that mechanisms other than β-cell apoptosis may also play a role in diabetes progression.
“The observed increase in the rate of apoptosis in diabetes is insufficient to explain the marked reduction in β-cell mass”
β-cell identity crisis in diabetes
The current understanding that loss of β-cell mass in diabetes is primarily induced by β-cell death has been recently challenged. The notion that β-cell identity, rather than β-cell apoptosis, may be compromised was first shown by Jonas et al [52] and others later suggested that this may involve loss of mature β-cell identity accompanied by dedifferentiation. Genetic and epigenetic analysis of endocrine and exocrine cells within the pancreas revealed a certain degree of cellular plasticity under pathological conditions (reviewed in [53]).
Maintenance of cell identity requires active regulation of gene expression, evidenced by the finding that deletion of Pdx1 from post-natal islets resulted in loss of the β-cells phenotype [54], and that expression of Pdx1 in endocrine progenitors drives these cells to adopt a β-cell like fate. A number of other transcription factors have been also identified as essential for the development and maintenance of functional β-cells [55]. Loss of mature β-cell identity has been shown in several rodent models of diabetes. Chronic hyperglycemia in rats is accompanied by the loss of β-cell transcription factors [52]. β-cells from mice that genetically lack FoxO1 in β-cells (under conditions of metabolic stress) or mouse models of type 2 diabetes demonstrated a marked reduction in both message and protein levels of the mature β-cell transcription factors MafA, Nkx6.1 and Pdx1 [45]. Furthermore, in islets from severely diabetic, genetically modified mouse models of human KATP-induced neonatal diabetes, we and others have recently shown a marked reduction in the mature β-cell transcription factors MafA, Pdx1, Nkx6.1 as well as insulin [50,51]. Importantly and in correlation with this, two independent recent reports showed that pancreatic islets from type 2 diabetic cadaveric organ donors have a marked and selective loss of transcription factors involved in mature β-cell identity including MafA, Nkx6.1, Pdx1; this decrease was associated with a marked reduction in insulin immunostaining in these samples [37,38]. Furthermore, Nkx6.1 and MafB (found in human β-cells but not mouse β-cells) were reduced in human islet-to-mouse grafts under conditions of chronic hyperglycemia and insulin resistance, whereas hyperglycemia alone resulted in reduced MafB but not Nkx6.1 in human islets [27]. Thus, it seems that in diabetes both human and rodent β-cells lose transcription factors that are necessary to maintain mature cell identity, possibly changing cell fate (Figure 1).
“Loss of mature β-cell identity is a key feature found in different forms of diabetes, from monogenic to type 2 diabetes”
Is it a change in β-cell fate in diabetes?
Decrease in β-cell mass likely occurs in a gradual way, with a progressive loss of the hallmark of one cell type (as shown above) and the ability to express genes of an alternate cell type, i.e. precursor cells (dedifferentiation) or other mature cell type (transdifferentiation). Increasing evidence suggests that β-cells respond to challenges by transitioning to novel phenotypes, but it is still debated if the new cells carry all the features of a fully differentiated cell during organogenesis. β-cell dedifferentiation was inferred early on from partial pancreatectomy studies [52] and observed in in vitro studies [15]. β-cell dedifferentiation with reversion to pancreatic progenitor-like cells expressing Neurogenin3, Oct4, Nanog and L-Myc was recently demonstrated in β-cells from FoxO1 knockout mouse during stress induced hyperglycemia by Accili's group [45] (Figure 1). Changes that indicate β-cell dedifferentiation in response to the insult have been shown in a mouse model of pancreatic ductal ligation [56].
Importantly, concomitant with the decrease in mature β-cell markers, we have recently demonstrated β-cell dedifferentiation to islet-like progenitor cells (insulin negative and neurogenin3 positive cells) in a novel mouse model of KATP-induced human neonatal diabetes [50] (Figure 1). However, we did not find evidence of presence of pancreatic-progenitor markers such as Oct4, Nanog and L-Myc in this other mouse model of diabetes. While these results in mice suggest that β-cell dedifferentiation could be a mechanism of loss of β-cell mass in several forms of diabetes, whether this process is significant in human diabetes is still controversial [57]. As mentioned above, there was a marked inactivation of mature β-cell markers in islets obtained from type 2 diabetic cadaveric organ donors, however no increases in β-cell progenitor markers (including neurogenin3) were found in this study [37]. Conversely, a recent report shows evidence of β-cell dedifferentiation as assessed by the presence of aldehyde dehydrogenase 1A3 (a newly identified progenitor marker, as technical problems arose to detect Neurogenin3) concomitant with decreased mature β-cell markers in islets from type 2 diabetic organ donors [38] (Figure 1). Moreover, β-cell-specific transcription factors were ectopically found in glucagon- and somatostatin-producing cells of diabetic subjects [38]. Thus, studies examining different types of stress, genetic or metabolic, have converged on a new perspective suggesting loss of mature β-cell identity with dedifferentiation as a potential cause for the progressive loss of β-cell mass and β-cell failure in monogenic, type 2, and possibly type 1 diabetes.
“Dedifferentiation to progenitor cells and transdifferentiation to glucagon-producing α-cells are important mechanisms of β-cell failure in diabetes”
β-Cell dedifferentiation vs degranulation in diabetes
The possible presence of empty β-cells containing no insulin in diabetes is of interest but important questions still remain. How many empty β-cells are in the pancreas and how much do they contribute to the pathology? Sections from partially pancreatectomized rats as well as a baboon model of diabetes induced by streptozotocin do not show evidence for significant numbers of empty β-cells in the spite of the chronic hyperglycemia. Based on parallel light and electron microscopy examinations of pancreatic samples from nondiabetic and diabetic donors, it appears that a proportion of β-cells in type 2 diabetic islets may not be detectable by standard immunohistochemistry, possibly due to insulin degranulation [38], potentially leading to an overestimation of β-cell loss. At least some type 2 diabetic pancreatic islets also showed a marked loss of GSIS without an actual loss of β-cells [42]. This was also observed in human islet-to-mouse studies under conditions of metabolic stress [27]. Recently, it has been shown that there is degranulation in some human islets obtained from pancreatic type 2 diabetic organ donors, which was accompanied by some β-cells showing evidence of dedifferentiation and transdifferentiation [38] (Figure 1). The changes are probably caused by hyperglycemia and there is a good reason to think that tight glycemic treatment will restore the normal phenotype of these β-cells, whether degranulated or dedifferentiated.
Are transdifferentiation and hyperglucagonemia part of the problem in diabetes?
In various mouse models of diabetes, a percentage of former β-cells can also adopt the mature identity of glucagon-producing α-cells under hyperglycemic conditions [45,51]. We also found a small percentage of dedifferentiated cells (former β-cells) adopted the features of glucagon-secreting α-cells (transdifferentiation) [50] (Figure 1). These findings are consistent with previous reports that human β-cells can undergo transdifferentiation into α-cells indistinguishable from native α-cells during islet dispersion and re-aggregation, even in the absence of any specific transcription factor or genetic manipulation; and that they can maintain this phenotype after transplantation in vivo [58]. These results are also consistent with reports on islets from type 2 diabetic humans and macaques demonstrating co-staining for insulin and glucagon in some cells as well as that glucagon+ cells that are also positive for Nkx6.1 and Pdx1, transcription factors normally present in β-cells [59]. Failure of insulin signaling to suppress glucagon secretion and action (particularly hepatic gluconeogenesis) is significant in the progression of diabetes; and disrupting glucagon signaling can correct glucose tolerance and hyperglycemia in the absence of insulin secretion. In diabetic patients, hyperglucagonemia and failure of glucagon secretion to respond to meal intake are significant factors in hyperglycemia, though whether transdifferentiation affects glucagon production, endogenous α-cell function, or other factors in these signaling pathways beyond the effects of insulin loss remains unclear.
The temperamental β-cell: does bariatric surgery play a role?
The concept of reversibility of β-cell dysfunction in diabetes has also been borne out by the remarkable restoration of glucose-dependent insulin secretion after bariatric surgery in type 2 diabetic patients [60]. β-cell functional defects also play a major role in the pathophysiology of diabetes and improvements at this level may better explain, for instance, why patients who have undergone bariatric surgery may show diabetes remission within a few days after surgery and with only a 1–2% weight loss [61]. In rats, Roux-en-Y gastric bypass partially reversed loss of insulin content, rescued expression of β-cell specific differentiation markers, and prevented degranulation of insulin-containing cells [62]. Several mechanisms are proposed by which gastric bypass could affect pancreatic function, including increased incretin effects, via increased GLP-1 secretion, decreased dopamine secretion, indirect restoration of insulin sensitivity and appetite regulation, as well as increased pancreatic blood flow and improved lipid metabolism (reviewed in [63]). Whether recovery of β-cell identity is a significant factor in the effectiveness of bariatric surgery is unclear.
Preservation of β-cell identity in diabetes: therapeutic implications
It has been proposed that early and intensive correction of glucotoxicity during the diabetic progression of β-cell dysfunction might preserve endogenous β-cells [64-66]. Although insulin and sulfonylurea monotherapy were more effective in maintaining good glycemic control in the UK prospective study [14], such an approach did not prevent the natural decline of β-cell function and diabetic complications. Several short-term studies have shown that intensive insulin therapy for 2-3 weeks at the time of diagnosis leads to rapid improvement of insulin secretion and preserves β-cell function [66-69]. Insulin therapy achieves greater improvement in β-cell function compared with oral monotherapy [70-72], and insulin in combination with oral agents preserve β-cell function better than insulin alone [73]. Short-term glycemic control by intravenous insulin infusion restored sulfonylurea sensitivity in unresponsive type 2 diabetic subjects, with a management of diabetes with glibenclamide alone for the following 6 months [74].
Strikingly, we recently demonstrated restoration of β-cell mass and insulin content in severely diabetic mice following intensive insulin therapy [50]. Lineage tracing analysis demonstrates that reversing hyperglycemia by insulin therapy led the same dedifferentiated cells to re-differentiate to mature β-cells [50] (Figure 1). While re-differentiated KATP-GOF β-cells have recovered insulin content after insulin therapy, they will still fail to secrete insulin in response to glucose challenge, but they should have recovered insulin secretion in response to antidiabetic sulfonylureas. In agreement with this prediction, no insulin secretion was found in response to glucose, but these islets do show insulin secretion in response to the sulfonylurea glibenclamide in vitro [50]. As predicted, no C-peptide secretion (as a surrogate measurement for insulin secretion) was found in KATP-GOF diabetic mice in response to acute injection of glibenclamide in vivo (Figure 1). However, C-peptide levels were considerably higher in the same mice after 10 days of insulin treatment, and were even higher after 40 days of insulin therapy [50]. Thus, restoration of β-cells in diabetes leads to reestablishment of antidiabetic drug-responsivity in mice. These results may provide a potential explanation for the gradual decrease in β-cell mass observed in patients with long-lasting and poorly-controlled diabetes, as well as for the recovery of β-cell function and sulfonylurea responsivity in type 2 diabetic patients following β-cell ‘rest’ after a short period of intensive insulin therapy [14,66,68,75-77] (Figure 1).
“Early and aggressive antidiabetic therapies can restore mature β-cell identity in diabetes”
Concluding remarks
Our understanding of the impact of β-cell plasticity and function continues to grow. Fully differentiated β-cells have revealed more cellular fate flexibility than previously thought. In response to genetic or epigenetic factors, and environmental cues, β-cells can transition to different stages, including dedifferentiation and transdifferentiation, with re-differentiation when the conditions are more appropriate (i.e. lowering of blood glucose by anti-diabetic drugs). These processes could be transient or permanent depending on the severity and duration of the insult/stimuli; therefore early intervention may be important to avoid the prolonged exposure to stressors that will prevent cells to return to their mature cell identity, and consequently cause further β-cell dysfunction.
The cell adoption of a resting or dedifferentiated state can temporarily allow an interruption of the normal cellular function as a preventive mechanism to circumvent damage or death, as illustrated by β-cells undergoing dedifferentiation, or, alternatively, transdifferentiation to another cell type, rather than apoptosis in cases of metabolic stress in diabetes. Revising our current understanding of β-cell function to include the view that differentiated cells retain this inherent plasticity and that dedifferentiation may be a mechanism to enhance β-cell survival, but the long-term exposure to hyperglycemia/stressors cause β-cell deterioration and damage. Together, identifying ways to inhibit or reverse these stages by acute interventions early on will substantially increase the opportunities for developing novel therapies for restoring β-cell function in diabetes.
Acknowledgements
CE and MSR wrote the review. This work was supported in part by grants from NIH R01 DK098584 to MSR and Diabetes Research Center Grant 5P60 DK020579 to MSR.
Footnotes
Conflict of Interest
The authors have declared no conflicts of interest.
References
- 1.Weir GC, Bonner-Weir S. Islet beta cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci. 2013;1281:92–105. doi: 10.1111/nyas.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke GW, Vendrame F, Virdi SK, et al. Lessons from pancreas transplantation in type 1 diabetes: recurrence of islet autoimmunity. Curr Diabetes Rep. 2015;15:1–9. doi: 10.1007/s11892-015-0691-5. [DOI] [PubMed] [Google Scholar]
- 3.Potter KJ, Westwell-Roper CY, Klimek-Abercrombie AM, Warnock GL, Verchere CB. Death and dysfunction of transplanted β-cells: Lessons learned from type 2 diabetes? Diabetes. 2014;63:12–19. doi: 10.2337/db12-0364. [DOI] [PubMed] [Google Scholar]
- 4.Leibowitz G, Kaiser N, Cerasi E. Beta-cell failure in type 2 diabetes. J Diabetes Invest. 2011;2:82–91. doi: 10.1111/j.2040-1124.2010.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahren B. Type 2 diabetes, insulin secretion and beta-cell mass. Curr Mol Med. 2005;5:275–286. doi: 10.2174/1566524053766004. [DOI] [PubMed] [Google Scholar]
- 6.Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/nfat signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 7.Jhala US, Canettieri G, Screaton RA, et al. Camp promotes pancreatic beta-cell survival via creb-mediated induction of irs2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poitout V, Robertson RP. Glucolipotoxicity: Fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 11.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Sakuraba H, Mizukami H, Yagihashi N, et al. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of japanese type ii diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 13.Del Prato S, Bianchi C, Marchetti P. Beta-cell function and anti-diabetic pharmacotherapy. Diabetes Metab Res Rev. 2007;23:518–527. doi: 10.1002/dmrr.770. [DOI] [PubMed] [Google Scholar]
- 14.UKPDS-group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33).Uk prospective diabetes study group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 15.Weinberg N, Ouziel-Yahalom L, Knoller S, Efrat S, Dor Y. Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic beta-cells. Diabetes. 2007;56:1299–1304. doi: 10.2337/db06-1654. [DOI] [PubMed] [Google Scholar]
- 16.Bonner-Weir S. Life and death of the pancreatic beta cells. Trends Endocrinol Metab. 2000;11:375–378. doi: 10.1016/s1043-2760(00)00305-2. [DOI] [PubMed] [Google Scholar]
- 17.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation.[see comment]. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 18.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Fiaschi-Taesch NM, Vasavada RC, et al. Diabetes mellitus advances and challenges in human [beta]-cell proliferation. Nat Rev Endocrinol. 2015;11:201–212. doi: 10.1038/nrendo.2015.9. [DOI] [PubMed] [Google Scholar]
- 20.Takane KK, Kleinberger JW, Salim FG, Fiaschi-Taesch NM, Stewart AF. Regulated and reversible induction of adult human β-cell replication. Diabetes. 2012;61:418–424. doi: 10.2337/db11-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushner JA. The role of aging upon beta cell turnover. J Clin Invest. 2013;123:990–995. doi: 10.1172/JCI64095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cano DA, Rulifson IC, Heiser PW, et al. Regulated beta-cell regeneration in the adult mouse pancreas. Diabetes. 2008;57:958–966. doi: 10.2337/db07-0913. [DOI] [PubMed] [Google Scholar]
- 24.Rohatgi N, Remedi MS, Kwon G, et al. Therapeutic strategies to increase human β-cell growth and proliferation by regulating mtor and gsk-3/β-catenin pathways. Open Endocrinol J. 2010;1:40–54. doi: 10.2174/1874216501004010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human beta-cell proliferation and intracellular signaling: Driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53:2167–2176. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai C, Kayton NS, Shostak A, et al. Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J Clin Invest. 2016;126:1857–1870. doi: 10.1172/JCI83657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao Y, Le Lay J, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human β-cell replication in the transplant setting. Diabetes. 2014;63:1283–1288. doi: 10.2337/db13-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espes D, Martinell M, Liljebäck H, Carlsson P-O. Betatrophin in diabetes mellitus: the epidemiological evidence in humans. Curr Diabetes Rep. 2015;15:1–8. doi: 10.1007/s11892-015-0676-4. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 31.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonal C, Avril I, Herrera PL. Experimental models of beta-cell regeneration. Biochem Soc Trans. 2008;36:286–289. doi: 10.1042/BST0360286. [DOI] [PubMed] [Google Scholar]
- 33.Hanley NA, Hanley KP, Miettinen PJ, Otonkoski T. Weighing up beta-cell mass in mice and humans: Self-renewal, progenitors or stem cells? Mol Cell Endocrinol. 2008;288:79–85. doi: 10.1016/j.mce.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Maedler K. Beta cells in type 2 diabetes – a crucial contribution to pathogenesis. Diabetes Obes Metab. 2008;10:408–420. doi: 10.1111/j.1463-1326.2007.00718.x. [DOI] [PubMed] [Google Scholar]
- 35.Matveyenko AV, Butler PC. Relationship between β-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10:23–31. doi: 10.1111/j.1463-1326.2008.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reers C, Erbel S, Esposito I, et al. Impaired islet turnover in human donor pancreata with aging. Eur J Endocrinol. 2009;160:185–191. doi: 10.1530/EJE-08-0596. [DOI] [PubMed] [Google Scholar]
- 37.Guo S, Dai C, Guo M, et al. Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cinti F, Bouchi R, Kim-Muller JY, et al. Evidence of beta-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab. 2015;101:1044–1054. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3:758–768. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- 40.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in european subjects with type 2 diabetes. Diabetes Obes Metab. 2008;1032(Suppl):42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 41.Hanley SC, Austin E, Assouline-Thomas B, et al. {beta}-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology. 2010;151:1462–1472. doi: 10.1210/en.2009-1277. [DOI] [PubMed] [Google Scholar]
- 42.Marselli L, Suleiman M, Masini M, et al. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia. 2014;57:362–365. doi: 10.1007/s00125-013-3098-3. [DOI] [PubMed] [Google Scholar]
- 43.Laybutt DR, Glandt M, Xu G, et al. Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem. 2003;278:2997–3005. doi: 10.1074/jbc.M210581200. [DOI] [PubMed] [Google Scholar]
- 44.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 45.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flanagan SE, Clauin S, Bellanne-Chantelot C, et al. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30:170–180. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]
- 47.Remedi MS, Kurata HT, Scott A, et al. Secondary consequences of beta cell inexcitability: Identification and prevention in a murine model of k(atp)-induced neonatal diabetes mellitus. Cell Metab. 2009;9:140–151. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koster JC, Knopp A, Flagg TP, et al. Tolerance for ATP-insensitive KATP channels in transgenic mice. Circ Res. 2001;89:1022–1029. doi: 10.1161/hh2301.100342. [DOI] [PubMed] [Google Scholar]
- 49.Girard CA, Wunderlich FT, Shimomura K, et al. Expression of an activating mutation in the gene encoding the katp channel subunit kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 2014;19:872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brereton MF, Iberl M, Shimomura K, et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun. 2014;5:4639. doi: 10.1038/ncomms5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jonas JC, Sharma A, Hasenkamp W, et al. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- 53.Puri S, Hebrok M. Cellular plasticity within the pancreas--lessons learned from development. Dev Cell. 2010;18:342–356. doi: 10.1016/j.devcel.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse ipf1/pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szabat M, Lynn FC, Hoffman BG, et al. Maintenance of beta-cell maturity and plasticity in the adult pancreas: Developmental biology concepts in adult physiology. Diabetes. 2012;61:1365–1371. doi: 10.2337/db11-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao X, Chen Z, Shiota C, et al. No evidence for beta cell neogenesis in murine adult pancreas. J Clin Invest. 2013;123:2207–2217. doi: 10.1172/JCI66323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Butler AE, Dhawan S, Hoang J, et al. Beta-cell deficit in obese type 2 diabetes, a minor role of beta-cell dedifferentiation and degranulation. Journal Clin Endocrinol Metab. 2016;101:523–532. doi: 10.1210/jc.2015-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spijker HS, Ravelli RBG, Mommaas-Kienhuis AM, et al. Conversion of mature human β-cells into glucagon-producing α-cells. Diabetes. 2013;62:2471–2480. doi: 10.2337/db12-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spijker HS, Song H, Ellenbroek JH, et al. Loss of β-cell identity occurs in type 2 diabetes and is associated with islet amyloid deposits. Diabetes. 2015;64:2928–2938. doi: 10.2337/db14-1752. [DOI] [PubMed] [Google Scholar]
- 60.Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52:1098–1103. doi: 10.2337/diabetes.52.5.1098. [DOI] [PubMed] [Google Scholar]
- 61.Bradley D, Magkos F, Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology. 2012;143:897–912. doi: 10.1053/j.gastro.2012.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian B, Zhou X, Li B, et al. Reduction of pancreatic β-cell dedifferentiation after gastric bypass surgery in diabetic rats. J Mol Cell Biol. 2014;6:531–534. doi: 10.1093/jmcb/mju042. [DOI] [PubMed] [Google Scholar]
- 63.Arble DM, Sandoval DA, Seeley RJ. Mechanisms underlying weight loss and metabolic improvements in rodent models of bariatric surgery. Diabetologia. 2014;58:211–220. doi: 10.1007/s00125-014-3433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776–785. doi: 10.1089/dia.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Page KA, Reisman T. Interventions to preserve beta-cell function in the management and prevention of type 2 diabetes. Current Diab Rep. 2013;13:252–260. doi: 10.1007/s11892-013-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: A multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 67.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type ii diabetes mellitus. Diabetes. 1985;34:222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- 68.Ilkova H, Glaser B, Tunckale A, Bagriacik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–1356. doi: 10.2337/diacare.20.9.1353. [DOI] [PubMed] [Google Scholar]
- 69.Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–1032. doi: 10.2337/diacare.27.5.1028. [DOI] [PubMed] [Google Scholar]
- 70.Chen HS, Wu TE, Jap TS, et al. Beneficial effects of insulin on glycemic control and beta-cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy. Diabetes Care. 2008;31:1927–1932. doi: 10.2337/dc08-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 72.Chon S, Oh S, Kim SW, et al. The effect of early insulin therapy on pancreatic beta-cell function and long-term glycemic control in newly diagnosed type 2 diabetic patients. Korean J Intern Med. 2010;25:273–281. doi: 10.3904/kjim.2010.25.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yki-Jarvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care. 2001;24:758–767. doi: 10.2337/diacare.24.4.758. [DOI] [PubMed] [Google Scholar]
- 74.Sinagra D, Greco D, Amato MC, D'Acquisto G, Galluzzo A. A 12-h intravenous insulin infusion restores the beta-cell response torpidity to sulfonylureas in patients affected by type 2 diabetes. Diabetes Care. 2000;23:1857–1858. doi: 10.2337/diacare.23.12.1857. [DOI] [PubMed] [Google Scholar]
- 75.Alvarsson M, Sundkvist G, Lager I, et al. Effects of insulin vs. Glibenclamide in recently diagnosed patients with type 2 diabetes: A 4-year follow-up. Diabetes Obes Metab. 2008;10:421–429. doi: 10.1111/j.1463-1326.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 76.Torella R, Salvatore T, Cozzolino D, et al. Restoration of sensitivity to sulfonylurea after strict glycaemic control with insulin in non-obese type 2 diabetic subjects. Diabete Metab. 1991;17:443–447. [PubMed] [Google Scholar]
- 77.Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]