Abstract
Aim
To assess the role of imaging in the early management of encephalitis and the agreement on findings in a well-defined cohort of suspected encephalitis cases enrolled in the Prospective Aetiological Study of Encephalitis conducted by the Health Protection Agency (now incorporated into Public Health England).
Materials and methods
Eighty-five CT examinations from 68 patients and 101 MRI examinations from 80 patients with suspected encephalitis were independently rated by three neuroradiologists blinded to patient and clinical details. The level of agreement on the interpretation of images was measured using the kappa statistic. The sensitivity, specificity, and negative and positive predictive values of CT and MRI for herpes simplex virus (HSV) encephalitis and acute disseminated encephalomyelitis (ADEM) were estimated.
Results
The kappa value for interobserver agreement on rating the scans as normal or abnormal was good (0.65) for CT and moderate (0.59) for MRI. Agreement for HSV encephalitis was very good for CT (0.87) and MRI (0.82), but only fair for ADEM (0.32 CT; 0.31 MRI). Similarly, the overall sensitivity of imaging for HSV encephalitis was ∼80% for both CT and MRI, whereas for ADEM it was 0% for CT and 20% for MRI. MRI specificity for HSV encephalitis between 3–10 days after symptom onset was 100%.
Conclusion
There is a subjective component to scan interpretation that can have important implications for the clinical management of encephalitis cases. Neuroradiologists were good at diagnosing HSV encephalitis; however, agreement was worse for ADEM and other alternative aetiologies. Findings highlight the importance of a comprehensive and multidisciplinary approach to diagnosing the cause of encephalitis that takes into account individual clinical, microbiological, and radiological features of each patient.
Highlights
-
•
We assessed the role of imaging in encephalitis.
-
•
We assessed the agreement between raters on scan interpretation.
-
•
Diagnosis for herpes simplex encephalitis (HSE) was good.
-
•
Agreement was worse for ADEM and other alternative aetiologies.
-
•
HSE can be dismissed if MRI normal 72 hours after neurological symptom onset (with negative CSF tests).
Introduction
The prodrome of encephalitis is often non-specific, making it clinically difficult at an early stage to distinguish from other neurological syndromes. Both infectious and immune-mediated pathogeneses must be considered.1 Approximately one-third of cases are immune-mediated, most frequently acute disseminated encephalomyelitis (ADEM), but also antibody-associated encephalitides, such as N-methyl-d-aspartate receptor antibody (NMDAR antibody) and voltage-gated potassium channel-complex antibody (VGKC-complex antibody) encephalitis.2, 3, 4 Despite over 100 recognised causes, most studies fail to identify an aetiology in the majority of suspected cases.5
Management of acute encephalitis includes empirical initiation of antimicrobials. Following diagnostic investigations, these are rationalised and/or additional treatment instituted. Factors that complicate clinical decision making include falsely negative cerebrospinal fluid (CSF) polymerase chain reaction (PCR) results (particularly in herpes simplex virus [HSV] encephalitis) arising either from sampling too early or late or after institution of treatment; the availability of investigations; and lag time to results.6
Neuroimaging has a critical role in the evaluation of such patients. It aids diagnosis of encephalitis aetiology as well as mimicker conditions; it identifies complications (such as intracranial mass effect); and it may help prognostication. In patients with low probability of HSV encephalitis, Tyler7 suggested that magnetic resonance imaging (MRI) findings later in the illness along with other clinical parameters could guide halting acyclovir. This is now incorporated in UK guidelines.8
Patterns of imaging abnormalities are described in cohorts of more common encephalitis aetiologies, such as HSV encephalitis.9 In Japanese encephalitis, thalamic lesions on MRI are commonly seen, but the diagnosis is not excluded by their absence.10 Most patients with autoimmune or paraneoplastic limbic encephalitis, for example, those associated with antibodies against the gamma-aminobutyric acid-B receptor (GABABR) or leucine-rich glioma inactivated protein 1 (LGI1), have an increased signal in the medial temporal lobes. The brain MRI is normal in approximately 60% of patients with anti-NMDAR encephalitis.11 Very few studies have systematically investigated neuroimaging in all-cause encephalitis. Research is needed to better define associations between neuroimaging results and specific encephalitis aetiologies.
The aim of the present study was to assess the role of imaging in the early management of encephalitis and agreement on scan interpretation in a well-defined series of suspected encephalitis cases enrolled in the Prospective Aetiological Study of Encephalitis conducted by the Health Protection Agency (now incorporated into Public Health England [PHE]).
Materials and methods
Specific details regarding the study have been published elsewhere.12 Patients with suspected encephalitis were recruited over a 2-year period (staged start between October 2005 and November 2006) from 24 hospitals in three geographical areas of England. Detailed clinical information was collected and each patient underwent extensive laboratory investigation. The case definition for encephalitis included any person of any age admitted to hospital with encephalopathy (altered level of consciousness persisting >24 hours, including lethargy, irritability, or a change in personality and behaviour) and two or more of the following: fever or history of fever (≥38°C) during the presenting illness; seizures and/or focal neurological findings (with evidence of brain parenchyma involvement); CSF pleocytosis (>4 white blood cells/μl); electroencephalogram (EEG) findings compatible with encephalitis; abnormal results of neuroimaging (computed tomography [CT]/MRI) in keeping with encephalitis.
Wherever possible, CT and MRI images were collected from each patient. This was only possible in a subset of recruiting centres due to practical complexities, including computer and software compatibility issues; however, all regions were represented. Available CT and MRI images were independently rated by three consultant neuroradiologists with expertise in reporting adult and paediatric neuroimaging. The raters were blinded to patient and clinical details and used a pre-defined proforma (see Electronic Supplementary Material), which included a check-boxed list of possible abnormalities.
Age, gender, and aetiology of patients with available CT and MRI images were compared to those in the entire PHE study cohort to assess sample representativeness. Differences in proportions were assessed by chi-squared or Fisher's exact test with a p-value of <0.05 considered statistically significant.
Intra- and interobserver agreement
Intra-observer agreement was measured using interclass correlation coefficient (ICC) for reliability (Winer reliability).13 Interobserver agreement between neuroradiologists on the interpretation of images was measured using the kappa statistic. The following were considered: (1) whether the CT and MRI images were rated as normal or abnormal; (2) whether the scans fitted the diagnostic categories of ADEM, HSV encephalitis, normal, or “other” abnormalities. ‘Other’ abnormalities included those with another specific aetiology (e.g., non-encephalitis mimic) and those with a non-disease-specific scan abnormality; (3) whether the following regions were judged normal or abnormal: frontal, temporal, parietal, and occipital lobes; basal ganglia/thalami; brainstem; cerebellum; limbic structures.
Kappa values have traditionally been defined as <0=no agreement, 0.0–0.20=slight agreement, 0.21–0.40=fair agreement, 0.41–0.60=moderate agreement, 0.61–0.80=good agreement, and 0.81–1.00=very good agreement.14 As a sensitivity analysis ICCs between observers were also calculated.15
Sensitivity, specificity, and negative and positive predictive values
The sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of CT and MRI for HSV and ADEM (the two groups with enough cases) was estimated. Ninety-five percent confidence intervals (95% CI) were calculated according to the efficient-score method corrected for continuity.16, 17 A consensus result from the three raters was used for these purposes, i.e., if two raters recorded “looks like HSV” and one recorded “does not look like HSV”, the consensus was “looks like HSV”. PHE study “final diagnoses” were used as the reference standard.1 HSV encephalitis diagnosis was defined as HSV DNA/antigen detected in any CSF/brain specimens or a HSV-specific intrathecal antibody response.1 ADEM diagnosis was based on agreement reached by an expert multidisciplinary panel's assessment of the patient's clinical details accompanied by a MRI fulfilling the Schwarz ADEM criteria (i.e., one or multiple supra- or infratentorial demyelinating lesions and the absence of “black holes” on T1-weighted imaging).1, 18 These analyses were repeated stratified by age (children <18 years; adults ≥18 years) and by time of scan in relation to onset of symptoms (Day 0–2; Day 3–10; after Day 10). The latter was restricted to HSV due to the smaller sample size of ADEM cases.
The proportion of patients who had undergone CT within 24 hours of hospital admission (as recommended by the UK guidance) was calculated.8 For the same patient, CT examinations were matched to any MRI examinations carried out within 24 hours of the CT examinations to assess any difference in techniques at identification of abnormality.
Descriptive features
The proportion of first scans with structural abnormalities from all-cause encephalitis was calculated for T2-weighted (TW2) imaging, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI) MRI sequences, as was the proportion of scans with structural abnormalities from HSV and unknown encephalitis patients. The unknown group was included to assess whether any specific features were present on neuroimaging that might allude to a specific aetiology. The structures evaluated included grey matter, white matter, temporal lobe, frontal lobe, parietal lobe, occipital lobe, basal ganglia/thalami, brainstem, cerebellum, and limbic structures. These analyses were based on a consensus result from the three raters.
Ethics
Approval was granted by the North and East Devon Multicentre Research Ethics Committee (05/Q2102/22). Written informed consent was obtained from all patients or from their next of kin.
Results
Description of data
Eighty-five CT examinations from 68 patients and 101 MRI examinations from 80 patients with suspected encephalitis were assessed. Fifty-one patients had both a CT and MRI examination carried out, whereas 46 underwent either CT or MRI but not both. Sixteen percent of patients had serial CT examinations, whereas 17% had serial MRI examinations. The aetiological and gender breakdown of patients for whom images were available was similar to that of the main PHE study cohort (n=268; Table 1). A lower proportion of >65 year olds had MRI examinations available for rating than the proportion of >65 year olds recruited to the main study cohort (5/80 [6%] versus 46/268 [17%]; p=0.02). The former were more likely (p=0.04) to have encephalitis of unknown aetiology than the >65 year olds in the main study cohort (4/5 [80%] versus 13/46 [28%]).
Table 1.
Demographics and aetiologies for patients with scans available for rating.
| Patients with CT (n=68) | Patients with MRI (n=80) | Total in main study (n=268) | |
|---|---|---|---|
| Aetiology | |||
| ADEM | 5 (7%) | 10 (12.5%) | 23 (9%) |
| Antibody-associated | 4 (6%) | 6 (7.5%) | 16 (6%) |
| Bacterial | 4 (6%) | 4 (5%) | 13 (5%) |
| HSV | 11 (16%) | 11 (14%) | 38 (14%) |
| Mycobacterium tuberculosis | 5 (7%) | 3 (4%) | 10 (4%) |
| Non-encephalitis | 11a (16%) | 15b (19%) | 59 (22%) |
| Other | 5a (7%) | 4b (5%) | 21 (8%) |
| Unknown | 20 (29%) | 24 (30%) | 76 (28%) |
| VZV | 3 (4%) | 3 (4%) | 12 (5%) |
| Age (years) | |||
| <1 | 3 (4%) | 7 (9%) | 17 (6%) |
| 1–4 | 7 (10%) | 9 (11%) | 29 (11%) |
| 5–19 | 14 (21%) | 20 (25%) | 55 (20.5%) |
| 20–44 | 17 (25%) | 20 (25%) | 66 (25%) |
| 45–64 | 17 (25%) | 19 (24%) | 55 (20.5%) |
| ≥65 | 10 (15%) | 5 (6%) | 46 (17%) |
| Sex | |||
| Male | 33 (49%) | 34 (42.5%) | 146 (54%) |
| Female | 35 (51%) | 46 (57.5%) | 122 (46%) |
ADEM, acute disseminated encephalomyelitis; HSV, herpes simplex virus; VZV, varicella zoster virus.
One other and six non-encephalitis with CT did not meet case definition.
One other and seven non-encephalitis with MRI did not meet case definition.
Intra- and interobserver agreement
Intra-observer reliability was good for both CT (0.85 both for binary and four-category outcomes) and MRI (0.81 and 0.72 for binary and four-category outcomes, respectively). The kappa value for interobserver agreement on rating the scans as normal or abnormal was good (0.65) for CT and moderate (0.59) for MRI. The raters did not agree on whether the scan appeared normal or abnormal for 22 CT examinations (26%) from 18 patients and 30 MRI examinations (30%) from 26 patients. These examinations were predominantly from patients with encephalitis of unknown cause or those with suspected encephalitis who had an alternative non-encephalitic diagnosis (9/18 persons [50%] CTs; 17/26 persons [65%] MRIs).
The overall kappa value for interobserver agreement on whether the examinations fitted one of four diagnostic groups was good (0.68) for CT and moderate (0.54) for MRI (Table 2). Agreement for HSV encephalitis was very good for both CT (0.87) and MRI (0.82), but only fair for ADEM (0.32 for CT and 0.31 for MRI; Table 2; Figure 1, Figure 2). Kappa results were similar when examinations from patients who did not meet the case definition or were deemed non-encephalitis were excluded from the analyses (data not shown).
Table 2.
Kappa statistic for interobserver agreement amongst three neuroradiologists in categorising computed tomography (CT) and magnetic resonance imaging (MRI) examinations into four diagnostic groups and in reporting abnormalities in different areas of the brain.
| CT (n=85) | MRI (n=100a) | |||
|---|---|---|---|---|
| Four aetiological categories | 0.68 | 0.54 | ||
| ADEM | 0.32 | 0.31 | ||
| HSV | 0.87 | 0.82 | ||
| Normal | 0.65 | 0.55 | ||
| Other | 0.62 | 0.42 | ||
| CT (n=85) | MRI (n=101) |
|||
|---|---|---|---|---|
| TW2 | FLAIR | DWI | ||
| Structures | ||||
| Frontal lobe | 0.65 | 0.73 | 0.76 | 0.53 |
| Temporal lobe | 0.80 | 0.73 | 0.73 | 0.73 |
| Parietal lobe | 0.29 | 0.54 | 0.54 | 0.21 |
| Occipital lobe | 0.15 | 0.37 | 0.50 | 0.24 |
| Basal ganglia/thalami | 0.54 | 0.75 | 0.62 | 0.20 |
| Brainstem | –b | 0.67 | 0.53 | 0.18 |
| Cerebellum | 0.32 | 0.50 | 0.57 | 0.38 |
| Limbic changes | 0.58 | 0.61 | 0.65 | 0.75 |
ADEM, acute disseminated encephalomyelitis; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; HSV, herpes simplex virus; TW2, T2-weighted imaging.
One MRI excluded as there was no relevant result for one rater. These kappa analyses also include scans from patients who did not meet the case definition or were deemed non-encephalitis.
No brainstem abnormalities on CT.
Figure 1.
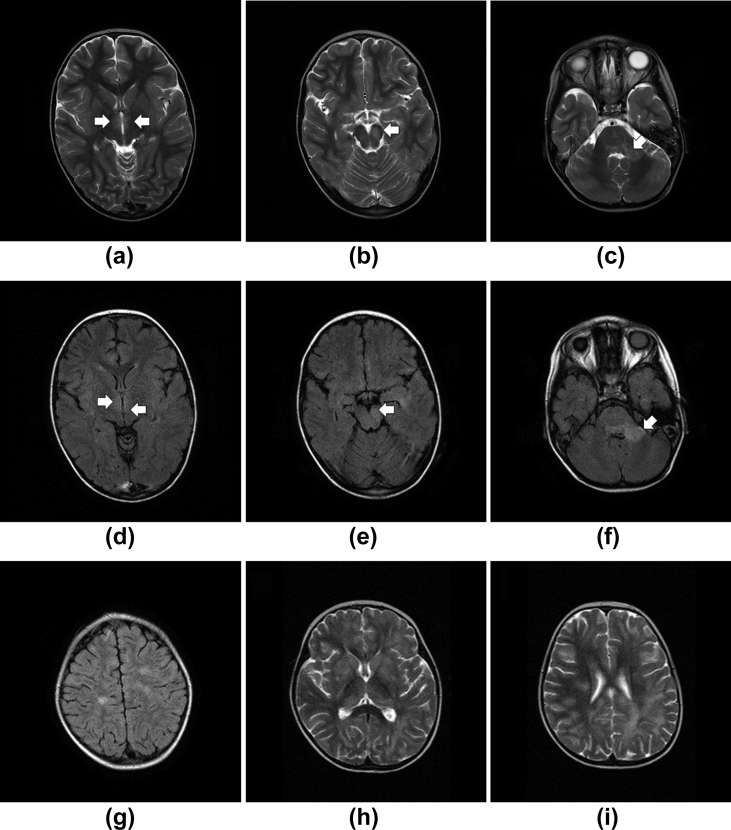
Cases where there was consensus agreement of ADEM diagnosis when reviewing neuroimaging. A 5-year-old male patient (Case 1b26023) with a 5-day history of pyrexia, increasing lethargy, and mild cough/cold; on presentation he had left-sided weakness, ptosis, and unequal pupils noted. (a–c) Axial T2 and (d–f) FLAIR images demonstrating hyperintensity within medial thalamus (arrows; a, d), left cerebral peduncle (arrow; b, c) and left dentate nucleus (not shown) extending in to cerebellar pontine angle (arrow; c, f); with some more widespread white matter lesions as seen on axial flair images in centrum semiovale (g). (h–i) A 5-year-old female patient (Case 1b26029) with a history of lethargy, leg weakness, and unsteadiness walking; also had recent episodes of urinary incontinence. (h) Axial T2 imaging demonstrating bilateral basal ganglia and thalamic lesions and (i) widespread deep and subcortical white matter lesions.
Figure 2.
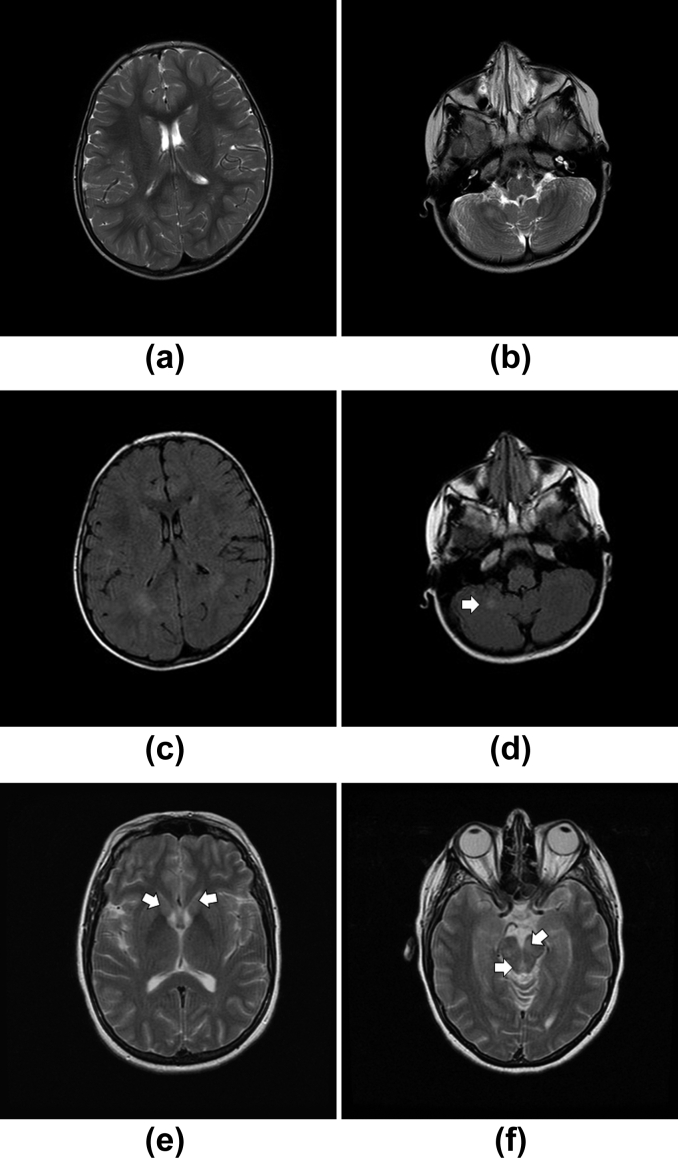
Cases where there was no consensus agreement of ADEM diagnosis when reviewing neuroimaging. A 2-year-old female patient (Case 1b26010) with a 2-day history of unsteadiness; on assessment noted to be ataxic with intention tremor and reluctance to weight bear. (a,b) Axial T2 and (c,d) FLAIR images demonstrating periventricular hyperintensity, which might have been interpreted as terminal zones of myelination (a,c). A solitary, high signal lesion within the cerebellar white matter was seen (arrow; b,d). (e–f) A 40-year-old woman (Case 1c31011) presented with fever, cough, diplopia, dysarthria, and paraesthesia in the feet ascending to the trunk. (e,f) Axial T2 and flair (not shown) images demonstrating symmetrical high signal lesions within the basal ganglia and thalamus (e) cerebral peduncle and periaqueductal grey matter (f). Here, the homogeneous and symmetrical lesion would have prompted the consideration of a neurometabolic disorder, such as a mitochondrial cytopathy, when reviewing the images.
Interobserver agreement in reporting abnormalities in different areas of the brain using CT was highest for the temporal (0.80) and frontal (0.65) lobes; agreement was moderate to only slight agreement for other structures (Table 2). Kappa values were generally higher for MRI than CT; however, interobserver agreement in reporting abnormalities in the parietal and occipital lobes and the cerebellum using MRI was still moderate, fair or slight. Kappa values for reporting structural abnormalities were higher using TW2 and FLAIR sequences than DWI. ICC for interobserver reliability was similar using ICC values ranging from 0.60 to 0.65 (data not shown).
Sensitivity, specificity, NPV and PPV
The overall sensitivity of imaging for HSV encephalitis was ∼80% for both CT and MRI (Table 3). There was little evidence that the sensitivity was lower in children than adults (p=0.5). The overall sensitivity for ADEM was 0% for CT and 20% for MRI, with little evidence of a higher sensitivity in children than adults (p=1.0). The specificity and NPV of CT and MRI for HSV and ADEM was >95% and >80%, respectively, for both children and adults. The PPV of CT and MRI for HSV was 100% for both age groups. The results were similar when scans from patients who did not meet the case definition or were deemed non-encephalitis were excluded from the analyses (data not shown).
Table 3.
Sensitivity, specificity, NPV and PPV of computed tomography (CT) and magnetic resonance imaging (MRI) examinations for HSV and ADEM.
| CT | MRI | |
|---|---|---|
| Both children and adults | (n=85) | (n=101) |
| HSV | ||
| Sensitivity | 80% (95% CI 51–95) | 81% (95% CI 54–95) |
| Specificity | 100% (93–100) | 100% (95–100) |
| NPV | 96% (88–99) | 97% (90–99) |
| PPV | 100% (74–100) | 100% (75–100) |
| ADEM | ||
| Sensitivity | 0% (0–54) | 20% (3–56) |
| Specificity | 99% (94–100) | 99% (93–100) |
| NPV | 94% (86–98) | 92% (84–96) |
| PPV | N/A | 67% (9–99) |
| Children | (n=23) | (n=37) |
| HSV | ||
| Sensitivity | 67% (12–98) | 67% (12–98) |
| Specificity | 100% (80–100) | 100% (87–100) |
| NPV | 95% (74–100) | 97% (83–100) |
| PPV | 100% (16–100) | 100% (16–100) |
| ADEM | ||
| Sensitivity | 0% (0–60) | 29% (5–70) |
| Specificity | 100% (79–100) | 97% (81–100) |
| NPV | 83% (60–94) | 85% (68–94) |
| PPV | N/A | 67% (9–99) |
| Adults | (n=62) | (n=64) |
| HSV | ||
| Sensitivity | 83% (51–97) | 85% (54–97) |
| Specificity | 100% (91–100) | 100% (91–100) |
| NPV | 96% (86–99) | 96% (86–99) |
| PPV | 100% (69–100) | 100% (72–100) |
| ADEM | ||
| Sensitivity | 0% (0–94) | 0% (0–69) |
| Specificity | 100% (93–100) | 100% (93–100) |
| NPV | 98% (90–100) | 95% (86–99) |
| PPV | N/A | N/A |
PPV of CT and MRI for ADEM and PPV of MRI for ADEM in adults only could not be calculated due to zero patients in these categories with a positive screening test (i.e., scan that looks like ADEM).
ADEM, acute disseminated encephalomyelitis; 95% CI, 95% confidence interval; HSV, herpes simplex virus; N/A, not available; NPV, negative predictive value; PPV, positive predictive value.
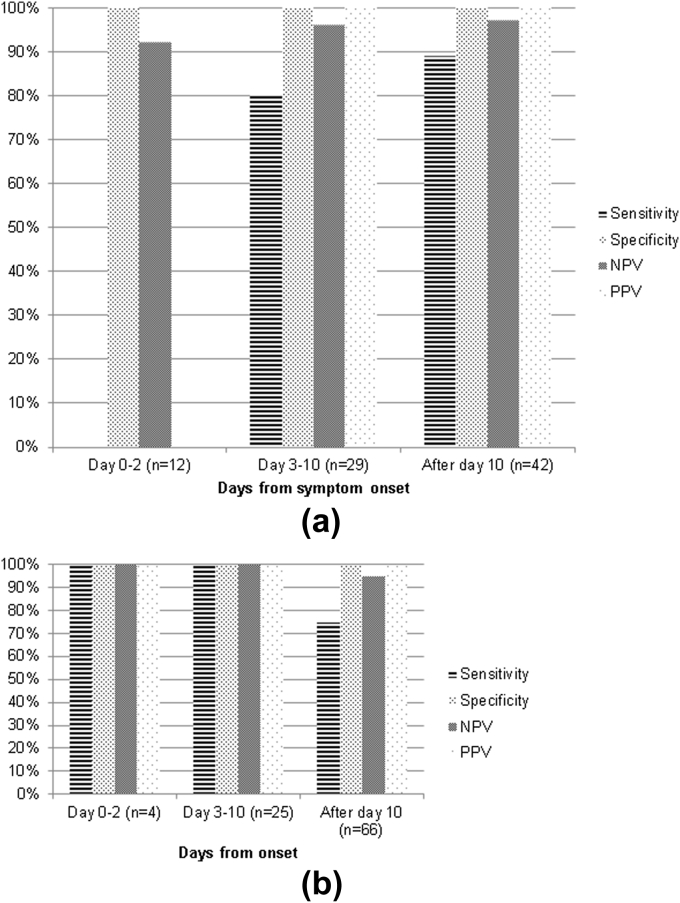
CT sensitivity for HSV encephalitis increased with time from symptom onset: 0% (95% CI: 0–94) at Day 0–2, 80% (95% CI: 30–99) at Day 3–10, and 89% (95% CI: 51–99) after Day 10. MRI sensitivity for HSV encephalitis decreased after 10 days from symptom onset: 100% (95% CI: 5–100) at Day 0–2, 100% (95% CI: 31–100) at Day 3–10, and 75% (43–93) after Day 10 (Fig 3). Three HSV encephalitis patients with serial MRI examinations had early images that were characteristic of HSV encephalitis. The median time to MRI in the >10-day group was 37.5 days (range 11–793 days). The same pattern was seen when days since hospital admission was used, and similar results were obtained when the outlier (time to MRI=793 days) was excluded from the analyses (data not shown). CT and MRI specificity, NPV, and PPV for HSV was >90% irrespective of scan timing.
Figure 3.
Sensitivity, specificity, NPV, and PPV of CT (a) and MRI (b) for HSV by timing of the scan from symptom onset. PPV in 0–2 day category could not be calculated due to zero patients in this timing category with a CT that looks like HSV (i.e. zero denominator).
Almost 50% (33/68) of patients in this study had a CT carried out within 24 hours of hospital admission. Twelve patients had a MRI carried out within 24 hours of a CT. Of these, three (25%) initial CT examinations were abnormal. Four patients (two ADEM; one HSV; one non-encephalitis) who had a normal CT examinations had an abnormal MRI within 24 hours.
Descriptive features
The median time from hospital admission to first MRI was 6 days (range 0–791). There was no significant difference between TW2 and FLAIR sequences in the structural abnormalities seen (Table 4). Detection of basal ganglia/thalamus abnormalities differed between TW2 and DWI (14% versus 2%; p=0.03) and FLAIR and DWI (14% versus 2%; p=0.03).
Table 4.
Proportion of first scans with structural abnormalities from all-cause encephalitis and proportion of scans with structural abnormalities from HSV and unknown encephalitis patients.
| Structural abnormality | First MRI (n=80) |
HSV (n=16) |
Unknown (n=29) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| TW2 (n=80) | FLAIR (n=69) | DWI (n=49) | TW2 (n=15) | FLAIR (n=13) | DWI (n=10) | TW2 (n=29) | FLAIR (n=24) | DWI (n=18) | |
| Grey matter | 21 (26%) | 21 (30%) | 9 (18%) | 15 (100%) | 13 (81%) | 10 (100%) | 1 (3%) | 3 (13%) | 1 (6%) |
| White matter | 22 (28) | 21 (30) | 8 (16) | 13 (87) | 11 (69) | 8 (80) | 4 (14) | 6 (25) | 1 (6) |
| Temporal | 15 (19) | 16 (23) | 9 (18) | 14 (93) | 12 (92) | 9 (90) | 1 (3) | 1 (4) | 1 (6) |
| Frontal | 25 (31) | 22 (32) | 10 (20) | 15 (100) | 13 (100) | 9 (90) | 4 (14) | 4 (17) | 1 (6) |
| Parietal | 12 (15) | 13 (19) | 3 (6) | 2 (13) | 2 (15) | 2 (20) | 2 (7) | 2 (8) | 0 (0) |
| Occipital | 3 (4) | 5 (7) | 1 (2) | 1 (7) | 2 (15) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| Basal ganglia/thalami | 11 (14) | 10 (14) | 1 (2) | 5 (33) | 5 (38) | 2 (20) | 1 (3) | 1 (4) | 0 (0) |
| Brainstem | 9 (11) | 7 (10) | 3 (6) | 0 (0) | 0 (0) | 0 (0) | 2 (7) | 2 (8) | 1 (6) |
| Cerebellum | 7 (9) | 6 (9) | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (7) | 2 (8) | 0 (0) |
| Limbic changes | 14 (18) | 14 (20) | 7 (14) | 13 (87) | 12 (92) | 7 (70) | 0 (0) | 0 (0) | 1 (6) |
DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; HSV, herpes simplex virus; TW2, T2-weighted imaging.
All HSV encephalitis cases had frontal lobe changes on MRI (TW2 and FLAIR) and >90% had temporal lobe changes (Table 4). Approximately one-third had basal ganglia/thalamic abnormalities. No HSV encephalitis cases had brainstem/cerebellar changes. Fewer structural abnormalities were seen in encephalitis cases of unknown aetiology (Table 4). Frontal lobe abnormalities were most common (14% on TW2 and 17% on FLAIR); none had limbic changes.
HSV encephalitis cases were more likely than encephalitis of unknown cause to have abnormalities detected in the grey matter, white matter, frontal lobe, temporal lobe, and limbic structures for all sequences (p<0.001). For TW2 and FLAIR they were more likely to have basal ganglia/thalamic abnormalities (p=0.01). There were no significant differences between the HSV encephalitis and unknown groups for TW2 and FLAIR regarding abnormalities in the parietal lobe, occipital lobe, brainstem, and cerebellum.
ADEM cases (n=10) were more likely than HSV encephalitis to have abnormalities detected in the brainstem on TW2 (p=0.01). Only one (8%) case of antibody-associated encephalitis (n=13) had limbic changes (TW2 and FLAIR).
Discussion
The present study constitutes one of the largest studies of neuroimaging in encephalitis, leveraging one of the highest-quality case series, and provides novel data on interobserver agreement among neuroradiologists across a range of neuroimaging parameters.
Interobserver agreement
Agreement among raters when interpreting examinations as normal or abnormal was good for CT, but only moderate for MRI. Poor agreement was seen for examinations from patients with encephalitis of unknown cause or with suspected encephalitis, which ultimately had a non-encephalitic diagnosis. These groups likely consisted of mixed aetiologies (some known and others unknown), unfamiliar imaging patterns, and were perhaps more complex cases than those with known encephalitis aetiologies. Studies of interobserver agreement on neuroradiological investigations have reported varying kappa values. One investigating intracerebral haemorrhage detection on DWI reported excellent agreement between raters (0.84).19 Another where CT examinations obtained within 24 hours of mild traumatic brain injury were rated by three neuroradiologists, reported good agreement on the presence of structural injury, but poor agreement (0.3) with regard to the specific classification of injury.20
HSV encephalitis
The imaging abnormalities that most commonly occur in HSV encephalitis are well-described.9 This was reflected in the high agreement between raters in classifying an image as “looking like HSV.” The sensitivity of both CT and MRI for HSV encephalitis was high, especially for adults, confirming that appropriately timed examinations are a very useful adjunct to support the CSF-confirmed diagnosis. One case of HSV encephalitis in the present study had a normal CT, whereas the MRI carried out within 24 hours of it was abnormal. This, along with the observation that the sensitivity of early CT for HSV encephalitis was 0% while for early MRI it was 100%, confirms that MRI is preferable to CT to detect early changes. Within the 3–10 day period post-symptom onset, the 100% sensitivity of MRI in detecting HSV encephalitis changes supports its use as one of the criteria to exclude that diagnosis.
ADEM
Interobserver agreement for the radiological diagnosis of ADEM was poor. Correct image interpretation in clinical practice is heavily influenced by clinical presentation and progression, especially for ADEM. The lack of access of raters to any clinical details other than suspected encephalitis might account, in part, for the poor agreement. Alternatively, the poor agreement might be an artefact of the small sample size of ADEM cases. Most likely, however, is that despite having undergone two major revisions within 5 years, the diagnosis of ADEM in children and adults still relies on excluding imaging pathognomonic of other conditions, such as multiple sclerosis, rather than inclusion based on neuroimaging criteria for ADEM.18, 21, 22 Imaging criteria should thus be developed and included in subsequent revisions. Similarly, the sensitivity of imaging for the diagnosis of ADEM was low in the present study: 20% overall for MRI (slightly higher in children). This is surprising considering the final diagnosis of ADEM in the PHE study was also based upon a clinicoradiological definition; however, in the PHE study, the expert panel, comprising a multidisciplinary team of experts spanning adult and paediatric specialties, reviewed and discussed the images together with the full clinical details before agreeing the diagnosis.
Structural abnormalities
The agreement amongst raters in detecting abnormalities in different structures of the brain was highest for the temporal and frontal lobes. This corresponds to the high agreement seen in diagnosing HSV encephalitis radiologically. There was better agreement amongst the neuroradiologists when using the TW2 and FLAIR sequences than DWI. The number of scans involving areas on DWI where agreement was poor (basal ganglia and brainstem) was small, so any variation in rating could affect agreement. Furthermore, the interpretation of smaller basal ganglia and brainstem lesions on DWI can be difficult, as it requires a correlation with the much noisier apparent diffusion coefficient image, thus increasing subjectivity, and may contribute to interobserver differences.
As previously mentioned, the structural abnormalities evident in cases of HSV encephalitis in the present study confirm that these occur predominantly in the frontal and temporal lobes. There were few structural abnormalities seen in cases of unknown aetiology. Interestingly, no cases of unknown aetiology had limbic changes suggestive of an autoimmune aetiology; however, only 8% of the antibody-associated encephalitis cases in the PHE study had limbic changes on MRI. Moreover, in a recent study of 164 Australian children presenting with encephalitis, patients with an identified autoimmune aetiology predominantly had non-specific white matter changes.23 As such, limbic changes are likely to be more specific than sensitive in identifying an immune aetiology. Abnormalities were more likely to be detected on TW2 or FLAIR than on DWI. The present observations support those of a recent Indian study, which assessed the usefulness of various MRI sequences in the diagnosis of viral encephalitis.24 Others studies have reported on the utility of DWI in the radiological diagnosis of HSV encephalitis and other central nervous system infections.7, 25, 26
Approximately half of patients in this study underwent CT within 24 hours of hospital admission, which falls short of UK guidance8; however, these guidelines were published in 2012, after the PHE study was performed. The UK has good hospital provision of CT and for suspected acute stroke, immediate CT is recommended and widely provided.27 It is likely that the low level of CT performed in the present study has since improved as the use of CT has increased since the study was conducted.
Strengths and limitations
This is one of the largest studies to date of imaging in encephalitis and the first to assess interobserver agreement. Scans were not obtainable for all patients in the main PHE study as this was practically impossible in some hospitals; however, the aetiological, age, and gender breakdown of patients for whom images were available was similar to that of the main PHE study cohort indicating representativeness of this population. Despite being one of the largest studies to date, one limitation was the small sample size, especially when analyses were broken down by aetiology. Secondly, for some of the conditions assessed there is no non-invasive reference standard diagnostic test, making it hard to estimate accurate imaging diagnostic sensitivity and specificity. Thirdly, it should be noted that the PPV in particular is so high because the study population comprised patients suspected of having encephalitis, and the prevalence of true disease is high. The PPV might be very different applying the same criteria to scanning different populations of patients with a different prevalence of encephalitis. Finally, depriving neuroradiologists of clinical information, which intended to make the study more objective, sacrificed the real-life clinical setting where images are interpreted in the context of this information.
In conclusion, the present study demonstrates that there is a subjective component to scan interpretation, which can have important implications for the clinical management of encephalitis cases. The agreement between raters was good for CT but only moderate for MRI. Agreement varied with diagnosis; for blinded reading radiologists were good at diagnosing HSV; however, agreement was worse for ADEM and other alternative aetiologies. The study showed that imaging data are affected by imaging timing and technique, and there was poor agreement on some regional abnormalities. The findings of the present study support the current UK guidance dismissing the diagnosis of HSV encephalitis when the MRI is normal 72 hours after neurological symptom onset with appropriate negative CSF tests. There was no evidence that DWI is better at early detection than TW2 or FLAIR in HSV encephalitis. Further research is needed to better define common radiological abnormalities in order to define diagnostic criteria for other encephalitis diagnoses besides HSV encephalitis.
Acknowledgements
We gratefully acknowledge the patients and their next of kin for providing consent to participate; the staff at participating centres; the Department of Health for funding; the UK Clinical Virology Network; and the Encephalitis Society. This report is independent research commissioned and funded by the Department of Health Policy Research Programme (Enhanced Diagnostic and Management Strategies to Improve the Identification and Outcome of Individuals with Encephalitis, 047/1084). The views expressed in this publication are those of the author(s) and not necessarily those of the Department of Health. M.L. receives research grants from Action Medical Research, DES society, GOSH charity, NIHR, MS Society, SPARKS charity and; receives research support grants from the London Clinical Research Network and Evelina Appeal; has received consultation fees from CSL Behring; received travel grants from Merck Serono; and awarded educational grants to organize meetings by Novartis, Biogen-Idec, Merck Serono and Bayer. N.D. has received an educational grant from Biogen-Idec.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.crad.2016.03.015.
Contributor Information
J. Granerod, Email: juliagranerod@hotmail.com.
UK Public Health England Aetiology of Encephalitis Study Group:
Helen E. Ambrose, Jonathan P. Clewley, Amanda L. Walsh, Dilys Morgan, Richard Cunningham, Mark Zuckerman, Kenneth J. Mutton, Katherine N. Ward, Michael P.T. Lunn, Sarosh R. Irani, Angela Vincent, Craig Ford, Emily Rothwell, William Tong, Jean-Pierre Lin, Javeed Ahmed, David Cubitt, Cheryl Hemingway, David Muir, Hermione Lyall, Ed Thompson, Geoff Keir, Viki Worthington, Paul Griffiths, Susan Bennett, Rachel Kneen, and Paul Klapper
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Granerod J., Cunningham R., Zuckerman M. Causality in acute encephalitis: defining aetiologies. Epidemiol Infect. 2010;138(6):783–800. doi: 10.1017/S0950268810000725. [DOI] [PubMed] [Google Scholar]
- 2.Johnson R.T. The virology of demyelinating diseases. Ann Neurol. 1994;36(Suppl.):S54–S60. doi: 10.1002/ana.410360715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irani S.R., Alexander S., Waters P. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133(9):2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J., Lancaster E., Martinez-Hernandez E. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granerod J., Tam C.C., Crowcroft N.S. Challenge of the unknown: a systematic review of acute encephalitis in non-outbreak situations. Neurology. 2010;75(10):924–932. doi: 10.1212/WNL.0b013e3181f11d65. [DOI] [PubMed] [Google Scholar]
- 6.Davies N.W.S., Brown L.J., Gonde J. Factors influencing PCR detection of viruses in cerebrospinal fluid of patients with suspected CNS infections. J Neurol Neurosurg Psychiatry. 2005;76:82–87. doi: 10.1136/jnnp.2004.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyler K.L. Update on herpes simplex encephalitis. Rev Neurol Dis. 2004;1(4):169–178. [PubMed] [Google Scholar]
- 8.Solomon T., Michael B.D., Smith P.E. Management of suspected viral encephalitis in adults. Association of British Neurologists and British Infection Association National Guidelines. J Infect. 2012;64(4):347–373. doi: 10.1016/j.jinf.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Tunkel A.R., Glaser C.A., Bloch K.C. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47(3):303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 10.Dung N.M., Turtle L., Chong W.K. An evaluation of the usefulness of neuroimaging for the diagnosis of Japanese encephalitis. J Neurol. 2009;256(12):2052–2060. doi: 10.1007/s00415-009-5249-5. [DOI] [PubMed] [Google Scholar]
- 11.Armanque T., Leypoldt F., Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. 2014;27(3):361–368. doi: 10.1097/WCO.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granerod J., Ambrose H.E., Davies N.W. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 13.Winer B.J. McGraw Hill; New York: 1971. Statistical principles in experimental design. [Google Scholar]
- 14.Brinjikji W., Kallmes D.F., White J.B. Inter- and intraobserver agreement in CT characterization of nonaneurysmal perimesencephalic subarachnoid haemorrhage. AJNR Am J Neuroradiol. 2010;31(6):1103–1105. doi: 10.3174/ajnr.A1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 16.Newcombe R.G. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stats Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Wilson E.B. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- 18.Schwarz S., Mohr A., Knauth M. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001;56(10):1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 19.Keigler G., Goldberg I., Eichel R. Diffusion-weighted imaging at b1000 for identifying intracerebral hemorrhage: preliminary sensitivity, specificity, and inter-rater variability. J Stroke Cerebrovasc Dis. 2014 Aug;23(7):1934–1938. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Huff J.S., Jahar S. Differences in interpretation of cranial computed tomography in ED traumatic brain injury patients by expert neuroradiologists. Am J Emerg Med. 2014;32(6):606–608. doi: 10.1016/j.ajem.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Tenembaum S., Chitnis T., Ness J. International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology. 2007;68(16 Suppl. 2):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 22.Krupp L.B., Tardieu M., Amato M.P. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 23.Pillai S.C., Hacohen Y., Tantsis E. Infectious and autoantibody-associated encephalitis: clinical features and long-term outcome. Pediatrics. 2015;135(4):e974–984. doi: 10.1542/peds.2014-2702. [DOI] [PubMed] [Google Scholar]
- 24.Misra U.K., Kalita J., Phadke R.V. Usefulness of various MRI sequences in the diagnosis of viral encephalitis. Acta Trop. 2010;116(3):206–211. doi: 10.1016/j.actatropica.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Baringer J.R. Herpes simplex virus encephalitis. In: Davis L.E., Kennedy P.G.E., editors. Infectious diseases of the nervous system. Butterworth-Heinemann; Oxford: 2000. [Google Scholar]
- 26.Kuker W., Nagele T., Schmidt E. Diffusion weighted MRI in herpes simplex encephalitis: a report of 3 cases. Neuroradiology. 2004;46:122–125. doi: 10.1007/s00234-003-1145-3. [DOI] [PubMed] [Google Scholar]
- 27.Brazzelli M., Shuler K., Quayyum Z. Clinical and imaging services for TIA and minor stroke: results of two surveys of practice across the UK. BMJ Open. 2013;3(8) doi: 10.1136/bmjopen-2013-003359. pii: e003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.