Abstract
Introduction
For women with chronic or gestational hypertension in CHIPS (Control of Hypertension In Pregnancy Study, NCT01192412), we aimed to examine whether clinical predictors collected at randomization could predict adverse outcomes.
Material and methods
This was a planned, secondary analysis of data from the 987 women in the CHIPS Trial. Logistic regression was used to examine the impact of 19 candidate predictors on the probability of adverse perinatal (pregnancy loss or high level neonatal care for >48 h, or birthweight <10th percentile) or maternal outcomes (severe hypertension, preeclampsia, or delivery at <34 or <37 weeks). A model containing all candidate predictors was used to start the stepwise regression process based on goodness of fit as measured by the Akaike information criterion. For face validity, these variables were forced into the model: treatment group (“less tight” or “tight” control), antihypertensive type at randomization, and blood pressure within 1 week before randomization. Continuous variables were represented continuously or dichotomized based on the smaller p‐value in univariate analyses. An area‐under‐the‐receiver‐operating‐curve (AUC ROC) of ≥0.70 was taken to reflect a potentially useful model.
Results
Point estimates for AUC ROC were <0.70 for all but severe hypertension (0.70, 95% CI 0.67–0.74) and delivery at <34 weeks (0.71, 95% CI 0.66–0.75). Therefore, no model warranted further assessment of performance.
Conclusions
CHIPS data suggest that when women with chronic hypertension develop an elevated blood pressure in pregnancy, or formerly normotensive women develop new gestational hypertension, maternal and current pregnancy clinical characteristics cannot predict adverse outcomes in the index pregnancy.
Keywords: Preexisting hypertension, chronic hypertension, gestational hypertension, prediction, adverse outcome, maternal, perinatal
Abbreviations
- ART
artificial reproductive technology
- AUC ROC
area under the receiver‐operating‐characteristic
- BMI
body mass index
- BP
blood pressure
- dBP
diastolic blood pressure
- HDP
hypertensive disorder of pregnancy
- OR
odds ratio
- PNV
prenatal vitamin
- sBP
systolic blood pressure
Key Message.
CHIPS data suggest that it is not possible to predict adverse maternal or perinatal outcomes in pregnancy at the time that a woman becomes hypertensive in that pregnancy.
Introduction
The hypertensive disorders of pregnancy (HDPs) are a leading cause of maternal and perinatal mortality and morbidity worldwide. Preeclampsia is the HDP associated with the greatest risk. As such, there is a large literature devoted to the study of the prediction of preeclampsia in different patient populations and at various time points in pregnancy. To date, no model has demonstrated sufficient accuracy to be applied in clinical practice. Under active study are multivariable approaches that combine clinical information, ultrasonographic results, and/or biomarker levels 1, 2.
Ascertaining prognosis once a HDP has developed has been less well‐studied. The published literature focuses on preeclampsia. Among such women, those with a heightened risk of adverse maternal outcome can be identified up to 1 week following admission to hospital in well and under‐resourced settings using maternal demographics, symptoms, signs, and standard maternal laboratory and fetal ultrasonographic testing 3, 4. The added value of angiogenic markers has also been demonstrated for timing delivery 5, 6.
Is it possible to predict adverse outcomes among women with chronic or gestational hypertension? Individual risk markers for adverse outcomes have been identified, for outcomes that include preeclampsia, preterm birth, severe hypertension, and birthweight <10th centile. However, there are no robust multivariable models. Among women with a history of chronic hypertension who become hypertensive in pregnancy, risk markers for adverse outcomes have not been studied; when pregnant women were identified as hypertensive prior to pregnancy (whether or not they had become hypertensive in pregnancy), markers for adverse outcomes later in pregnancy have included duration of hypertension of 4 years or more and preeclampsia in a prior pregnancy (for prediction of preeclampsia), as well as baseline proteinuria (for prediction of preterm delivery and birthweight <10th percentile) 7. Among women with gestational hypertension, risk markers for adverse outcomes (including preeclampsia, delivery at <34 weeks, severe hypertension, and birthweight <10th percentile) have included: gestational age <32 weeks at presentation with hypertension, severe hypertension, and higher blood pressure (BP) and serum uric acid; however, even when gestational age‐standardized values were used for BP and uric acid, the likelihood ratios for prediction of adverse outcomes were poor at best 8, 9.
In the CHIPS (Control of Hypertension In Pregnancy Study) international randomized trial, 987 women were allocated to a target diastolic BP (dBP) of 100 mmHg (“less tight” control) or 85 mmHg (“tight” control) 10. “Tight” control was of benefit to the mother (at minimum, by decreasing the incidence of severe hypertension), without increasing (or decreasing) risk to the baby. We sought to examine, for women with chronic or gestational hypertension enrolled in CHIPS, whether major adverse outcomes could be predicted by clinical characteristics at the time women were hypertensive and eligible to join the Trial. Our hypothesis was that predictors of adverse outcome may be more powerful at this point in time for women with chronic hypertension who made up approximately 75% of the CHIPS cohort.
Material and methods
In brief, CHIPS was an open pragmatic international multicenter trial (ISRCTN 71416914, NCT01192412, http://pre-empt.cfri.ca/CHIPS) approved by the Research Ethics Board at the University of British Columbia as the Co‐ordinating Centre (H08‐00882) and at all study sites. Women at 14+0 to 33+6 weeks' gestation with non‐proteinuric preexisting or gestational hypertension, elevated BP (office dBP 90–105 mmHg, or 85–105 mmHg if on antihypertensives), and a live fetus were randomized (centrally and stratified by center and hypertension type) to “less tight” (target dBP 100 mmHg) or “tight” control (target dBP 85 mmHg) 10. Women could be recruited on an antihypertensive agent (other than atenolol from ≥14+0 weeks' gestation). Post‐randomization, labetalol was the recommended antihypertensive of first choice.
Candidate predictors
The 19 candidate predictor variables were measured at baseline to determine eligibility and to document status at randomization. These were variables either demonstrated to increase maternal and/or perinatal risk in prior studies 11, 12, 13, as follows: treatment group (“less tight” or “tight” control), maternal age (years, continuously and as <35/≥35), mother's self‐declared ethnicity (Caucasian/Asian/other or Black/Hispanic), body mass index (BMI) (kg/m2, continuously and as unknown/<25/≥25), conceived through use of artificial reproductive technology (ART), gestational age at randomization (weeks, continuously and as <20/≥20), nulliparity, type of hypertension (preexisting/gestational), prior severe hypertension in this pregnancy, antihypertensive therapy at randomization, type of antihypertensive therapy at randomization (none/labetalol with or without another antihypertensive other than methyldopa/methyldopa with or without another antihypertensive other than labetalol/other), systolic BP (mmHg) within 1 week before randomization (mmHg, continuously and as <140/140–149/≥150), dBP within 1 week before randomization (mmHg, continuously and as <90/90–94/95–99/≥100), in hospital at enrolment, gestational diabetes at randomization, cigarette smoking during this pregnancy, aspirin at enrolment, folic acid and/or a prenatal vitamin (PNV) at enrolment, perinatal mortality ratio of recruiting country (low defined as <10/1000 births or high defined as ≥10/1000 births). As these variables were collected prior to randomization, none was concealed from the attending clinicians and all variables were known prior to any post‐randomization adverse outcomes. No serum or urinary biomarkers were collected in CHIPS.
Outcomes
The composite primary outcome in CHIPS was pregnancy loss or high level neonatal care (greater than normal newborn care) for >48 h in the first 28 days of life. The composite secondary outcome in CHIPS was serious maternal complications before 6 weeks postpartum or until hospital discharge, whichever was later. Serious maternal complications included death, stroke, eclampsia, blindness, uncontrolled hypertension, the use of inotropic agents, pulmonary edema, respiratory failure, myocardial ischemia or infarction, hepatic dysfunction, hepatic hematoma or rupture, renal failure, and transfusion. Additional outcomes were severe hypertension, birthweight <10th percentile, and preterm delivery at <34 or <37 weeks. Further details can be found in the CHIPS protocol (http://pre-empt.cfri.ca/CHIPS), the main CHIPS publication 10 and Table S2.
Sample size
This was a secondary analysis of an existing trial data set. Based on the trial size of 987 women and adverse outcomes rates of 14.1–47.3%, our 19 candidate predictor variables could be considered according to the recommendation of a minimum of five to 10 events per variable 14. With only 28 (2.9%) secondary maternal outcomes and 22 (2.2%) abruptions, these outcomes were not considered for predictive modeling.
Statistical analyses
Candidate predictors were compared between women with an adverse outcome and those without, using the Chi‐squared test, Fisher's exact test or Wilcoxon rank sum test as appropriate.
Logistic regression was used to examine the impact of each candidate predictor on the probability of each outcome. A stepwise regression technique based on goodness of fit (as measured by the Akaike information criterion) was used to determine the subset of covariates most predictive of each outcome. A model containing the 19 candidate variables was used to start stepwise regression. These variables were forced into the model regardless of their impact on the model goodness of fit: treatment group (“less tight” or “tight” control), type of antihypertensive therapy at randomization (labetalol, methyldopa, “other” or “none”), and both systolic BP (sBP) and dBP within 1 week before randomization (continuous variable). For variables that could be expressed as both continuous and dichotomous, we used the representation of the variable that had the smaller p‐value in univariable analyses. An odds ratio (OR) >1 suggested a higher odds of experiencing the outcome. In a sensitivity analysis, for each outcome, we examined whether there was an interaction between antihypertensive therapy at enrolment (as yes or no) and any variables in the final model.
For each outcome, the final model was evaluated based on discrimination ability using the area‐under‐the‐receiver‐operating‐characteristic (AUC ROC) curve; an AUC ROC ≥0.70 was considered evidence of good discrimination 15. The model or models with good discrimination were to be assessed for calibration, stratification capacity, predictive performance, and internal validity 16.
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R 3.2.0 (R Development Core Team, Vienna, Austria).
Results
Of the 987 women enrolled in the CHIPS Trial, six women were lost to follow up for the primary and secondary outcomes, leaving 981 women (99.4%) who were included in this predictive modeling analysis. Among these, 305 (31.1%) had primary outcomes, 175 (17.9%) babies with birthweight <10th percentile, 334 (34.0%) developed severe hypertension and 464 (47.3%) preeclampsia, 138 (14.1%) delivered at <34 weeks, and 328 (33.4%) at <37 weeks, as previously reported 10.
The baseline maternal characteristics according to the occurrence of perinatal and maternal outcomes are examined in Tables 1 and 2, respectively. In general, in women who suffered an adverse outcome, compared with those who did not, a number of characteristics differed. Although the specifics depended on the adverse outcome, those pre‐randomization characteristics most closely associated with adverse maternal and perinatal outcomes post‐randomization were: conception through ART, prior severe hypertension in that pregnancy, taking antihypertensive therapy at time of randomization, higher sBP and dBP at randomization, and being an inpatient at enrolment.
Table 1.
Baseline maternal characteristics considered as candidate predictors for the occurrence of adverse perinatal outcomes [n (%) unless otherwise specified]
| Variable | Primary outcomea | p b | Birthweight <10th percentile | p b | ||
|---|---|---|---|---|---|---|
| No (n = 676) | Yes (n = 305) | No (n = 801) | Yes (n = 175) | |||
| Treatment group | ||||||
| “Less tight” | 338 (68.6) | 155 (31.4) | 0.81 | 411 (83.9) | 79 (16.1) | 0.14 |
| “Tight” | 338 (69.3) | 150 (30.7) | 390 (80.2) | 96 (19.8) | ||
| Age (years) | ||||||
| Mean (SD) | 33.7 (5.8) | 34.0 (5.8) | 0.54 | 33.7 (5.8) | 34.4 (5.9) | 0.06 |
| <35 | 388 (69.8) | 168 (30.2) | 0.50 | 467 (84.3) | 87 (15.7) | 0.04 |
| ≥35 | 288 (67.8) | 137 (32.2) | 334 (79.1) | 88 (20.9) | ||
| Ethnicity | ||||||
| Caucasian/Asian/Other | 505 (68.4) | 233 (31.6) | 0.57 | 602 (82.0) | 132 (18.0) | 0.94 |
| Black/Hispanic | 171 (70.4) | 72 (29.6) | 199 (82.2) | 43 (17.8) | ||
| BMI (kg/m2) | ||||||
| Mean (SD) | 31.0 (7.3) | 31.2 (8.3) | 0.81 | 31.5 (7.6) | 29.2 (7.5) | <0.001 |
| <25 | 148 (64.6) | 81 (35.4) | 0.11 | 168 (73.7) | 60 (26.3) | <0.001 |
| ≥25 | 522 (70.3) | 221 (29.7) | 625 (84.6) | 114 (15.4) | ||
| Unknown | 6 (0.9) | 3 (1.0) | 8 (1.0) | 1 (0.6) | ||
| Conceived through ART | 21 (50.0) | 21 (50.0) | 0.007 | 34 (81.0) | 8 (19.0) | 0.86 |
| Unknown | 12 (1.8) | 5 (1.6) | 15 (1.9) | 2 (1.1) | ||
| Gestational age at randomization (week) | ||||||
| Mean (SD) | 24.3 (6.4) | 24.5 (6.1) | 0.85 | 24.3 (6.3) | 24.7 (6.6) | 0.42 |
| <20 | 209 (70.1) | 89 (29.9) | 0.58 | 241 (82.0) | 53 (18.0) | 0.96 |
| ≥20 | 467 (68.4) | 216 (31.6) | 560 (82.1) | 122 (17.9) | ||
| Nulliparous | 207 (62.9) | 122 (37.1) | 0.004 | 258 (78.9) | 69 (21.1) | 0.07 |
| Type of non‐proteinuric hypertension | ||||||
| Gestational hypertension | 162 (65.1) | 87 (34.9) | 0.13 | 196 (78.7) | 53 (21.3) | 0.11 |
| Preexisting hypertension | 514 (70.2) | 218 (29.8) | 605 (83.2) | 122 (16.8) | ||
| Prior sBP ≥160 or dBP ≥110 mmHg in this pregnancy | 83 (58.9) | 58 (41.1) | 0.005 | 114 (81.4) | 26 (18.6) | 0.83 |
| Antihypertensive use at randomization | 368 (65.5) | 194 (34.5) | 0.007 | 460 (82.4) | 98 (17.6) | 0.73 |
| Antihypertensive type at randomization | ||||||
| Labetalol ± other (not methyldopa) | 144 (60.8) | 93 (39.2) | 0.001 | 183 (78.2) | 51 (21.8) | 0.06 |
| Methyldopa ± other (not labetalol) | 174 (72.2) | 67 (27.8) | 210 (87.5) | 30 (12.5) | ||
| Other | 50 (59.5) | 34 (40.5) | 67 (79.8) | 17 (20.2) | ||
| sBP within 1 week before randomization (mmHg) | ||||||
| Mean (SD) | 139.6 (10.0) | 141.1 (9.0) | 0.04 | 140.3 (9.6) | 139.4 (10.0) | 0.31 |
| <140 | 272 (72.3) | 104 (27.7) | 0.18 | 299 (80.2) | 74 (19.8) | 0.10 |
| 140–149 | 266 (67.2) | 130 (32.8) | 336 (85.3) | 58 (14.7) | ||
| ≥150 | 138 (66.0) | 71 (34.0) | 166 (79.4) | 43 (20.6) | ||
| dBP within 1 week before randomization (mmHg) | ||||||
| Mean (SD) | 92.2 (4.7) | 92.9 (5.4) | 0.04 | 92.3 (4.9) | 93.0 (5.3) | 0.12 |
| <90 | 135 (71.8) | 53 (28.2) | 0.02 | 154 (83.2) | 31 (16.8) | 0.27 |
| 90–94 | 340 (70.1) | 145 (29.9) | 406 (83.9) | 78 (16.1) | ||
| 95–99 | 131 (71.2) | 53 (28.8) | 144 (78.7) | 39 (21.3) | ||
| ≥100 | 70 (56.5) | 54 (43.5) | 97 (78.2) | 27 (21.8) | ||
| In hospital at enrolment | 21 (34.4) | 40 (65.6) | <0.001 | 42 (70.0) | 18 (30.0) | 0.01 |
| GDM prior to randomization | 44 (69.8) | 19 (30.2) | 0.87 | 53 (84.1) | 10 (15.9) | 0.66 |
| Smoking during this pregnancy | 38 (60.3) | 25 (39.7) | 0.13 | 45 (71.4) | 18 (28.6) | 0.02 |
| Aspirin at enrollment | 178 (69.3) | 79 (30.7) | 0.89 | 221 (86.3) | 35 (13.7) | 0.04 |
| Folic acid or PNV vitamin at enrolment | 450 (70.5) | 188 (29.5) | 0.13 | 526 (83.0) | 108 (17.0) | 0.31 |
| Unknown | 1 (0.1) | 0 | 1 (0.1) | 0 | ||
| PMR recruiting countryc | ||||||
| Low | 560 (68.2) | 261 (31.8) | 0.28 | 671 (82.1) | 146 (17.9) | 0.91 |
| High | 116 (72.5) | 44 (27.5) | 130 (81.8) | 29 (18.2) | ||
ART, artificial reproductive technology; BMI, body mass index; dBP, diastolic blood pressure; Del, delivery; GDM, gestational diabetes mellitus; Htn, hypertension; PET, preeclampsia; PMR, perinatal mortality ratio; PNV, prenatal vitamin; sBP, systolic blood pressure).
The primary outcome was pregnancy loss or high level neonatal care for >48 h (until primary discharge home or 28 days of life, whichever was later) (Table S2).
The p‐values are based on Chi‐squared test, Fisher's exact test or Wilcoxon rank sum test, as appropriate.
Low PMR was defined as <10 perinatal deaths/1000 births and high PMR as ≥10 perinatal deaths/1000 births.
Table 2.
Baseline characteristics considered as covariates for prediction of adverse maternal outcomes
| Variable | Severe hypertension | Pre‐eclampsia | Delivery at <34 weeks | Delivery at <37 weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (n = 647) | Yes (n = 334) | p | No (n = 515) | Yes (n = 464) | p | No (n = 840) | Yes (n = 138) | p | No (n = 650) | Yes (n = 328) | p | |
| Treatment group | ||||||||||||
| “Less tight” | 293 (59.4) | 200 (40.6) | <0.001 | 250 (50.9) | 241 (49.1) | 0.29 | 415 (84.3) | 77 (15.7) | 0.16 | 317 (64.4) | 175 (35.6) | 0.18 |
| “Tight” | 354 (72.5) | 134 (27.5) | 265 (54.3) | 223 (45.7) | 425 (87.4) | 61 (12.6) | 333 (68.5) | 153 (31.5) | ||||
| Age (years) | ||||||||||||
| Mean (SD) | 33.6 (5.8) | 34.3 (5.8) | 0.09 | 34.1 (5.9) | 33.5 (5.7) | 0.16 | 33.8 (5.8) | 33.9 (5.5) | 0.77 | 33.9 (5.8) | 33.7 (5.7) | 0.63 |
| <35 | 380 (68.3) | 176 (31.7) | 0.07 | 277 (49.9) | 278 (50.1) | 0.05 | 478 (86.1) | 77 (13.9) | 0.81 | 364 (65.6) | 191 (34.4) | 0.51 |
| ≥35 | 267 (62.8) | 158 (37.2) | 238 (56.1) | 186 (43.9) | 362 (85.6) | 61 (14.4) | 286 (67.6) | 137 (32.4) | ||||
| Ethnicity | ||||||||||||
| Caucasian/Asian/Other | 496 (67.2) | 242 (32.8) | 0.14 | 389 (52.9) | 347 (47.1) | 0.79 | 637 (86.5) | 99 (13.5) | 0.30 | 494 (67.1) | 242 (32.9) | 0.45 |
| Black/Hispanic | 151 (62.1) | 92 (37.9) | 126 (51.9) | 117 (48.1) | 203 (83.9) | 39 (16.1) | 156 (64.5) | 86 (35.5) | ||||
| BMI (kg/m2) | ||||||||||||
| Mean (SD) | 31.2 (7.7) | 30.7 (7.2) | 0.35 | 31.1 (7.7) | 31.0 (7.4) | 0.90 | 31.2 (7.6) | 30.2 (7.4) | 0.13 | 31.3 (7.5) | 30.7 (7.6) | 0.15 |
| Unknown | 6 (0.9) | 3 (0.9) | 0.87 | 5 (1.0) | 4 (0.9) | 0.44 | 8 (1.0) | 1 (0.7) | 0.09 | 6 (0.9) | 3 (0.9) | 0.04 |
| <25 | 150 (65.5) | 79 (34.5) | 125 (54.8) | 103 (45.2) | 188 (82.5) | 40 (17.5) | 139 (61.0) | 89 (39.0) | ||||
| ≥25 | 491 (66.1) | 252 (33.9) | 385 (51.9) | 357 (48.1) | 644 (86.9) | 97 (13.1) | 505 (68.2) | 236 (31.8) | ||||
| ART | 19 (45.2) | 23 (54.8) | 0.004 | 19 (45.2) | 23 (54.8) | 0.31 | 31 (73.8) | 11 (26.2) | 0.02 | 23 (54.8) | 19 (45.2) | 0.10 |
| Unknown | 11 (1.7) | 6 (1.8) | 6 (1.2) | 11 (2.4) | 15 (1.8) | 2 (1.4) | 11 (1.7) | 6 (1.8) | ||||
| Gestational age (week) | ||||||||||||
| Mean (SD) | 24.4 (6.4) | 24.3 (6.2) | 0.73 | 24.1 (6.2) | 24.6 (6.5) | 0.27 | 24.5 (6.4) | 23.7 (5.6) | 0.13 | 23.9 (6.4) | 25.2 (6.2) | 0.006 |
| <20 | 196 (65.8) | 102 (34.2) | 0.94 | 154 (51.7) | 144 (48.3) | 0.70 | 252 (85.4) | 43 (14.6) | 0.78 | 212 (71.9) | 83 (28.1) | 0.02 |
| ≥20 | 451 (66.0) | 232 (34.0) | 361 (53.0) | 320 (47.0) | 588 (86.1) | 95 (13.9) | 438 (64.1) | 245 (35.9) | ||||
| Nulliparous | 210 (63.8) | 119 (36.2) | 0.32 | 169 (51.5) | 159 (48.5) | 0.63 | 278 (84.8) | 50 (15.2) | 0.47 | 199 (60.7) | 129 (39.3) | 0.006 |
| Type non‐proteinuric Htn | ||||||||||||
| Gestational | 170 (68.3) | 79 (31.7) | 0.37 | 115 (46.4) | 133 (53.6) | 0.02 | 206 (82.7) | 43 (17.3) | 0.10 | 139 (55.8) | 110 (44.2) | <0.001 |
| Preexisting | 477 (65.2) | 255 (34.8) | 400 (54.7) | 331 (45.3) | 634 (87.0) | 95 (13.0) | 511 (70.1) | 218 (29.9) | ||||
| Prior sBP ≥ 160 or dBP ≥ 110 mmHg in this pregnancy | 63 (44.7) | 78 (55.3) | <0.001 | 59 (41.8) | 82 (58.2) | 0.006 | 108 (76.6) | 33 (23.4) | 0.001 | 71 (50.4) | 70 (49.6) | <0.001 |
| Antihypertensive | 357 (63.5) | 205 (36.5) | 0.06 | 296 (52.8) | 265 (47.2) | 0.91 | 471 (84.3) | 88 (15.7) | 0.09 | 362 (64.8) | 197 (35.2) | 0.19 |
| Antihypertensive type | ||||||||||||
| Labetalol ± othera | 157 (66.2) | 80 (33.8) | <0.001 | 132 (55.9) | 104 (44.1) | 0.23 | 194 (82.6) | 41 (17.4) | 0.12 | 140 (59.6) | 95 (40.4) | 0.01 |
| MD ± otherb | 162 (67.2) | 79 (32.8) | 128 (53.1) | 113 (46.9) | 209 (87.1) | 31 (12.9) | 172 (71.7) | 68 (28.3) | ||||
| Other | 38 (45.2) | 46 (54.8) | 36 (42.9) | 48 (57.1) | 68 (81.0) | 16 (19.0) | 50 (59.5) | 34 (40.5) | ||||
| sBP (mmHg) within last week | ||||||||||||
| Mean (SD) | 138.8 (9.7) | 142.5 (9.3) | <0.001 | 139.1 (9.9) | 141.2 (9.5) | 0.002 | 139.8 (9.8) | 141.9 (8.9) | 0.02 | 139.5 (10.0) | 141.3 (9.1) | 0.005 |
| <140 | 284 (75.5) | 92 (24.5) | <0.001 | 215 (57.2) | 161 (42.8) | 0.02 | 331 (88.3) | 44 (11.7) | 0.17 | 269 (71.7) | 106 (28.3) | 0.02 |
| 140–149 | 247 (62.4) | 149 (37.6) | 207 (52.4) | 188 (47.6) | 336 (85.3) | 58 (14.7) | 254 (64.5) | 140 (35.5) | ||||
| ≥150 | 116 (55.5) | 93 (44.5) | 93 (44.7) | 115 (55.3) | 173 (82.8) | 36 (17.2) | 127 (60.8) | 82 (39.2) | ||||
| dBP (mmHg) within last week | ||||||||||||
| Mean (SD) | 91.9 (4.7) | 93.2 (5.4) | <0.001 | 92.1 (5.0) | 92.7 (5.0) | 0.047 | 92.2 (4.9) | 93.6 (5.3) | 0.008 | 92.0 (4.8) | 93.1 (5.1) | 0.004 |
| <90 | 130 (69.1) | 58 (30.9) | <0.001 | 111 (59.0) | 77 (41.0) | 0.09 | 163 (87.6) | 23 (12.4) | 0.02 | 132 (71.0) | 54 (29.0) | 0.001 |
| 90–94 | 340 (70.1) | 145 (29.9) | 253 (52.2) | 232 (47.8) | 423 (87.4) | 61 (12.6) | 333 (68.8) | 151 (31.2) | ||||
| 95–99 | 115 (62.5) | 69 (37.5) | 97 (52.7) | 87 (47.3) | 159 (86.4) | 25 (13.6) | 122 (66.3) | 62 (33.7) | ||||
| ≥100 | 62 (50.0) | 62 (50.0) | 54 (44.3) | 68 (55.7) | 95 (76.6) | 29 (23.4) | 63 (50.8) | 61 (49.2) | ||||
| In hospital | 36 (59.0) | 25 (41.0) | 0.24 | 18 (29.5) | 43 (70.5) | <0.001 | 36 (60.0) | 24 (40.0) | <0.001 | 20 (33.3) | 40 (66.7) | <0.001 |
| GDM | 46 (73.0) | 17 (27.0) | 0.22 | 34 (54.8) | 28 (45.2) | 0.72 | 54 (85.7) | 9 (14.3) | 0.97 | 42 (66.7) | 21 (33.3) | 0.97 |
| Smoking | 36 (57.1) | 27 (42.9) | 0.13 | 28 (44.4) | 35 (55.6) | 0.18 | 52 (82.5) | 11 (17.5) | 0.43 | 40 (63.5) | 23 (36.5) | 0.61 |
| Aspirin | 150 (58.4) | 107 (41.6) | 0.003 | 133 (51.8) | 124 (48.2) | 0.75 | 211 (82.4) | 45 (17.6) | 0.06 | 172 (67.2) | 84 (32.8) | 0.78 |
| Folic acid or PNV | 427 (66.9) | 211 (33.1) | 0.36 | 353 (55.4) | 284 (44.6) | 0.01 | 552 (86.8) | 84 (13.2) | 0.26 | 424 (66.7) | 212 (33.3) | 0.83 |
| Unknown | 1 (0.2) | 0 | 1 (0.2) | 0 | 1 (0.1) | 0 | 1 (0.2) | 0 | ||||
| PMR recruiting countryc | ||||||||||||
| Low | 528 (64.3) | 293 (35.7) | 0.01 | 422 (51.5) | 397 (48.5) | 0.13 | 703 (85.8) | 116 (14.2) | 0.91 | 549 (67.0) | 270 (33.0) | 0.39 |
| High | 119 (74.4) | 41 (25.6) | 93 (58.1) | 67 (41.9) | 137 (86.2) | 22 (13.8) | 101 (63.5) | 58 (36.5) | ||||
ART, artificial reproductive technology; BMI, body mass index; GDM, gestational diabetes mellitus; Htn, hypertension; MD, methyldopa; PMR, perinatal mortality ratio; PNV, prenatal vitamin.
Other antihypertensive agents could NOT include methyldopa.
Other antihypertensive agents could NOT include labetalol.
Low PMR was defined as <10 perinatal deaths/1000 births and high PMR as ≥10 perinatal deaths/1000 births.
The risk markers with the strongest association with each of the outcomes, and the discrimination ability of the model, are presented for each of the perinatal (Figure 1a,b) and maternal outcomes (Figure 1c–f); numeric data are presented in Tables S3a,b.
Figure 1.
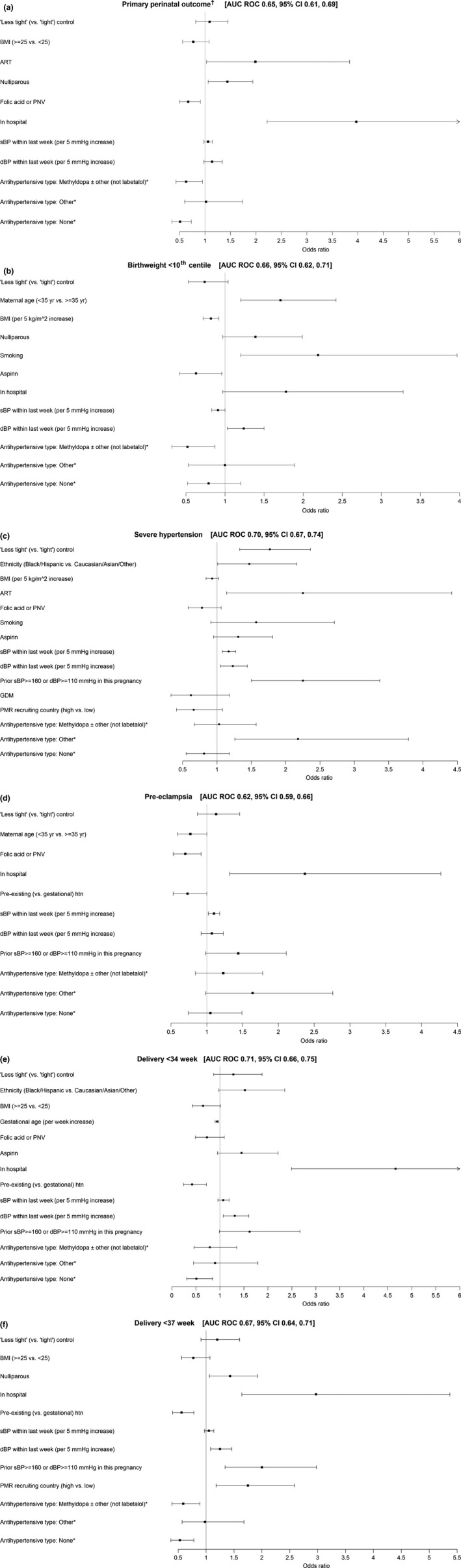
Risk markers associated with (a) CHIPS primary perinatal outcome, (b) birthweight <10th centile, (c) severe hypertension, (d) preeclampsia, (e) delivery at <34 weeks and (f) delivery at <37 weeks in the final multivariable regression model. *Labetalol with or without other (not methyldopa) as the reference category. †The primary perinatal outcome was pregnancy loss or high level neonatal care for >48 h (until primary discharge home or 28 days of life, whichever was later).
The CHIPS primary perinatal outcome was more common among women who conceived by ART, were nulliparous or were in hospital at enrolment; the primary outcome was less common among women who used folic acid and/or a PNV at enrolment, were not on any antihypertensive therapy, or were taking methyldopa at enrolment (Figure 1a). Birthweight <10th percentile was more common among women who were older, smokers, and those with higher diastolic BP at enrolment, and less common among women with higher maternal BMI or those taking aspirin or using methyldopa as their antihypertensive agent at enrolment (Figure 1b). For each of the CHIPS primary outcome and birthweight <10th percentile, there was no statistical interaction between antihypertensive therapy at enrolment and any variable in the model (Table S4).
Adverse maternal outcomes were most closely related to the woman being in hospital at enrolment and having gestational (vs. preexisting) hypertension.
Severe hypertension was associated with “less tight” BP control [as reported 10], Black/Hispanic ethnicity (vs. Caucasian/Asian/other), conception by ART, higher BP, prior severe hypertension in the index pregnancy, and antihypertensive therapy other than labetalol or methyldopa (as previously reported)] (Figure 1c). Sensitivity analysis revealed one interaction – increasing sBP at enrolment had a greater impact on the likelihood of severe hypertension among women who were not on any antihypertensive at enrolment compared with the effect on those who were on antihypertensives (Table S4).
Preeclampsia was more common among women who were in hospital at enrolment and had higher BP, and less common among women taking folic acid and/or a PNV at enrolment (Figure 1d). No significant interactions were identified.
Very preterm delivery at <34 weeks was more common among women who were in hospital at enrolment or had higher BP, and less common among women were enrolled at later gestational ages, had preexisting (vs. gestational hypertension) or were taking methyldopa as their antihypertensive agent at enrolment (as previously reported) 17 (Figure 1e). No significant interactions were identified.
Delivery at <37 weeks was more common among women who were nulliparous or in hospital at enrolment, had higher BP or prior severe hypertension in the index pregnancy, or who were cared for in a country with a high perinatal mortality ratio. Preterm delivery was less likely among women with preexisting (vs. gestational) hypertension, and those either on no antihypertensive therapy or taking methyldopa [as previously reported 17] (Figure 1f). Sensitivity analysis (Table S4) revealed three interactions of note: (i) higher dBP at enrolment was associated with more preterm delivery among women on antihypertensive therapy at enrolment (OR 1.07, p < 0.001) in contrast to those not on antihypertensives (OR = 1.01, p = 0.85); (ii) although increased BMI (≥25 vs. <25 kg/m2) was not associated with preterm delivery overall, these overweight/obese women had a lower risk of preterm delivery when taking antihypertensive therapy at enrolment (OR 0.54, p < 0.004), but a nonsignificant, increased risk of preterm delivery when not on antihypertensive therapy at enrolment (OR 1.26, p = 0.38); and (iii) being in hospital at enrolment was associated with preterm delivery among women on antihypertensive therapy at enrolment (OR 5.44, p < 0.001) but less so in women not on antihypertensive therapy at enrolment (OR 1.48, p = 0.39). Inclusion of the interactions did not have a meaningful effect on the other terms in the models.
The point estimate for the AUC was <0.70 for all outcomes except severe hypertension (0.70, 95% CI 0.67–0.74) and delivery at <34 weeks (0.71, 95% CI 0.66–0.75) for which AUC ROC was borderline (Figure 2). As no model was considered potentially useful for clinical practice, no further analyses were done as regards model evaluation or internal validation.
Figure 2.
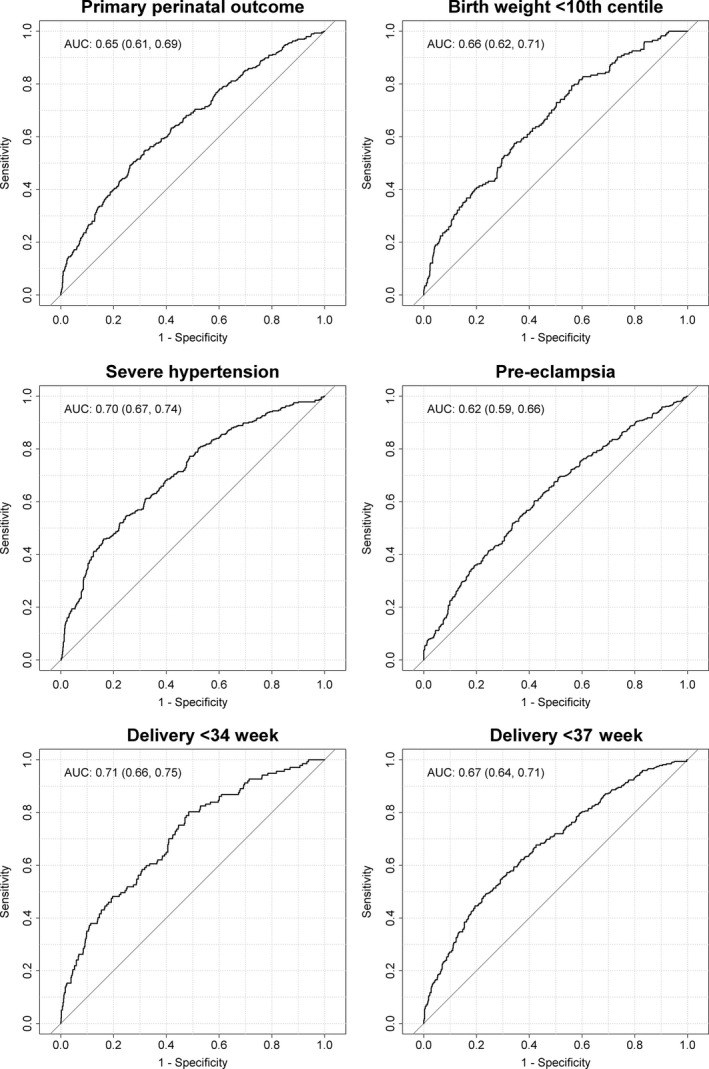
AUC ROC for prediction of major adverse pregnancy outcomes based on baseline characteristics of women enrolled in CHIPS.
Discussion
This planned secondary analysis of CHIPS Trial data suggests that at the time that a pregnant woman with chronic hypertension becomes hypertensive in the index pregnancy, or a formerly normotensive women develops gestational hypertension, it is not possible to use maternal or pregnancy clinical characteristics (including the absolute BP level) to predict adverse outcomes. Using models containing all candidate predictors to start the stepwise regression, and forcing into the model, treatment group, antihypertensive therapy type at randomization, and BP within 1 week before randomization, the point estimate for the AUC was <0.70 for all outcomes (primary perinatal, birthweight <10th percentile, preeclampsia, and delivery at <37 weeks) except severe hypertension (AUC ROC 0.70, 95% CI 0.67–0.74) and delivery at <34 weeks (AUC ROC 0.71, 95% CI 0.66–0.75) for which the AUC ROC was borderline.
A particular strength of our study is that CHIPS was a high‐quality international RCT. Also, our analyses were focused on whether we could identify which women were at increased risk of adverse outcomes when antihypertensive treatment decisions needed to be made for the duration of the pregnancy.
The CHIPS data set has limitations for predictive modeling, related to focused data collection in an international pragmatic trial. CHIPS collected no information about prior pregnancy 7, 15, 16, 18 or family history (of hypertension), each of which has been associated with various adverse outcomes, ranging from preeclampsia to preterm delivery 16, 19. Another weakness of CHIPS data is that all the candidate predictors were revealed to the managing clinicians, who may have incorporated those predictors into clinical decision‐making. This means that prediction of adverse outcomes for each item is susceptible to “treatment paradox,” meaning relationships between candidate predictors and outcomes may be confounded when the clinician uses these predictors in decision‐making. This treatment paradox may mask an association between the variable and outcome, or create an association when none actually exists. For example, a variable that may be predictive of severe hypertension may not be identified as such if the presence of that variable leads to antihypertensive therapy that avoids the severe hypertension and its complications; this may be why BP level was not predictive of adverse maternal outcome in the PIERS model of prognosis among women hospitalized with preeclampsia 3. Another example of this is the lack of a demonstrated association between corticosteroids and reduced neonatal mortality and morbidity in data of babies admitted to neonatal intensive care in the Canadian Neonatal Network 20. Many other predictive databases suffer from the same weakness, which should indicate the need for caution in interpreting predictive models from data sets in which clinicians know some or all of the variables analyzed. Although this issue has not been adequately addressed to date, it should be noted that our model used baseline characteristics that clinicians will know, such as demographics and baseline BP, as opposed to biomarkers or investigational ultrasonographic results, which can be masked from clinicians.
Although a multivariable model that could predict adverse outcome was not identified, in univariable analyses, baseline factors significantly associated with adverse outcomes were consistent with published literature that has included adverse prognostic factors of older maternal age, conception by ART, nulliparity, smoking, no use of low‐dose aspirin, earlier gestational age (at diagnosis with hypertension), higher BP, severe hypertension in that pregnancy, use of antihypertensive therapy, and higher serum uric acid 9, 11, 12, 13. Use of antihypertensive therapy at randomization magnified the risk associated with higher BP for progression to severe hypertension and preterm delivery, and being in hospital at enrolment for preterm delivery. Interestingly, use of antihypertensive therapy was associated with a decrease in preterm delivery among overweight/obese women, suggesting that clinicians time delivery differently in these women.
There were a few other findings of specific note.
First, women with high BMI less frequently had babies with birthweight <10th percentile, likely representing the interplay between hypertension‐related fetal growth restriction and obesity‐related macrosomia.
Also, the association of preexisting hypertension with better outcomes compared with gestational hypertension, was related to the fact that gestational age at presentation had to be <34 weeks in CHIPS, so these women with gestational hypertension were a high‐risk subgroup of hypertensive pregnant women with a higher risk of progression to preeclampsia 21, 22, 23, 24, 25.
In addition, analyses of the CHIPS data set showing better prognosis with methyldopa (vs. labetalol) antihypertensive therapy have been reported and discussed previously 17. In brief, women treated with methyldopa (vs. labetalol) may have had better outcomes, particularly women with preexisting hypertension, accounting for centre (and thereby, differences in practice) and baseline participant differences; however, these non‐randomized comparisons may be subject to residual confounding.
With regard to preventative therapy, aspirin use in this high‐risk cohort of women was low. Although 75% of women in CHIPS had preexisting hypertension, only ~25% of women were prescribed aspirin at enrolment at an average gestational age of 24 weeks 10. This underuse of aspirin is not in keeping with recommendations to use it for preeclampsia prevention in women at increased risk (such as those with preexisting hypertension) 26, 27, 28, 29, 30, 31 with no guideline recommending against it. Aspirin was not associated with a reduction in preeclampsia in this cohort but CHIPS was underpowered to find the small effect that could be anticipated. Also, taking a folate‐containing PNV was associated with lower rates of the primary outcome and preeclampsia; the Folic Acid Clinical Trial (NCT01355159) is examining whether high‐dose folic acid decreases preeclampsia.
Finally, preterm delivery was increased in countries with a high perinatal mortality ratio, possibly related to general factors associated with preterm birth, such as poor nutrition or socioeconomic status; these were not measured in CHIPS.
In conclusion, it was not possible to identify which women were at increased risk of perinatal or maternal adverse outcomes based on maternal and current pregnancy clinical characteristics at the time of CHIPS Trial enrolment. All such women must be followed closely and counseled accordingly.
Funding
CHIPS was funded by the Canadian Institutes of Health Research (MCT 87522).
Supporting information
Table S1. CHIPS Study Group.
Table S2. Definitions of CHIPS outcomes.
Table S3. (a) Multivariable models for prediction of adverse perinatal. (b) Multivariable models for prediction maternal outcomes.
Table S4. Interaction between antihypertensive therapy and variables in final models for each outcome, as listed in Tables S3a,b.
Acknowledgments
We sincerely thank all of the women who generously participated in CHIPS. This manuscript is dedicated to the memory of our friend and colleague, Dr. Andrée Gruslin.
Magee LA, von Dadelszen P, Singer J, Lee T, Rey E, Ross S, et al. Can adverse maternal and perinatal outcomes be predicted when blood pressure becomes elevated? Secondary analyses from the CHIPS (Control of Hypertension In Pregnancy Study) randomized controlled trial. Acta Obstet Gynecol Scand 2016; 95:763–776.
Conflict of interest
Dr. von Dadelszen receives consultancy fees and placental growth factor (PlGF) cartridges for research from Alere International. The other authors have stated explicitly that there are no conflicts of interest in connection with this article.
References
- 1. Androutsopoulos G, Gkogkos P, Decavalas G. Mid‐trimester maternal serum HCG and alpha fetal protein levels: clinical significance and prediction of adverse pregnancy outcome. Int J Endocrinol Metab. 2013;11:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hund M, Allegranza D, Schoedl M, Dilba P, Verhagen‐Kamerbeek W, Stepan H. Multicenter prospective clinical study to evaluate the prediction of short‐term outcome in pregnant women with suspected preeclampsia (PROGNOSIS): study protocol. BMC Pregnancy Childbirth. 2014;14:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Dadelszen P, Payne B, Li J, Ansermino JM, Broughton PF, Cote AM, et al. Prediction of adverse maternal outcomes in pre‐eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377:219–27. [DOI] [PubMed] [Google Scholar]
- 4. Payne BA, Hutcheon JA, Ansermino JM, Hall DR, Bhutta ZA, Bhutta SZ, et al. A risk prediction model for the assessment and triage of women with hypertensive disorders of pregnancy in low‐resourced settings: the miniPIERS (Pre‐eclampsia Integrated Estimate of RiSk) multi‐country prospective cohort study. PLoS Med. 2014;11:e1001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128:2121–31. [DOI] [PubMed] [Google Scholar]
- 6. Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sibai BM, Lindheimer M, Hauth J, Caritis S, Vandorsten P, Klebanoff M, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal‐Fetal Medicine Units. N Engl J Med. 1998;339:667–71. [DOI] [PubMed] [Google Scholar]
- 8. Anumba DO, Lincoln K, Robson SC. Predictive value of clinical and laboratory indices at first assessment in women referred with suspected gestational hypertension. Hypertens Pregnancy. 2010;29:163–79. [DOI] [PubMed] [Google Scholar]
- 9. Buchbinder A, Sibai BM, Caritis S, Macpherson C, Hauth J, Lindheimer MD, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66–71. [DOI] [PubMed] [Google Scholar]
- 10. Magee LA, von DP, Rey E, Ross S, Asztalos E, Murphy KE, et al. Less‐tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–17. [DOI] [PubMed] [Google Scholar]
- 11. Duckitt K, Harrington D. Risk factors for pre‐eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milne F, Redman C, Walker J, Baker P, Bradley J, Cooper C, et al. The pre‐eclampsia community guideline (PRECOG): how to screen for and detect onset of pre‐eclampsia in the community. BMJ. 2005;330:576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. North RA, McCowan LM, Dekker GA, Poston L, Chan EH, Stewart AW, et al. Clinical risk prediction for pre‐eclampsia in nulliparous women: development of model in international prospective cohort. BMJ. 2011;342:d1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–8. [DOI] [PubMed] [Google Scholar]
- 15. Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013;42:634–43. [DOI] [PubMed] [Google Scholar]
- 16. Tandu‐Umba B, Mbangama MA, Kamongola KM, Kamgang Tchawou AG, Kivuidi MP, Kasonga MS, et al. Pre‐pregnancy high‐risk factors at first antenatal visit: how predictive are these of pregnancy outcomes? Int J Womens Health. 2014;6:1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magee LA, von DP, Rey E, Ross S, Asztalos E, Murphy KE, et al. Do labetalol and methyldopa have different effects on pregnancy outcome? Analysis of data from the Control of Hypertension In Pregnancy Study (CHIPS) trial. BJOG. 2015; doi: 10.1111/1471‐0528.13569. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MV, Martins‐Costa S, et al. The impact of prior preeclampsia on the risk of superimposed preeclampsia and other adverse pregnancy outcomes in patients with chronic hypertension. Am J Obstet Gynecol. 2011;204:345–6. [DOI] [PubMed] [Google Scholar]
- 19. Syngelaki A, Bredaki FE, Vaikousi E, Maiz N, Nicolaides KH. Body mass index at 11–13 weeks' gestation and pregnancy complications. Fetal Diagn Ther. 2011;30:250–65. [DOI] [PubMed] [Google Scholar]
- 20. Synnes AR, Chien LY, Peliowski A, Baboolal R, Lee SK. Variations in intraventricular hemorrhage incidence rates among Canadian neonatal intensive care units. J Pediatr. 2001;138:525–31. [DOI] [PubMed] [Google Scholar]
- 21. Barton JR, O'Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184:979–83. [DOI] [PubMed] [Google Scholar]
- 22. Brown MA, Buddle ML. The importance of nonproteinuric hypertension in pregnancy. Hypertens Pregnancy. 2002;14:57–65. [Google Scholar]
- 23. Magee LA, von DP, Bohun CM, Rey E, El‐Zibdeh M, Stalker S, et al. Serious perinatal complications of non‐proteinuric hypertension: an international, multicentre, retrospective cohort study. J Obstet Gynaecol Can. 2003;25:372–82. [DOI] [PubMed] [Google Scholar]
- 24. Magee LA, von Dadelszen P, Chan S, Gafni A, Gruslin A, Helewa M, et al. The Control of Hypertension In Pregnancy Study Pilot Trial. BJOG. 2007;114:770, e13–770, e20. [DOI] [PubMed] [Google Scholar]
- 25. Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre‐eclampsia? Br J Obstet Gynaecol. 1998;105:1177–84. [DOI] [PubMed] [Google Scholar]
- 26. The management of hypertensive disorders during pregnancy: NICE clinical guideline 107. Available online at: http://www.niceorg/cg1072010 (accessed March 24, 2016).
- 27. WHO recommendations for the prevention and treatment of pre‐eclampsia and eclampsia. Geneva: WHO, 2011. apps.who.int (accessed March 24, 2016). [Google Scholar]
- 28. American College of Obstetricians and Gynecologists . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 29. Gillon TE, Pels A, von DP, MacDonell K, Magee LA. Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS ONE. 2014;9:e113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. HDP CPG Working Group . Association of Ontario midwives. Hypertensive disorders of pregnancy (Clinical Practice Guideline No. 15). 2012. http://www.aomonca/Health_Care_Professionals/Clinical_Practice_Guidelines (accessed March 24, 2016).
- 31. Magee LA, Pels A, Helewa M, Rey E, von Dadelszen DP, on behalf of the Canadian Hypertensive Disorders of Preganncy (HDP) Working Group . Diagnosis, evaluation and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4:105–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. CHIPS Study Group.
Table S2. Definitions of CHIPS outcomes.
Table S3. (a) Multivariable models for prediction of adverse perinatal. (b) Multivariable models for prediction maternal outcomes.
Table S4. Interaction between antihypertensive therapy and variables in final models for each outcome, as listed in Tables S3a,b.


