Sterol-containing ordered membrane domains
Membrane lipids can form co-existing membrane domains which are distinct in terms of both lipid composition and lipid physical state [1]. The liquid disordered (Ld) state is most common in natural membranes. As its name implies, lipids in the Ld state undergo rapid lateral diffusion and have relatively disordered, loosely packed hydrocarbon chains. In contrast, lipids in the solid-like gel state are highly ordered, tightly packed with each other, and undergo only very slow lateral diffusion. In lipid mixtures containing sterols such as cholesterol, a third physical state can form, the liquid ordered (Lo) state. This state is intermediate in properties relative to the gel and Ld states. Like the Ld state, the Lo state is characterized by fast lateral diffusion, although slightly slower than in the Ld state. At the same time, the lipids in the Lo state are tightly packed, as in the gel state. The Lo state can form most readily when sterols are mixed with lipids lacking cis double bonds in their hydrocarbon chains, e.g. sphingolipids.
It is believed that in many natural membranes, Ld domains rich in unsaturated lipids containing cis double bonds co-exist with Lo domains rich in sphingolipids and sterols [2–4]. The term “lipid raft” or “membrane raft” has been widely adopted to denote cellular Lo domains. A wide variety of cellular functions have been postulated to be dependent upon the presence of lipid rafts. Raft-dependence has been most well-studied in the cases of sorting of membrane proteins and lipids to different subcellular compartments and/or domains, infection by certain bacteria and viruses, and signal transduction in the immune system [5–15]. However, just about every process that involves the sphingolipid-rich plasma membrane of cells has been claimed to be raft-dependent in one study or another.
The raft field has been embroiled in much controversy, mainly because under most conditions fully unambiguous methods to directly detect lipid rafts in natural membranes have been lacking. It appears that, at least in most cases, domains are too small (existing as nanometer scale sized “nanodomains”) for direct detection in cells by available methods. There are exceptions to this [16–18], and microscopy methods that detect regions of high membrane order have proven valuable to detect ordered membrane domains in cells when they are large, or present as nanodomains that are concentrated in one portion of a membrane [19]. In contrast to the situation in cells, membrane domains are readily detected in model membranes (i.e. membrane lipid dispersions), in which conditions can easily be manipulated to create large membrane domains, and for which methods to directly detect nanodomains are available [20, 21]. As a result, much is known about the physical properties of ordered lipid domains.
Biochemical assays and modifying sterol levels to probe the functional importance of ordered membrane domain formation
As a result of the inability to directly detect ordered membrane domains in cells, many indirect methods have been used to identify if a specific cellular function is likely to be raft-dependent. For example, whether membrane proteins are associated with detergent resistant membranes (DRM) has been widely used to define whether a membrane protein is likely to associate with ordered domains [22]. The logic behind this experiment is that Lo membrane domains are detergent resistant, while Ld domains are not [2]. Such experiments can be ambiguous because the degree of solubilization of Lo and Ld domains can depend on experimental conditions such as detergent concentration. In addition, the level of ordered membrane domain formation is highly temperature dependent, and solubilization studies are usually carried out at 4°C, which would increase ordered domain formation relative to that at 37°C [23]. Finally, detergent may perturb ordered domain properties. The extent to which this is an issue is not settled. Although it has been claimed that the detergent Triton X-100 can induce ordered domain formation under some conditions [24], other studies, using the same conditions, indicate that Triton X-100 only induces coalescence of pre-existing ordered domains into larger domains [21]. Certainly many studies have shown that when there are co-existing ordered and disordered Ld domains Triton X-100 selectively dissolves Ld domains [25, 26].
Another widely used approach has been to determine if partial removal of cholesterol (e.g. by treatment of cells with cyclodextrins such as MβCD or HPβCD) abolishes specific biological functions, and whether restoring cholesterol levels recovers these functions. Cyclodextrins are polymeric rings formed by six or more glucose molecules. Their circular structure has a hydrophobic cavity which can bind lipid, while their exterior is hydrophilic, allowing them to be dissolved in water. As a result, they can bind sterols, and can both extract and deliver sterols to membranes [27]. Removal of cholesterol is often combined with experiments showing that DRM are lost upon removal of cholesterol, the assumption being that loss of DRM is an indication that Lo domains are abolished upon removal of cholesterol. This behavior is difficult to interpret. Because ordered domains can dissolve in detergents, including Triton X-100, when they have low cholesterol content [25, 26, 28], loss of detergent insolubility does not demonstrate loss of Lo domain formation. In other words, partial removal of cholesterol may only have made DRM less resistant to detergent. In addition, removal of cholesterol, which is a major lipid component of plasma membranes, influences a variety of membrane physical properties in addition to domain formation, e.g. lipid packing and bilayer width/thickness. A protein that undergoes conformational changes sensitive to these parameters might show altered function, and thus alter a biological process for reasons not directly related to the presence of co-existing Lo and Ld domains. Another obvious possibility is that protein conformation and function may be altered by specific binding interactions with cholesterol, interactions lost when cholesterol is removed. Finally, there may be changes in overall membrane properties and functions simply related to the change in overall lipid levels in the cell/plasma membrane when cholesterol is removed.
An alternative to removal of cholesterol with cyclodextrins is treatment of cells with polyene antibiotics (e.g. nystatin) that interact with and sequester cholesterol [29]. Interpretation of such studies can be complicated by the toxic effects of these antibiotics and the uncertainty concerning the properties of the sequestered sterol, although agreement between polyene antibiotic and cyclodextrin effects on a biological function increase the confidence that the function is influenced by cholesterol.
Modifying sterol type to probe the functional importance of ordered membrane domains
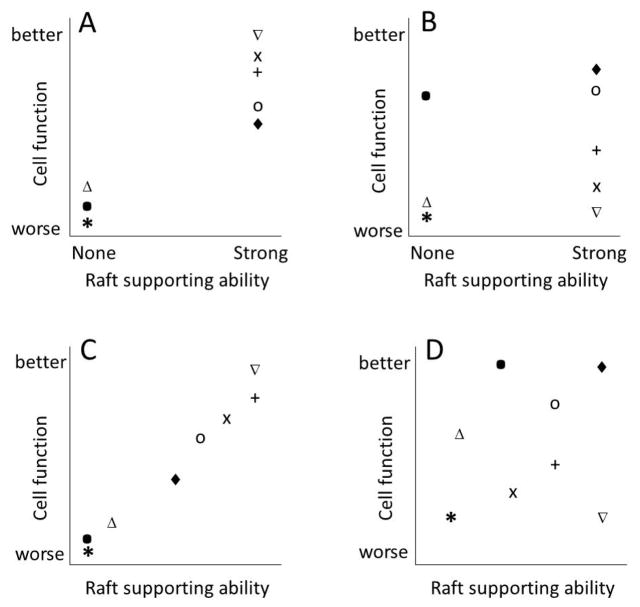
Bloch and colleagues investigated the correlation between overall membrane order and sterol structure in studies in Mycoplasma [30–33]. We suggested that sterol substitution might be a useful strategy for defining the importance of ordered domains in cells [28]. In this strategy, instead of cholesterol depletion/repletion one carries out cholesterol depletion/substitution, in which cholesterol is (partially) replaced by a sterol with different chemical and/or physical properties. In some cases sterol incorporation into cells is spontaneous [17, 34], but in most cases this can be carried out using cyclodextrins both to remove cholesterol, and when loaded with sterol, to carry out the substitution step [27, 35–38]. The basic concept behind this strategy is that cellular functions that respond to membrane domain formation will be supported by those sterols with chemical structures that maintain domain formation, while those functions that depend on other sterol properties will exhibit a different dependence upon sterol structure. In other words, if a sterol having the ability to maintain ordered domains is necessary and sufficient to support a biological function, then all sterols that support domain formation should support that function, while no sterol that does not support domain formation should support that function (Figure 1A). Similarly, studying the effect of a set of sterols with a range of abilities to support the formation of ordered domains should show a very tight correlation between the ability of a sterol to support ordered domain formation and biological function if the function is dependent upon ordered domains (Figure 1C). If a function is dependent upon specific sterol interactions with particular proteins rather than upon ordered domains, a strong correlation between the ability of a sterol to form ordered domains and function should not be observed. In this case, the relationship between sterol structure and biological function can reveal what part of the sterol molecule is critical for protein interaction.
Figure 1.
Hypotheticalterol-dependence patterns for biological functions that are or are not dependent upon ordered domain formation. Each symbol represents a different sterol. (Top) A. Effect of sterol ordered domain forming properties upon biological function in a case for which they are both necessary and sufficient to support function. B. Effect of sterol ordered domains forming properties upon biological function in a case for which they are not necessary, nor sufficient, to support function. (Bottom). C. Effect of sterol ordered domain forming properties upon a biological function that is dependent on ordered domain formation. D. Effect of sterol ordered domain forming properties upon a biological function NOT dependent upon ordered domain formation.
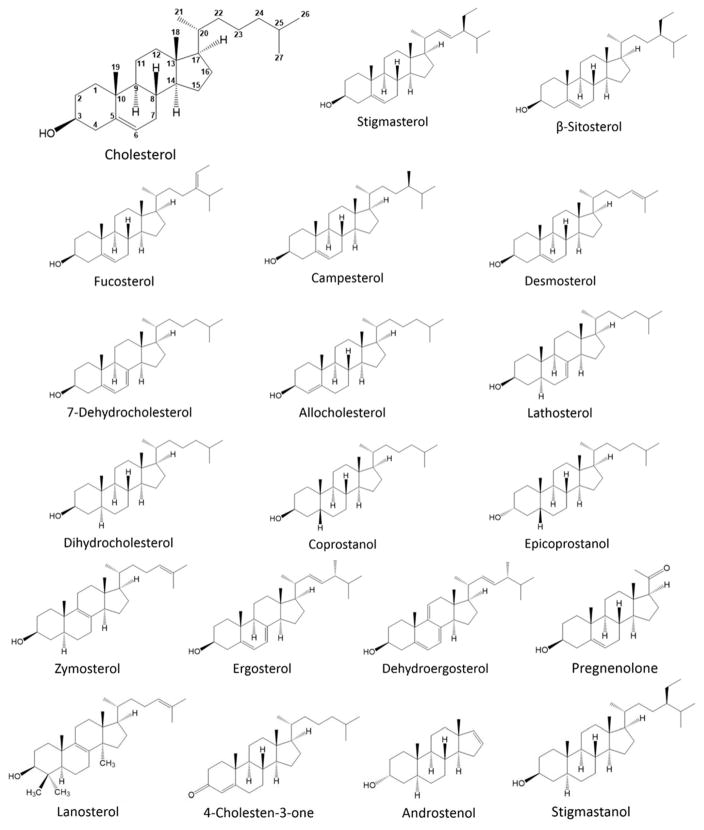
The key to carrying out such experiments in the most informative fashion is the use of a large number of sterols with a wide variety of abilities to form ordered domains. The abilities of over 25 sterols and closely related steroids with a wide range of structures and ability to support ordered domain formation have been investigated in a series of studies in model membrane vesicles (ours plus Barrantes [28, 39–43]). Sterols can have a wide range of chemical structures (Figure 2) and their variations will greatly alter any specific interactions with sterol binding sites on proteins, as well as alter their abilities to pack tightly with other lipids, restrict membrane permeability, H-bond, etc. Although some of these properties may also influence the degree to which sterols support segregation of ordered domains from disordered domains, sterols/steroids with similar ability (or inability) to support/stabilize ordered domain formation can differ in the presence or absence of a free 3OH group, OH group configuration, double bond number and position in the steroid rings, alkyl tail structure, and overall enantiomeric configuration. Thus, with a wide enough selection of sterols, it should be possible to pinpoint functions most likely to be dependent on domain formation.
Figure 2.
Sterols used in prior sterol substitution studies. Cholesterol is shown with carbon atoms numbered. Sterols with variations on cholesterol studied have included those with substituents replacing or modifying the 3β OH group (named in parentheses, full structures of sterols not shown) including: cholestene (H), cholesterol formate (formate), chlorocholesterol (Cl), epicholesterol (3α-OH), cholesterol palmitate (palmitate), cholesterol ethyl ether (ethyl), 5-cholesten-3-one (3 keto); oxysterols (full structures not shown) with substituents including: 6 ketocholesterol, 7α-OH cholesterol, 7β-OH cholesterol, 19 ketocholesterol, 22 ketocholesterol, 25-OH cholesterol, 20α-OH cholesterol, 22α-OH cholesterol; alkyl chain variants including: stigmasterol, β-sitosterol, fucosterol, campesterol, desmosterol; ring double bond variants including: 7-dehydrocholesterol, allocholesterol, lathosterol; saturated sterol ring variants including: dihydrocholesterol, coprostanol; and sterols/steroids with multiple differences relative to cholesterol including: epicoprostanol, zymosterol, ergosterol, dehydroergosterol, pregnenolone, lanosterol, 4-cholesten-3-one, androstenol, stigmastanol.
Sterol substitution experiments require an efficient sterol substitution step. Although efficient cyclodextrin-induced sterol exchange seems to be possible for a wide variety of sterols, it should be noted that it is necessary to confirm this and for the most accurate results, quantify the extent of sterol substitution. Also, removal of cholesterol can be toxic to cells [44, 45], and to show that the deleterious effect of a sterol substitution on a specific cellular function is not due to some irreversible general toxicity to the cell it is best to demonstrate biological function is restored upon a second substitution step in which cholesterol is re-substituted for at least a large fraction of the sterol used in the first substitution step.
It should be stated explicitly that the sterol substitution strategy cannot be used by itself to absolutely prove that a process is dependent on ordered domains. For example, the greater the degree to which a sterol supports ordered domain formation, the greater the extent to which it should increase membrane order, and because acyl chains are most fully extended when membranes are highly ordered, the greater the membrane thickness. Thus, a protein with a function dependent upon membrane thickness might show a pattern of sterol structure dependence similar to that for a protein with a function dependent upon membrane domain formation. To distinguish these possibilities will require development of methods to independently alter membrane thickness, membrane order, and domain formation. For example, since ordered domain formation involves a loss of homogeneous lipid mixing, altering the structure of Ld lipids so that they mix more poorly with cholesterol and sphingolipid [46] is likely to be a useful strategy for promoting domain formation without increasing membrane order and bilayer thickness. Similarly, partial substitution of cholesterol with sterols that do not support ordered domain formation may increase the immiscibility of sphingolipids and unsaturated phospholipids and promote domain formation, while decreasing overall membrane order. A complication is that, in intact cells the level of plasma membrane substitution and total cellular cholesterol substitution may not be identical. Thus, evidence for dependence of a function on ordered domains would be strengthened by directly detecting changes in plasma membrane ordered domain levels upon sterol substitution, for example by FRET [47], rather than indirectly inferring such changes from the properties of the sterols used.
It should also be kept in mind that a protein that binds sterols in a fashion dependent upon sterol structure does not preclude it from having properties influenced by membrane domains. Pefringolysin O (PFO) binds to many sterols with a 3β-OH group, but not sterols with a 3α-OH group [48]. However, when it binds to cholesterol, a sterol which tends to locate in ordered domains PFO, locates in ordered membrane domains, while when it binds coprostanol, a sterol which tends to locate in disordered domains, PFO locates in disordered domains [49]. It appears that sterols bind to PFO in a fashion in which they can still interact with other membrane lipids, probably because binding only involves the portion of the sterol close to the polar end of the sterol molecule.
Despite such complications, by defining what functions have a sterol substitution dependence that matches that expected for a raft-dependent process, and what functions show a pattern indicating specific sterol-protein interactions is a key first step in distinguishing processes likely to be dependent upon ordered domains from those with a specific sterol requirement. In fact, the sterol substitution method may be the most powerful method for identifying biological functions involving specific sterol-protein interactions.
Studies in eukaryotic cell plasma membranes using sterol-modifying enzymes
A number of previous studies have used sterol substitution to investigate the effect of specific sterol and lipid rafts on cellular functions [1]. Two types of sterol substitution have been carried out in cells: 1. treatment with sterol-modifying enzymes, and 2. sterol-exchange in membrane by using sterol-carriers such as cyclodextrins, bovine serum albumin (BSA) or liposomes. For sterol modification, cholesterol oxidase has been used. The oxidase transforms cholesterol into 4-cholesten-3-one, a steroid that does not support ordered domain formation [28] and has both shifted ring double bond position and replacement of the 3β-OH group of cholesterol with a keto group.
Phalen et al studied the role of membrane cholesterol in host cells on infection by Semliki Forest virus (SFV) [50]. Cholesterol depletion from cells did not alter viral binding, internalization or acidification. However, SFV fusion with plasma or endosomal membranes of infected cells was inhibited by both cholesterol depletion and cholesterol oxidase treatment. Additionally, production of viral RNA and viral proteins were impaired by cholesterol depletion. The investigators tried to restore impaired functions by repletion of cholesterol or 4-cholesten-3-one. Bovine serum albumin was used as the steroid carrier for these experiments. Repletion with cholesterol partly restored inhibited functions, but repletion with 4-cholesten-3-one did not. They concluded that a high level of steroid having a 3β-hydroxyl group in host cellular plasma membrane (as is the case in normal cells) is important for SFV infection. These studies did not define the mechanism by which a high level of the 3β-hydroxyl sterol acts.
Okamoto et al observed that endothelin1 binding-induced internalization of endothelin receptor type A (ETA) mainly occurred through caveolin-coated pits in Chinese hamster ovary cells, but that upon cholesterol oxidase treatment, intracellular translocation of caveolin-1 occurred and ETA-internalization switched to a clathrin-coated pit pathway [51]. Since caveolin directly binds to cholesterol [3], it is possible that a loss of binding to caveolin due to a change in sterol structure directly regulates caveolae-mediated endocytosis. On the other hand, given the significantly different abilities of cholesterol and 4-cholesten-3-one to support raft domain formation and tight lipid packing in plasma membranes [28], an effect on endocytosis due to a change in membrane physical properties could also be involved.
Pucadyil and Chattopadhyay found decreasing levels of cholesterol in hippocampal membranes by treatment with MβCD reduced ligand binding by serotonin1A (5-HT1A) receptors [52]. Thus, the cholesterol level in plasma membrane seems to be important for ligand binding. Pucadyil et al then studied the effect of cholesterol oxidation on ligand binding by 5-HT1A receptors [53]. Cholesterol oxidase treatment decreased agonist and antagonist ligand binding compared to non-treated samples. They found that the fraction of cholesterol loss (about 28%) was too small to give rise to a large decrease in plasma membrane order (i.e. lipid packing) as measured by fluorescence polarization. Therefore, they concluded that ligand binding to receptor more likely to depend on direct interaction with cholesterol than the physical properties of plasma membrane.
Rouquette-Jazdanian et al studied the effect of cholesterol oxidation on T cell receptor (TCR) signaling [54]. They observed transformation of cholesterol into 4-cholesten-3-one did not affect localization of the TCR complex subunit CD3-ζ in rafts, tyrosine phosphorylation induced by CD3, production of CD3-induced second messengers such as Ca2+-fluxes and diacylglycerol, or CD-3 induced actin-polymerization. The 4-cholesten-3-one moved to non-DRM fractions, but they found that there were residual DRM (which they called cholesterol-depleted DRM) with a similar amount of sphingomyelin and GM1 as DRM from untreated cells. They concluded cholesterol in DRM (which they interpreted as reflective of cholesterol in ordered membrane domains) was not necessary for the CD3/TCR signaling. It should be noted that loss of cholesterol does not mean a loss of ordered domains. It is possible that cholesterol-depleted DRM could exist in the ordered gel state, rather than liquid ordered state. It would be interesting to determine whether a combination of cholesterol oxidation and sphingomyelinase (SMase) treatment, which might fully destroy ordered domains, would inhibit TCR signaling. It should be noted that the investigators found that the behavior of T cells upon MβCD treatment was somewhat different from that upon cholesterol oxidation. It may be that the change in membrane physical properties upon decreasing total sterol levels are different from those induced upon changing sterol structure.
Klink et al studied the role of cholesterol oxidase for infectivity of Mycobacterium tuberculosis (Mtb) [55]. Mtb lacking the cholesterol oxidase gene (ΔchoD) replicated inside macrophages to a lesser degree than wild type Mtb. When complement receptor 3 (CR3) and Toll-like receptor 2 (TLR2) were blocked with antibodies normal growth was recovered. Infection with ΔchoD resulted in higher nitric oxide (NO) and reactive oxygen species (ROS) production in macrophages than did wild type Mtb, suggesting that 4-cholesten-3-one formation might inhibit the production of NO and ROS. The loss of NO and ROS production appears to reflect their observation that phosphorylation events that induce NO and ROS production were only inhibited by wild type Mtb. They also found production of interleukin 10, an anti-inflammatory cytokine, is lower in ΔchoD-infected macrophages compared to wild-type infected ones. It is not clear whether these effects resulted from removal of cholesterol or an inhibitory effect of 4-cholesten-3-one. In addition, the possibility that changes in membrane physical properties were involved in these processes was not investigated.
Neuvonen et al studied effect of cholesterol oxidation upon membrane properties and cellular functions by simulation and cellular experiments [56]. Cholesterol oxidase or MβCD-loaded with steroid was used to increase 4-cholesten-3-one and decrease cholesterol levels in human dermal fibroblasts. The 4-cholesten-3-one was more easily displaced from membranes than cholesterol such that the efflux of 4-cholesten-3-one to extracellular acceptors was larger than for cholesterol. Cellular localization of cholesterol and 4-cholesten-3-one also differed, with 4-cholesten-3-one moving rapidly to intracellular compartments. In the fibroblasts, 4-cholesten-3-one decreased lamellipodium formation and cellular mobility beyond that induced by simple removal of cholesterol with MβCD. Even though they found a small decrease in membrane order after cholesterol oxidation, matching their predictions for a decrease based on molecular dynamics, it is not certain whether reduced cellular mobility was a result of reduced membrane order, or some other effect of 4-cholesten-3-one.
Studies in eukaryotic cell membranes using sterol substitution
As noted above, an alternative method for altering the type of sterols in cells involves sterol exchange with cyclodextrins. In some studies, pre-depletion of cholesterol was carried out before substitution using sterol-loaded cyclodextrins, under conditions resulting in similar levels of total sterol in the initial cells and after exchange [57–64]. Other studies simply incubated cells with sterol-loaded cyclodextrins, carrying out cholesterol extraction and sterol substitution simultaneously, and under conditions in which total amount of sterol in the membrane was largely unchanged [65–68]. However, in other studies treatment of cyclodextrins loaded with sterols was carried out under conditions that increased total membrane sterol without decreasing cholesterol content [69–73]. This difference in final sterol levels may reflect use of different experimental conditions, or variations in sterol affinity for different cells. In any case, it is important to consider final levels of both cholesterol and total sterol after exchange.
The sensitivity of several membrane channel proteins to sterol has been studied using cyclodextrin-induced sterol substitution. Romanenko et al studied the effect of sterol on inward-rectifier K+ channel in endothelial cells [66]. Over-loading cholesterol decreased the inwardly rectifying K+ current while depleting cholesterol increased it. Substitution of about 50% of membrane cholesterol by epicholesterol induced an even stronger current-increasing effect than cholesterol-depletion. However, this sterol composition change did not affect single-channel properties or cellular plasma membrane capacitance, suggesting the number of active channels was being affected by sterol. The authors conclude that, given the similarities in cholesterol and epicholesterol physical properties, a specific protein interaction is likely to be involved. Given the slightly weaker ability of epicholesterol to maintain ordered domain formation relative to cholesterol [28], confirming these results with additional cholesterol analogs would be desirable.
Romanenko et al also studied the effect of sterols upon volume-regulated anion current (VRAC) in bovine aortic endothelial cells [67]. Cholesterol depletion and sterol-substitution were carried out with MβCD. Depletion of cholesterol activated VRAC, while increasing cholesterol slightly decreased VRAC activity. Substitution of cholesterol with epicholesterol or β-sitosterol gave behavior similar to that in cells having a normal level of cholesterol. However, substitution with coprostanol induced VRAC activation similarly to cholesterol-depletion. This group concluded that membrane physical properties, rather than specific sterol interactions are important for regulation of VRAC activity. This could involve membrane domain formation because epicholesterol and β-sitosterol support ordered domain formation, while coprostanol inhibits ordered domain formation [28, 40]. A study of the role of sterol and cell/cytoskeletal stiffness in VRAC activity came to a similar conclusion [74].
In another study, Romanenko et al investigated the effect of sterol-substitution on interaction between intermediate-conductance (IK1) and large-conductance (maxi-K) Ca2+-activated K channels in mouse parotid acinar cells [65]. Activation of IK1 channels decreased the activity of maxi-K channel in cells having normal levels of cholesterol. Cholesterol-depletion diminished the inactivation of maxi-K channels by activated IK1 channels. Repletion with cholesterol or with coprostanol restored inactivation (as did destabilization of the actin cytoskeleton), but substitution of cholesterol with epicholesterol or epicoprostanol resulted in behavior similar to that observed upon cholesterol depletion. Given the inability of coprostanol to support membrane order, and the inability of epi sterols to substitute for cholesterol, it was concluded that a specific sterol interaction involving the 3β-OH group on the A-ring of sterol is critical for regulating the interaction between IK1 and maxi-K channels, rather than membrane physical properties.
Picazo-Juárez et al studied the effect of cholesterol on transient receptor potential vanilloid 1 (TRPV1) channels in 293 cells [72]. When they incubated cells with MβCD-cholesterol complexes, they found that the resulting increase in membrane cholesterol gave a lower TRPV1 channel activity. They did not see this decrease in activity when they incubated with MβCD-epicholesterol under similar condition. When they introduced various point mutations in gene sequence coding S5 helix of the TRPV1 channel, they found that cholesterol-sensitivity was decreased or abolished. They concluded the S5 helix may interact with cholesterol, and that both membrane cholesterol content and an intact cholesterol binding site of TRPV1 channel are important for channel activity. It should be noted that although the effect of cholesterol and epicholesterol on membrane physical properties are similar but not identical, the specific binding interaction involved could obscure any additional effect due to small changes in membrane physical properties.
Other studies have examined sterol effects on receptors. In early studies that are the first or among the first to use sterol substitution with cyclodextrins, Fahrenholz et al and Klein et al modulated cholesterol amount and steroid structure in myometrial cell membranes by using MβCD, and then observed the effect on the binding of oxytocin to its receptor [59, 75]. Cholesterol depletion decreased binding affinity and cholesterol repletion restored affinity to normal. Then they carried out sterol-substitution with 5-cholesten-3-one, pregnenolone, stigmasterol and 5-cholestene. A specific steroid structure requirement for the oxytocin binding was found. Substitution with 5-cholesten-3-one, pregnenolone and 5-cholestene (sterols that do not have 3β-hydroxyl group or aliphatic tail) did not reverse the reduced binding affinity after cholesterol depletion, while stigmasterol partially restored binding affinity. They concluded that cholesterol level and a specific steroid structure are important for oxytocin receptor binding. It is noteworthy that, unlike most of the steroids they tested, stigmasterol and cholesterol only differ in their alkyl tail structures, suggesting this part of a sterol is less crucial for binding than the ring structure or OH group. However, the steroids that were unable to substitute for cholesterol in this study are also unlikely to support membrane order or domain formation, while stigmaterol does [40]. Thus, studies using a wider variety of sterols would have been valuable, as was carried out in the follow-up study below.
Gimpl et al studied the effect of steroid upon oxytocin receptor (OTR) and brain cholecystokinin receptor (CCKR) function [58]. To change steroid amount and structure, they carried out cholesterol oxidase treatment, SMase treatment, filipin treatment, and/or sterol substitution by treatment using MβCD with/without loaded sterol. Twenty four sterols and steroids were studied. Sterol levels, membrane order assayed by fluorescence (DPH) anisotropy, and ligand binding affinities to receptors were analyzed. The use of a variety of techniques and range of steroids helped reveal patterns of dependence on steroid and membrane structure. They found that sterol regulation of ligand binding for the two receptors seemed to involve different mechanisms. Ligand binding to CCKR was correlated with membrane order, although only weakly affected by sterol type. Ligand binding to OTR was more sensitive to specific steroid structure than membrane order. Thus, they concluded that there are two mechanisms for regulating receptor function: one is membrane physical state and another is specific molecular interaction between steroid and receptor.
Cross et al researched the effect of sterol on sperm responsiveness to progesterone [76, 77]. During fertilization, sperm undergo an acrosome reaction, and this reaction is largely triggered by exposure to inducers such as progesterone. They incubated sperm in media having various steroids bound to phosphatidylcholine (PC) liposomes or bovine serum albumin (BSA), and then measured both spontaneous and progesterone-induced acrosome reaction. Relative to control medium, progesterone-induced reaction was reduced when sperm were incubated in cholesterol-enriched medium. This was reversible, such that the acrosome reaction returned to normal after cholesterol was washed out. It was found that cholesterol-3-sulfate and desmosterol also inhibited the progesterone-induced reaction, but cholesterol palmitate did not. They concluded that incubation of sperm with unesterified sterol blocks the acrosome reaction.
Nimmo and Cross studied the mechanism by which sterols/steroids affect two sperm maturation steps, capacitation and the acrosome reaction [78]. Human sperm were incubated in the medium with/without diverse sterols/steroids added from solvent or in a liposomal preparation for 24 hours and then total steroid content, the extent of capacitation and spontaneous acrosome reaction of the sperm were analyzed. Addition of sterols/steroids that promote ordered domain formation and lipid tight packing inhibited capacitation. Sterols that disrupt tight lipid packing, and most likely ordered domain formation, 4-cholesten-3-one, 5α-androstan-3-β-ol, and epicoprostanol, gave a different response. They either promoted capacitation (in the case of epicoprostanol) or increased the spontaneous acrosome reaction (4-cholesten-3-one and 5α-androstan-3-β-ol). Thus, they suggested that sterols control sperm maturation via their affect upon membrane order. It should be noted that these relatively early studies on sperm used liposomes or BSA as sterol carriers. This could introduce variables that might affect function, such as liposome-cell fusion or BSA-cell interactions that would not occur with cyclodextrins.
Pang et al studied the effect of sterol in membrane on the binding of galanin to galanin receptor (GalR2) [60]. Using MβCD, cholesterol in Chinese hamster ovary (CHO) cell membranes was depleted and repleted, and galanin binding to the receptor was analyzed. Membranes were treated with cholesterol oxidase or filipin or subjected for sterol-substitution. Cholesterol oxidase or filipin treatment interrupted binding of galanin to GalR2. Binding affinity to GalR2 was also reduced by cholesterol depletion, and restored by repletion. For sterol-substitution, 5-cholestene, 5-pregnen-3β-ol-20-one, 4-cholesten-3-one, and 5-cholesten-3-one were used. Only substitution with 5-cholesten-3-one restored galanin binding to receptor. The authors concluded that there was a specific interaction GalR2 and sterol needed for ligand binding to the receptor, and that binding is not affected by membrane fluidity. However, further studies are required because, in contrast to cholesterol, 5-pregnen-3β-ol-20-one and 4-cholesten-3-one do not increase membrane order or domain formation [28, 41], while in the other cases, the effects on membrane properties are unknown.
Sooksawate and Simmonds studied the effect of cholesterol or epicholesterol incorporation in plasma membrane of rat hippocampal neurons upon the response of the GABAA receptor to GABA [70]. The receptor showed decreased sensitivity to GABA upon cholesterol depletion or cholesterol enrichment. Interestingly, cholesterol repletion to normal levels after cholesterol depletion, restored receptor response to GABA. Enrichment with epicholesterol give the same decrease in GABA sensitivity as did cholesterol enrichment. However, repletion with epicholesterol after cholesterol depletion, did not restore receptor response to GABA. They concluded that a specific amount of cholesterol is important for optimal GABAA receptor response to GABA, and that the decreased sensitivity after cholesterol enrichment or additional incorporation of epicholesterol reflected increased membrane lipid order. In a later study, Sooksawate and Simmonds performed similar experiments to see how membrane sterols affect the regulation of GABAA receptor by potentiators or antagonists [69]. Again, some differences were observed between the effects of cholesterol and epicholesterol. Specifying the effect of membrane order or lipid domains would require studies with additional sterols.
Brown et al researched how cholesterol in ER membrane affects functions of sterol regulatory element binding protein (SREBP) cleavage-activating protein (SCAP) [71]. SCAP senses if there is depletion of cholesterol in ER membrane and then escorts SREBP to the Golgi complex [79–82]. SCAP interaction with cholesterol was found to induce a conformational change increasing its sensitivity to trypsin proteolysis. To investigate SCAP regulation by cholesterol, trypsin sensitivity was assayed after MβCD was used to change the amount of ER cholesterol or exogenously add a large variety of sterols to ER. A variety of sterols were able to substitute functionally for cholesterol, and support SCAP trypsin sensitivity. However, a number of sterols with additional oxygens, or those with the 3β-OH altered to a keto, Cl or 3α-OH did not support SCAP digestion by trypsin. Cholesterol-insensitive SCAP mutants with reduced accessibility to trypsin digestion were also identified. It was concluded that SCAP has a specific sterol interaction, and this interaction induces an activating conformational change.
Westover et al researched the regulation of epidermal growth factor receptor (EGFR) induced autophosphorylation by plasma membrane cholesterol in A431 cells [63]. When cholesterol was depleted in plasma membranes using MβCD, both basal and EGF-stimulated EGFR site-specific phosphorylation were enhanced. Repletion with cholesterol or with cholesterol-enantiomer (ent-cholesterol) reversed the enhancement of phosphorylation. Cholesterol and ent-cholesterol appeared to be similar in their abilities to pack with sphingomyelin and form ordered membrane domains as judged by DRM levels. However, they interacted differently with cholesterol oxidase, suggesting that a cholesterol binding site on a protein can distinguish between them. They concluded regulation of site specific-EGFR phosphorylation does not involve direct interaction between cholesterol and EGFR, but instead reflects their effect upon membrane physical properties. However, the degree to which cholesterol binding sites can distinguish between cholesterol and ent-cholesterol is likely to be protein dependent, so studies with additional sterols might strengthen these conclusions.
Campbell et al studied the effect of sterol structure on infectivity and DRM formation in human immunodeficiency virus type 1 (HIV-1) [57]. Conditions in which about 50% of sterol-substitution was achieved were defined. Under these conditions, HIV-1 infectivity seemed to be affected by the raft-forming ability of sterols, such that raft-promoting sterols induced higher infectivity of HIV-1 than raft-disrupting sterols. This suggests that some properties strongly correlated with domain formation is important in infectivity of HIV-1. The amount DRM derived from HIV-1 (using the detergent Brij 98, DRM were not obtained from Triton X-100 treatment) contained between 18% and 28% of cellular gp41 after sterol substitution. Even so, it is unclear to what degree sterol substitution altered HIV-1 ordered domain levels or properties.
Papanikolaou et al studied the effect of cholesterol on the enzyme activity of the plasma membrane protein ecto-nucleoside triphosphate diphosphohydrolase-1 (CD39) [61]. This protein is believed to be in caveolae and was partly associated with DRM, but its activity and plasma membrane localization was not altered by a caveolin-1 knockout. Cholesterol-depletion by MβCD or sequestering by filipin inhibited CD 39 activity. Activity could be restored by repletion with cholesterol, but not by steroid repletion using 4-cholesten-3-one, a steroid which does not support ordered domain formation. It was concluded that rafts are important for CD39 localization and activity. However, the studies do not rule out a specific cholesterol interaction.
Rentero et al studied the effect of sterol on T cell membrane lipid condensation and T cell activation [73]. It was suggested previously that membrane condensation (i.e. ordered domain formation) is related to T cell activity because diverse T cell receptor (TCR) signaling proteins were found in DRM fractions and tightly packed lipid domains located at T cell activation sites were observed [83, 84]. In the sterol substitution report, T cell cholesterol was substituted with 7-ketocholesterol (7KC). This resulted in somewhat less efficient ordered domain formation in the T cells. Substitution did not change calcium flux and early tyrosine phosphorylation events occurring upon TCR-stimulation. In contrast, the substitution negatively affected signaling complex formation, maintenance of phosphorylated signaling proteins within the plasma membrane, and actin reorganization at activation sites. This seemed to result in reduced downstream activation responses. It was concluded that membrane lipid condensation is critical for T cell activation. Because 7KC induced a modest decrease (about 30%) in cell viability it would be desirable to confirm this and rule out specific sterol interactions with other cholesterol analogs.
Singh et al studied whether desmosterol supports ligand binding to hippocampal serotonin1A receptor as well as cholesterol [62, 85]. In the first study [62], serotonin1A receptor function was studied in hippocampal membranes. Cholesterol-depletion/repletion or substitution with desmosterol was performed by using MβCD. Cholesterol depletion reduced receptor affinity for 8-OH-DPAT. Cholesterol repletion partially recovered binding, but repletion of sterol with desmosterol did not. In a second study [85], hippocampal membranes were solubilized using CHAPS, which induced some cholesterol loss. In these samples both replenishment with cholesterol and with desmosterol loaded onto MβCD restored ligand binding. The authors interpreted this result as reflecting a change when the membrane is solubilized by detergent. They proposed that in detergent lipids and proteins are more loosely packed than in intact membranes, and so desmosterol can integrate in nonannular binding sites important for recovery of ligand binding affinity. It should be noted that some of the DPH anisotropy experiments used to evaluate membrane order may have been complicated by the binding of DPH to MβCD.
Yamamoto et al studied the effect of the structure of sterols associated with hepatitis C virions upon infectivity, buoyant-density and binding to apolipoprotein [64]. Cholesterol depletion or sterol substitution was carried out with MβCD. Viral infectivity and internalization was significantly decreased by cholesterol depletion but recovered upon repletion with cholesterol. Among the sterols tested, substitution (>30%) with dihydrocholesterol and coprostanol were effective for recovery of the infectivity and internalization, but several other steroids lacking a 3β-OH or having a blocked 3β-OH, and those having an altered aliphatic tail did not. Since coprostanol does not support domain formation, this suggests that domain formation is not important for infectivity. Buoyant-density of the viruses was also affected by cholesterol amount or sterol structure. Cholesterol depletion increased viral density while cholesterol repletion or substitution with dihydrocholesterol, coprostanol, or 7-dehydrocholesterol restored it to near-normal levels. Substitution with the other steroids did not recover the buoyant-density. Normal buoyant-density was required for interaction of virions with apolipoprotein E. However, 7-dehydrocholesterol was not as effective as dihydrocholesterol and coprostanol in maintaining viral infectivity and internalization. It was concluded that the absence of sterol ring double bonds does not impair infectivity but an additional double bond in the rings does.
Wang et al studied the effect of β-sitosterol on cleavage of endogenous amyloid precursor protein (APP) in HT22 mouse hippocampal cells [68]. APP has two alternative cleavage pathways, one involving α-secretase and another involving β-secretase [86]. Only cleavage by β-secretase leads to production of Aβ peptide, which forms amyloid plagues in brains of Alzheimer’s disease patients. Cholesterol substitution with β-sitosterol was performed using HPβCD. Beta-sitosterol substitution or cholesterol depletion induced nonamyloidogenic cleavage of APP, decreasing Aβ production. Additionally, β-sitosterol substitution resulted in decreased association of APP with DRM. Treatment with HPβCD loaded with cholesterol or β-sitosterol did not significantly change in TMADPH fluorescence anisotropy compared to non-treated cells, while cholesterol depletion decreased anisotropy, suggesting only depletion decreased overall membrane order. This may suggest a specific sterol interaction is involved. It should be noted that β-sitosterol substitution efficiency was low (about 9%). This may be because they used HPβCD rather than MβCD [87].
Considered together, the above studies using sterol substitution can be broadly divided into two categories. Those that replace cholesterol with a single sterol, and those that used a series of sterols. Single sterol substitution experiments, including the cholesterol oxidase studies, are generally less definitive than those using a series of sterols. If a cell function is altered by substitution with a sterol that alters membrane order and/or domain formation, it is difficult to define whether the effect on membrane order/domain formation, even if the change in membrane properties has been directly measured, is responsible for the change in that cell function, or instead, if there is a contribution to altered cell function from changes in specific sterol-protein interactions. On the other hand, if the change in sterol alters membrane function without altering membrane order/domain formation, or if the sterol alters membrane order/domain formation without altering cellular function, one can make a stronger statement: that an effect of sterol structure other than its effect upon membrane order must be involved. Nevertheless, if the conclusions using a single sterol substitution are correct, then they should be able to predict the effects of additional sterol substitutions. Thus, the most robust of the studies above are those that make conclusions based upon the behavior observed after substitution with a wide variety of sterols.
Sterol substitution experiments in cholesterol-containing bacteria
Although most bacteria do not contain cholesterol, some obtain it from their hosts. Among such bacteria is Borrelia burgdorferi, the causative agent of Lyme disease [16]. B. burgdorferi contains cholesterol glycolipids as well as cholesterol [88]. Cholesterol glycolipids have also been identified in plants, and appear to be involved in ordered domain formation [89], although this has not been studied in detail. In B. burgdorferi LaRocca et al. identified lipid raft-like structures containing cholesterol glycolipids and large enough to detect by electron microscopy [16]. To confirm these structures were ordered lipid domains, in a second study LaRocca et al. carried out sterol substitution with a wide range of sterols [17]. To do this cholesterol and cholesterol glycolipids were partly depleted with MβCD, and then sterols added back. Because B. burgdorferi can efficiently take up sterols, the sterol addition/substitution step was carried out with cholesterol dispersed in media, rather than loaded onto MβCD. Sterol substitution showed a strong correlation between the ability to form ordered domains in model membranes and support the formation of cholesterol glycolipid-containing domains in B. burgdorferi. Sterols that strongly stabilize ordered domains showed the strongest clustering of cholesterol glycolipids into domains, sterols with an intermediate ability to stabilize ordered domains showed a lesser degree of clustering of cholesterol glycolipids, and sterols that tend to inhibit ordered domain formation did not show any clustering into cholesterol glycolipid domains. Changes in DRM levels with different sterols paralleled changes in domain levels as detected by electron microscopy. Analogous experiments were also carried out in living B. burgdorferi using fluorescence resonance energy transfer (FRET). Domain formation was detected in B. burgdorferi substituted with sterols that have a strong or intermediate ability to support ordered domain formation in model membranes, but not in the presence of sterols that tend to inhibit ordered domain formation. Interestingly, prolonged incubation of B. burgdorferi after sterol substitution resulted in membrane defects closely related to sterol domain forming abilities. The membrane defects, as detected by permeability and electron microscopy, were moderate with sterols that moderately support ordered domain formation and severe with sterols that inhibit ordered domain formation. In this case, the pattern of the dependence of both membrane ordered domain formation and membrane integrity comes close to the type of patterns seen in Figures 1A and 1C.
Varying sterol type in reconstituted model membranes
A strategy to define the role of membrane domains in membrane protein function that is related to sterol substitution is to reconstitute a membrane protein in artificial vesicles with various sterols. A number of studies have taken this approach [48, 49, 90–96]. Such experiments are often carried out by reconstituting proteins in model membranes of well-defined lipid composition and with different sterols. However, because membrane protein orientation and the efficiency of reconstitution can depend upon lipid, it should be pointed out that in many cases a sterol-exchange protocol carried out after the protein reconstitution step may be preferable. Although this protocol would require sterol quantitation, and result in only partial sterol exchange, it would have the advantage that, in almost all cases, the orientation and efficiency of reconstitution would not be variables complicating interpretation. (However, there is at least one case, the lactose permease of E. coli, in which it has been shown membrane protein orientation can reverse after reconstitution [97].)
Conclusions
As the studies described above demonstrate, a significant amount of information about the role of sterols and domains in biological functions have been gained by sterol substitution studies. In some cases there is a strong correlation between membrane physical properties and function, while in other cases, there is no such correlation, and a specific sterol-protein interaction is implicated in function instead. However, in many studies just a single or small number of substitutions was investigated, which allows alternate interpretations of the data. We believe that such studies carried out with an wider range of sterols could resolve much of the present confusion in the literature concerning when membrane domains are (and just as importantly are not) functionally important, and as a result more focused studies on the subset of processes most likely to be raft-dependent will become practical.
Acknowledgments
This work was supported by NIH grant GM 099892
References
- 1.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164(2):103–14. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 1994;91(25):12130–4. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed SN, Brown DA, London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36(36):10944–53. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 5.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–36. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta. 2014;1838(2):532–45. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lafont F, et al. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 2002;21(17):4449–57. doi: 10.1093/emboj/cdf457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baorto DM, et al. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389(6651):636–9. doi: 10.1038/39376. [DOI] [PubMed] [Google Scholar]
- 9.Duncan MJ, et al. Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem. 2004;279(18):18944–51. doi: 10.1074/jbc.M400769200. [DOI] [PubMed] [Google Scholar]
- 10.Konkel ME, et al. Characteristics of the internalization and intracellular survival of Campylobacter jejuni in human epithelial cell cultures. Microb Pathog. 1992;13(5):357–70. doi: 10.1016/0882-4010(92)90079-4. [DOI] [PubMed] [Google Scholar]
- 11.Seveau S, et al. Role of lipid rafts in E-cadherin-- and HGF-R/Met--mediated entry of Listeria monocytogenes into host cells. J Cell Biol. 2004;166(5):743–53. doi: 10.1083/jcb.200406078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3(10):a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 2006;21:430–9. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- 14.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 15.Campbell SM, Crowe SM, Mak J. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J Clin Virol. 2001;22(3):217–27. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- 16.LaRocca TJ, et al. Cholesterol lipids of Borrelia burgdorferi form lipid rafts and are required for the bactericidal activity of a complement-independent antibody. Cell Host Microbe. 2010;8(4):331–42. doi: 10.1016/j.chom.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaRocca TJ, et al. Proving lipid rafts exist: membrane domains in the prokaryote Borrelia burgdorferi have the same properties as eukaryotic lipid rafts. PLoS Pathog. 2013;9(5):e1003353. doi: 10.1371/journal.ppat.1003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toulmay A, Prinz WA. Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. J Cell Biol. 2013;202(1):35–44. doi: 10.1083/jcb.201301039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaus K, Zech T, Harder T. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol Membr Biol. 2006;23(1):41–8. doi: 10.1080/09687860500466857. [DOI] [PubMed] [Google Scholar]
- 20.Petruzielo RS, et al. Phase behavior and domain size in sphingomyelin-containing lipid bilayers. Biochim Biophys Acta. 2013;1828(4):1302–13. doi: 10.1016/j.bbamem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathak P, London E. Measurement of lipid nanodomain (raft) formation and size in sphingomyelin/POPC/cholesterol vesicles shows TX-100 and transmembrane helices increase domain size by coalescing preexisting nanodomains but do not induce domain formation. Biophys J. 2011;101(10):2417–25. doi: 10.1016/j.bpj.2011.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68(3):533–44. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 23.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275(23):17221–4. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 24.Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83(5):2693–701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.London E, Brown DA. Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim Biophys Acta. 2000;1508(1–2):182–95. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 26.London E. How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochim Biophys Acta. 2005;1746(3):203–20. doi: 10.1016/j.bbamcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768(6):1311–24. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000;39(5):843–9. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- 29.Holz RW. The effects of the polyene antibiotics nystatin and amphotericin B on thin lipid membranes. Ann N Y Acad Sci. 1974;235(0):469–79. doi: 10.1111/j.1749-6632.1974.tb43284.x. [DOI] [PubMed] [Google Scholar]
- 30.Dahl JS, Dahl CE, Bloch K. Sterols in membranes: growth characteristics and membrane properties of Mycoplasma capricolum cultured on cholesterol and lanosterol. Biochemistry. 1980;19(7):1467–72. doi: 10.1021/bi00548a032. [DOI] [PubMed] [Google Scholar]
- 31.Dahl CE, Dahl JS, Bloch K. Effect of alkyl-substituted precursors of cholesterol on artificial and natural membranes and on the viability of Mycoplasma capricolum. Biochemistry. 1980;19(7):1462–7. doi: 10.1021/bi00548a031. [DOI] [PubMed] [Google Scholar]
- 32.Dahl JS, Dahl CE, Bloch K. Effect of cholesterol on macromolecular synthesis and fatty acid uptake by Mycoplasma capricolum. J Biol Chem. 1981;256(1):87–91. [PubMed] [Google Scholar]
- 33.Dahl JS, Dahl CE, Bloch K. Role of membrane sterols in Mycoplasma capricolum. Rev Infect Dis. 1982;4(Suppl):S93–6. doi: 10.1093/clinids/4.supplement_1.s93. [DOI] [PubMed] [Google Scholar]
- 34.Odriozola JM, et al. Sterol requirement of Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1978;75(9):4107–9. doi: 10.1073/pnas.75.9.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levitan I, Singh DK, Rosenhouse-Dantsker A. Cholesterol binding to ion channels. Front Physiol. 2014;5:65. doi: 10.3389/fphys.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilsdonk EP, et al. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270(29):17250–6. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 37.Yancey PG, et al. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration Of kinetic pools and mechanism of efflux. J Biol Chem. 1996;271(27):16026–34. doi: 10.1074/jbc.271.27.16026. [DOI] [PubMed] [Google Scholar]
- 38.Atger VM, et al. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J Clin Invest. 1997;99(4):773–80. doi: 10.1172/JCI119223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Megha, London E. Relationship between sterol/steroid structure and participation in ordered lipid domains (lipid rafts): implications for lipid raft structure and function. Biochemistry. 2004;43(4):1010–8. doi: 10.1021/bi035696y. [DOI] [PubMed] [Google Scholar]
- 40.Xu X, et al. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem. 2001;276(36):33540–6. doi: 10.1074/jbc.M104776200. [DOI] [PubMed] [Google Scholar]
- 41.Wenz JJ, Barrantes FJ. Steroid structural requirements for stabilizing or disrupting lipid domains. Biochemistry. 2003;42(48):14267–76. doi: 10.1021/bi035759c. [DOI] [PubMed] [Google Scholar]
- 42.Beattie ME, et al. Sterol structure determines miscibility versus melting transitions in lipid vesicles. Biophys J. 2005;89(3):1760–8. doi: 10.1529/biophysj.104.049635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westover EJ, Covey DF. The enantiomer of cholesterol. J Membr Biol. 2004;202(2):61–72. doi: 10.1007/s00232-004-0714-7. [DOI] [PubMed] [Google Scholar]
- 44.Bang B, Gniadecki R, Gajkowska B. Disruption of lipid rafts causes apoptotic cell death in HaCaT keratinocytes. Exp Dermatol. 2005;14(4):266–72. doi: 10.1111/j.0906-6705.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 45.Gniadecki R. Depletion of membrane cholesterol causes ligand-independent activation of Fas and apoptosis. Biochem Biophys Res Commun. 2004;320(1):165–9. doi: 10.1016/j.bbrc.2004.05.145. [DOI] [PubMed] [Google Scholar]
- 46.Bakht O, Pathak P, London E. Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): identification of multiple raft-stabilization mechanisms. Biophys J. 2007;93(12):4307–18. doi: 10.1529/biophysj.107.114967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sengupta P, Holowka D, Baird B. Fluorescence resonance energy transfer between lipid probes detects nanoscopic heterogeneity in the plasma membrane of live cells. Biophys J. 2007;92(10):3564–74. doi: 10.1529/biophysj.106.094730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson LD, Johnson AE, London E. How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction. J Biol Chem. 2008;283(8):4632–42. doi: 10.1074/jbc.M709483200. [DOI] [PubMed] [Google Scholar]
- 49.Lin Q, London E. Transmembrane protein (perfringolysin o) association with ordered membrane domains (rafts) depends upon the raft-associating properties of protein-bound sterol. Biophys J. 2013;105(12):2733–42. doi: 10.1016/j.bpj.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phalen T, Kielian M. Cholesterol is required for infection by Semliki Forest virus. J Cell Biol. 1991;112(4):615–23. doi: 10.1083/jcb.112.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okamoto Y, et al. Cholesterol oxidation switches the internalization pathway of endothelin receptor type A from caveolae to clathrin-coated pits in Chinese hamster ovary cells. J Biol Chem. 2000;275(9):6439–46. doi: 10.1074/jbc.275.9.6439. [DOI] [PubMed] [Google Scholar]
- 52.Pucadyil TJ, Chattopadhyay A. Cholesterol modulates ligand binding and G-protein coupling to serotonin(1A) receptors from bovine hippocampus. Biochim Biophys Acta. 2004;1663(1–2):188–200. doi: 10.1016/j.bbamem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Pucadyil TJ, Shrivastava S, Chattopadhyay A. Membrane cholesterol oxidation inhibits ligand binding function of hippocampal serotonin(1A) receptors. Biochem Biophys Res Commun. 2005;331(2):422–7. doi: 10.1016/j.bbrc.2005.03.178. [DOI] [PubMed] [Google Scholar]
- 54.Rouquette-Jazdanian AK, et al. Revaluation of the role of cholesterol in stabilizing rafts implicated in T cell receptor signaling. Cell Signal. 2006;18(1):105–22. doi: 10.1016/j.cellsig.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 55.Klink M, et al. Cholesterol oxidase is indispensable in the pathogenesis of Mycobacterium tuberculosis. PLoS One. 2013;8(9):e73333. doi: 10.1371/journal.pone.0073333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neuvonen M, et al. Enzymatic oxidation of cholesterol: properties and functional effects of cholestenone in cell membranes. PLoS One. 2014;9(8):e103743. doi: 10.1371/journal.pone.0103743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell S, et al. The raft-promoting property of virion-associated cholesterol, but not the presence of virion-associated Brij 98 rafts, is a determinant of human immunodeficiency virus type 1 infectivity. J Virol. 2004;78(19):10556–65. doi: 10.1128/JVI.78.19.10556-10565.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gimpl G, Burger K, Fahrenholz F. Cholesterol as modulator of receptor function. Biochemistry. 1997;36(36):10959–74. doi: 10.1021/bi963138w. [DOI] [PubMed] [Google Scholar]
- 59.Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34(42):13784–93. doi: 10.1021/bi00042a009. [DOI] [PubMed] [Google Scholar]
- 60.Pang L, Graziano M, Wang S. Membrane cholesterol modulates galanin-GalR2 interaction. Biochemistry. 1999;38(37):12003–11. doi: 10.1021/bi990227a. [DOI] [PubMed] [Google Scholar]
- 61.Papanikolaou A, et al. Cholesterol-dependent lipid assemblies regulate the activity of the ecto-nucleotidase CD39. J Biol Chem. 2005;280(28):26406–14. doi: 10.1074/jbc.M413927200. [DOI] [PubMed] [Google Scholar]
- 62.Singh P, et al. Differential effects of cholesterol and desmosterol on the ligand binding function of the hippocampal serotonin(1A) receptor: implications in desmosterolosis. Biochim Biophys Acta. 2009;1788(10):2169–73. doi: 10.1016/j.bbamem.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Westover EJ, et al. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278(51):51125–33. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto M, et al. Structural requirements of virion-associated cholesterol for infectivity, buoyant density and apolipoprotein association of hepatitis C virus. J Gen Virol. 2011;92(Pt 9):2082–7. doi: 10.1099/vir.0.032391-0. [DOI] [PubMed] [Google Scholar]
- 65.Romanenko VG, et al. The role of cell cholesterol and the cytoskeleton in the interaction between IK1 and maxi-K channels. Am J Physiol Cell Physiol. 2009;296(4):C878–88. doi: 10.1152/ajpcell.00438.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romanenko VG, Rothblat GH, Levitan I. Modulation of endothelial inward-rectifier K+ current by optical isomers of cholesterol. Biophys J. 2002;83(6):3211–22. doi: 10.1016/S0006-3495(02)75323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romanenko VG, Rothblat GH, Levitan I. Sensitivity of volume-regulated anion current to cholesterol structural analogues. J Gen Physiol. 2004;123(1):77–87. doi: 10.1085/jgp.200308882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J, Wu F, Shi C. Substitution of membrane cholesterol with beta-sitosterol promotes nonamyloidogenic cleavage of endogenous amyloid precursor protein. Neuroscience. 2013;247:227–33. doi: 10.1016/j.neuroscience.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 69.Sooksawate T, Simmonds MA. Influence of membrane cholesterol on modulation of the GABA(A) receptor by neuroactive steroids and other potentiators. Br J Pharmacol. 2001;134(6):1303–11. doi: 10.1038/sj.bjp.0704360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001;40(2):178–84. doi: 10.1016/s0028-3908(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 71.Brown AJ, et al. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol Cell. 2002;10(2):237–45. doi: 10.1016/s1097-2765(02)00591-9. [DOI] [PubMed] [Google Scholar]
- 72.Picazo-Juarez G, et al. Identification of a binding motif in the S5 helix that confers cholesterol sensitivity to the TRPV1 ion channel. J Biol Chem. 2011;286(28):24966–76. doi: 10.1074/jbc.M111.237537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rentero C, et al. Functional implications of plasma membrane condensation for T cell activation. PLoS One. 2008;3(5):e2262. doi: 10.1371/journal.pone.0002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Byfield FJ, et al. Evidence for the role of cell stiffness in modulation of volume-regulated anion channels. Acta Physiol (Oxf) 2006;187(1–2):285–94. doi: 10.1111/j.1748-1716.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- 75.Fahrenholz F, Klein U, Gimpl G. Conversion of the myometrial oxytocin receptor from low to high affinity state by cholesterol. Adv Exp Med Biol. 1995;395:311–9. [PubMed] [Google Scholar]
- 76.Cross NL. Effect of cholesterol and other sterols on human sperm acrosomal responsiveness. Mol Reprod Dev. 1996;45(2):212–7. doi: 10.1002/(SICI)1098-2795(199610)45:2<212::AID-MRD14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 77.Cross NL. Effect of methyl-beta-cyclodextrin on the acrosomal responsiveness of human sperm. Mol Reprod Dev. 1999;53(1):92–8. doi: 10.1002/(SICI)1098-2795(199905)53:1<92::AID-MRD11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 78.Nimmo MR, Cross NL. Structural features of sterols required to inhibit human sperm capacitation. Biol Reprod. 2003;68(4):1308–17. doi: 10.1095/biolreprod.102.008607. [DOI] [PubMed] [Google Scholar]
- 79.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999;96(20):11041–8. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeBose-Boyd RA, et al. Transport-dependent proteolysis of SREBP: relocation of site-1 protease from Golgi to ER obviates the need for SREBP transport to Golgi. Cell. 1999;99(7):703–12. doi: 10.1016/s0092-8674(00)81668-2. [DOI] [PubMed] [Google Scholar]
- 81.Goldstein JL, Rawson RB, Brown MS. Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Arch Biochem Biophys. 2002;397(2):139–48. doi: 10.1006/abbi.2001.2615. [DOI] [PubMed] [Google Scholar]
- 82.Nohturfft A, et al. Regulated step in cholesterol feedback localized to budding of SCAP from ER membranes. Cell. 2000;102(3):315–23. doi: 10.1016/s0092-8674(00)00037-4. [DOI] [PubMed] [Google Scholar]
- 83.Dykstra M, et al. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–81. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 84.Gaus K, et al. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171(1):121–31. doi: 10.1083/jcb.200505047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh P, et al. Desmosterol replaces cholesterol for ligand binding function of the serotonin(1A) receptor in solubilized hippocampal membranes: support for nonannular binding sites for cholesterol? Biochim Biophys Acta. 2011;1808(10):2428–34. doi: 10.1016/j.bbamem.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 86.Goodenough S, Schafer M, Behl C. Estrogen-induced cell signalling in a cellular model of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2003;84(2–3):301–5. doi: 10.1016/s0960-0760(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 87.Huang Z, London E. Effect of cyclodextrin and membrane lipid structure upon cyclodextrin-lipid interaction. Langmuir. 2013;29(47):14631–8. doi: 10.1021/la4031427. [DOI] [PubMed] [Google Scholar]
- 88.Stubs G, et al. Acylated cholesteryl galactosides are specific antigens of borrelia causing lyme disease and frequently induce antibodies in late stages of disease. J Biol Chem. 2009;284(20):13326–34. doi: 10.1074/jbc.M809575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simon-Plas F, et al. An update on plant membrane rafts. Curr Opin Plant Biol. 2011;14(6):642–9. doi: 10.1016/j.pbi.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Sunshine C, McNamee MG. Lipid modulation of nicotinic acetylcholine receptor function: the role of membrane lipid composition and fluidity. Biochim Biophys Acta. 1994;1191(1):59–64. doi: 10.1016/0005-2736(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 91.Addona GH, et al. Low chemical specificity of the nicotinic acetylcholine receptor sterol activation site. Biochim Biophys Acta. 2003;1609(2):177–82. doi: 10.1016/s0005-2736(02)00685-5. [DOI] [PubMed] [Google Scholar]
- 92.Fong TM, McNamee MG. Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry. 1986;25(4):830–40. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- 93.Singh DK, et al. Direct regulation of prokaryotic Kir channel by cholesterol. J Biol Chem. 2009;284(44):30727–36. doi: 10.1074/jbc.M109.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bukiya AN, et al. Specificity of cholesterol and analogs to modulate BK channels points to direct sterol-channel protein interactions. J Gen Physiol. 2011;137(1):93–110. doi: 10.1085/jgp.201010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Razinkov VI, Cohen FS. Sterols and sphingolipids strongly affect the growth of fusion pores induced by the hemagglutinin of influenza virus. Biochemistry. 2000;39(44):13462–8. doi: 10.1021/bi0012078. [DOI] [PubMed] [Google Scholar]
- 96.Popot JL, et al. Interaction of the acetylcholine (nicotinic) receptor protein from Torpedo marmorata electric organ with monolayers of pure lipids. Eur J Biochem. 1978;85(1):27–42. doi: 10.1111/j.1432-1033.1978.tb12209.x. [DOI] [PubMed] [Google Scholar]
- 97.Vitrac H, Bogdanov M, Dowhan W. In vitro reconstitution of lipid-dependent dual topology and postassembly topological switching of a membrane protein. Proc Natl Acad Sci U S A. 2013;110(23):9338–43. doi: 10.1073/pnas.1304375110. [DOI] [PMC free article] [PubMed] [Google Scholar]