Abstract
Background. Plasmodium falciparum malaria remains a leading cause of childhood morbidity and mortality. There are important gaps in our understanding of the factors driving the development of antimalaria immunity as a function of age and exposure.
Methods. We used data from a cohort of 93 children participating in a clinical trial in Tororo, Uganda, an area of very high exposure to P. falciparum. We jointly quantified individual heterogeneity in the risk of infection and the development of immunity against infection and clinical disease.
Results. Results showed significant heterogeneity in the hazard of infection and independent effects of age and cumulative number of infections on the risk of infection and disease. The risk of developing clinical malaria upon infection decreased on average by 6% (95% confidence interval [CI], 0%–12%) for each additional year of age and by 2% (95% CI, 1%–3%) for each additional prior infection. Children randomly assigned to receive dihydroartemisinin-piperaquine for treatment appeared to develop immunity more slowly than those receiving artemether-lumefantrine.
Conclusions. Heterogeneity in P. falciparum exposure and immunity can be independently evaluated using detailed longitudinal studies. Improved understanding of the factors driving immunity will provide key information to anticipate the impact of malaria-control interventions and to understand the mechanisms of clinical immunity.
Keywords: malaria, epidemiology, immunity, heterogeneity in transmission
Despite progress in expanding the coverage of malaria control interventions [1], malaria remains a major cause of morbidity and mortality in Africa [1, 2]. An important feature of malaria epidemiology is the enormous range in transmission intensities, from 1 infectious bite per person per year to 1 per day [3–5]. Across the transmission spectrum, immunity to malaria develops gradually and provides only partial protection. This protection manifests as a decline in clinical malaria with increasing age and repeated infection, such that after a few years of repeated infections, clinical malaria is much less common [6–11]. An important challenge is to understand the total malaria burden accumulated over a lifetime for individuals in relation to transmission intensity, age, and the development of immunity. This is particularly important for planning and implementing interventions with the goal of reducing morbidity.
Epidemiologic data consistently suggest that clinical immunity to P. falciparum develops gradually in children who are highly exposed to infection, reducing the likelihood of disease and severity of symptoms arising from each infection. Immunity against infection, on the other hand, is slower to develop and at best weakly protective such that older individuals may continue to be reinfected (or superinfected) hundreds of times despite the absence of clinical malaria [12]. In high-transmission settings, the age-specific incidence of clinical malaria is generally seen to peak in the first few years of life and then decline, while the prevalence of parasitemia reaches a plateau later [13]. However, study of the underlying biology of these distinct epidemiologic patterns is limited by a lack of understanding of how the risk of infection per se and the risk of disease once infected are influenced by age and repeated infection. Interpretation of observed epidemiologic patterns is further complicated by the large, usually unmeasured, heterogeneity in individual exposure that exists across the transmission spectrum at all spatial scales [14–18].
Better quantification of individuals' exposure (ie, the rate at which an individual is reinfected) and immunity would be particularly useful for studies of the manifestations and underlying mechanisms of immunity to malaria. Studies aiming to identify immunologic mechanisms or biomarkers of protection have been hampered by the strong but poorly quantified association between exposure and protection. Individuals who get exposed and reinfected more frequently are likely to have higher immunologic responses, and this can lead to false-positive associations with protection [19] or seemingly paradoxical findings, such as associations between anti-malarial antibodies and a higher risk of disease [20]. Individual-level quantitative biomarkers of exposure would help with this identifiability but are not yet established [21]. Making matters more challenging, different manifestations of immunity (eg, immunity against disease, which limits clinical manifestations despite infection, vs immunity against parasite infection, which limits parasite replication and biomass) may develop at different rates, depending on exposure and age, such that associations between immunological markers of protection and measures of both infection and clinical disease incidence change throughout life.
Prior studies have used mathematical models to try and distinguish between multiple plausible immune mechanisms that could be driving the observed age-specific incidence curves [22–24]. Results of these studies are consistent with the idea that immunity against infection and clinical disease result from multiple processes and develop at different rates [25]. However, while most of these studies were calibrated to extensive epidemiological (aggregated age-associated prevalence and age-associated incidence) data, they failed to explicitly take into account the heterogeneity in individual exposure to parasite-infected mosquitoes that is characteristic in these settings.
Here, we used data from a detailed cohort study to jointly quantify individual heterogeneity in the risk of infection and development of immunity against clinical disease in Tororo, a district in southeastern Uganda where malaria transmission is holoendemic. By following children longitudinally over their first 5 years of life, we were able to quantify both exposure and protection and to identify important factors associated with each.
METHODS
Data
We used data from the Tororo Child Cohort study conducted in Tororo District, Uganda, where the annual entomological inoculation rate was estimated by human landing catches in separate studies performed a decade apart to be 562 infectious bites per person-year (during 2001–2002) and 125 infectious bites per person-year (during 2011–2012) [3, 5]. The population and methods of this study have been described extensively elsewhere [26–28]. In particular, we used data on recurrent P. falciparum infection and fever from children who were born to human immunodeficiency virus (HIV)–negative mothers and enrolled between 6 weeks and 10 months of age and followed up to 5 years of age.
Participants were followed for all medical problems at a dedicated study clinic open 7 days a week. Participants with a documented elevated temperature (≥38°C [tympanic]) or a history of fever in the previous 24 hours had blood obtained by finger prick for a thick blood smear. If the smear was positive for asexual parasites, the patient received a diagnosis of malaria, regardless of parasite density. At the time of their first uncomplicated malaria episode, children were randomly assigned to receive open-label artemether-lumefantrine (AL) or dihydroartemisinin-piperaquine (DP) for the current and all subsequent uncomplicated malaria episodes. Children were followed-up on days 1, 2, 3, 7, 14, 21, and 28 after diagnosis, and blood smears were performed at all follow-up visits. In addition, all participants had routine blood smears performed on a monthly basis. Individuals with positive blood smears in the absence of fever were classified as having asymptomatic parasitemia and were not treated.
For the purpose of this analysis, new clinical malaria episodes were defined as febrile episodes with parasite-positive blood smears occurring at least 14 days since the last treatment. Asymptomatic infections were defined as instances of parasitemia that were not followed by symptomatic malaria (ie, fever) in the next 7 days. Based on previous publications, we assumed all new episodes of malaria to be reinfections (rather than recrudescence) [28].
Ethical Approval
The study protocol was reviewed and approved by the Uganda National Council of Science and Technology and the institutional review boards of the University of California–San Francisco, Makerere University, and the Centers for Disease Control and Prevention. Informed consent was obtained from the parent or guardian of all participating children.
Characterizing Reinfection and Development of Immunity Against Clinical Malaria
To explore factors associated with recurrent P. falciparum infections and the risk of clinical malaria, we specified a simple probabilistic (recurrent event) model. Following any parasite negative visit, individuals may (1) remain uninfected (parasite negative), (2) get infected and develop clinical malaria, or (3) get infected and develop asymptomatic parasitemia. These outcomes can be expressed as products of probabilities, where the probability of remaining uninfected is equal to , the probability of getting infected and developing malaria is equal to [] × ϕ, and the probability of getting infected and developing asymptomatic parasitemia is equal to [] × [1 − ϕ], where λi is the hazard of infection (our metric of exposure) for individual i, is the probability of being infected during time t, and ϕ is the probability of developing malaria, conditional on having been infected. Thus, a higher λi implies that a child is at higher risk of infection, while a ϕ of <1 can be interpreted as indicative of some level of immunity against clinical disease. The probability of a new malaria episode is the product of the probabilities of being infected and developing malaria, given infection.
The hazard of infection of participant i at time t can be expressed as λi[t|zi] = λ0zi, where λ0 is the basal hazard, and zi is the individual frailty (relative hazard), which is assumed to follow a gamma distribution [z∼Γ(1/θ, 1/θ)], with a mean of 1 and a variance equal to θ. Thus, our models assume that recurrent events are independent, conditional on the individual frailty parameter.
While the outcomes that follow a parasite-negative visit are generally well defined, outcomes following an asymptomatic infection are not. In particular, we do not know whether an asymptomatic infection that is followed by an episode of malaria represents disease progression from the same infection or a new infection. Similarly, we do not know whether 2 consecutive visits in which an asymptomatic infection is detected represent the same infection that has not cleared or a new infection. For the purpose of this analysis, we assumed that episodes of malaria occurring >7 days after the detection of an asymptomatic infection, as well as consecutive episodes of asymptomatic parasitemia, represented new infections. While these undefined events accounted for <3% of the data set, we performed extensive sensitivity analyses to assess the impact of these assumptions.
To explore the effects of several individual-level covariates (eg, age, body surface area, treatment arm, and prior number of infections) on the probabilities of infection and clinical disease, we expressed the hazard (λ0) and the probability of malaria, given infection (ϕ), in terms of these covariates of interest.
All models were fitted using Bayesian Markov-chain Monte Carlo methods with the RStan package [29, 30]. We ran 4 chains of 1000 iterations each and used the last half of each chain to determine the posterior distributions. We used noninformative priors for all fixed effects and estimated variance components. Convergence was assessed using visual examination of chains and the Gelman and Rubin statistic [31]. We compared the fit of the models to the data, using the deviance information criterion (DIC) [32]; a smaller DIC indicates a better model. Further details on the assumptions and specific models explored are provided in the Supplementary Materials.
RESULTS
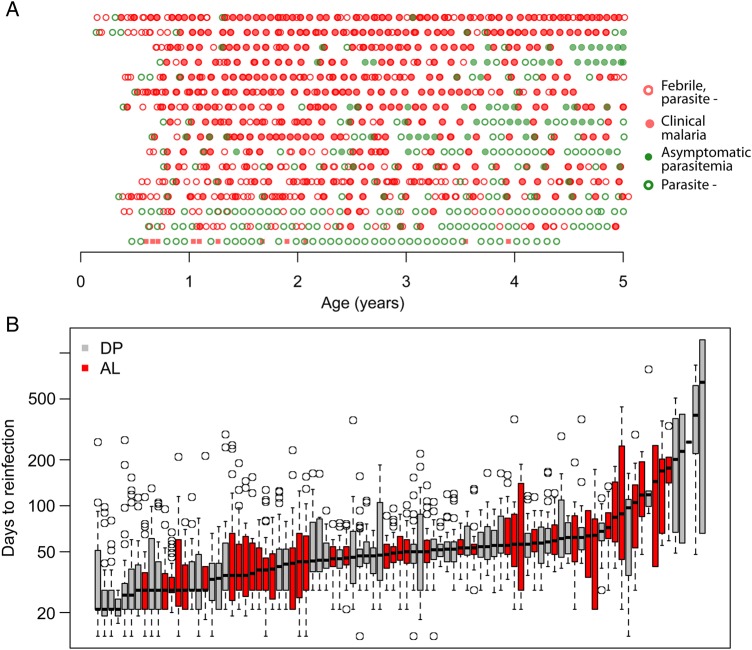
Between August 2007 and January 2008, 100 children born to HIV-negative mothers were enrolled in the study. Analyses below were limited to the 93 children who experienced ≥2 malaria episodes during follow-up. Of note, 4 of 7 excluded participants were followed for <1 year. A total of 2005 incident clinical malaria episodes and 221 episodes of asymptomatic parasitemia were recorded throughout the study period (Table 1). The median yearly incidence of clinical malaria was 5.9 episodes per person-year but varied significantly between participants, ranging between 0.63 and 11.3 episodes per person-year. Figure 1A illustrates the experiences of a subsample of children in the study.
Table 1.
Basic Characteristics of Study Participants
| Characteristic | Value (n = 93) |
|---|---|
| Female sex | 39 (42) |
| Rural residence | 75 (81) |
| Age, mo | |
| At enrollment | 5.3 (1.5–9.9) |
| At randomization | 9.6 (3.8–29.5) |
| At last follow-up | 60.0 (12–60.0) |
| Total person-time of follow-up, person-years | 376.1 |
| Randomization group | |
| DP | 51 (55) |
| AL | 42 (45) |
| Malaria episodes, no. | 2005 |
| Asymptomatic parasitemia episodes, no. | 221 |
Data are no. (%) of participants or median value (range), unless otherwise indicated.
Abbreviations: AL, artemether-lumefantrine; DP, dihydroartemisinin-piperaquine.
Figure 1.
A, Figure showing the infection history of a subset of participants throughout the 5-year follow-up. Each row represents the experience of a specific participant. Children are sorted on the basis of their estimated individual frailty, from low exposure to high exposure. B, Times between subsequent infections for children in the data set. Each box plot represents the distribution of gap times (times to reinfection) for a particular child. Abbreviations: AL, artemether-lumefantrine; DP, dihydroartemisinin-piperaquine.
Consistent with previous analyses of this data set [27], we found significant heterogeneity between study participants in the times to reinfection (ie, days between a treated malaria infection and the next detected infection; Figure 1B). The median time to reinfection ranged between 21 and 642 days (overall median, 44 days). The median time was significantly longer for children treated with DP (50 days; 95% confidence interval [CI], 48–53 days) as compared to those treated with AP (28 days; 95% CI, 28–36 days), which was expected, given the longer posttreatment prophylaxis effect of piperaquine versus lumefantrine [28, 33].
Factors Associated With the Hazard of Infection
To explore factors driving the large variation in times to reinfection, we fit a series of models in which the hazard of infection was expressed as a function of covariates (Table 2). Details of the specific models that were explored are provided in the Supplementary Materials and Supplementary Table 1.
Table 2.
Factors Associated With the Hazard of Infection
| Covariate | Univariate HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|
| Factors associated with the hazard of infection | ||
| Body surface area (per m2) | 3.7 (2.48–5.34) | …a |
| Age (per additional year) | 1.15 (1.11–1.18) | … |
| Residence | ||
| Urban | Reference | … |
| Rural | 3.16 (2.73–3.66) | 3.18 (2.20–4.42) |
| Treatment arm | ||
| DP | Reference | … |
| AL | 1.30 (1.19–1.41) | … |
| Prior infection (per additional no.) | 1.04 (1.04–1.05) | … |
| Variance of the individual random effect | 0.48 (.35—.65) | 0.36 (.26—.50) |
| Factors associated with the probability of clinical malaria, given infection | ||
| Age (per additional year) | ||
| Overall | 0.80 (.77–.84) | |
| Among patients receiving DP | 0.84 (.81–.88) | 0.92 (.85–.99) |
| Among patients receiving AL | 0.76 (.72–.80) | 0.83 (.77–.9) |
| Prior infection (per additional no.) | 0.97 (.97–.98) | 0.99 (.98–1.00) |
| Malaria treatment in past mo | 0.58 (.52–.65) | … |
| DP in past mo | 0.49 (.39–.60) | 0.44 (.35–.55) |
| AL in past mo | 0.61 (.54–.69) | 0.79 (.67–.91) |
Abbreviations: AL, artemether-lumefantrine; CI, confidence interval; DP, dihydroartemisinin-piperaquine; HR, hazard ratio.
a Adjusted for a nonlinear function of the body surface area and as such, coefficient is not interpretable.
In unadjusted analyses, the most important predictor of the hazard of infection was living in a rural residence. Children residing in rural houses experienced a hazard that was 3.2 times (95% CI, 2.7–3.7 times) that experienced by children living in an urban setting. In agreement with previous studies [34], increasing body surface area was also associated with shorter times to infection (hazard ratio [HR], 3.7 per m2 increase in surface area; 95% CI, 2.5–5.3 per m2 increase in surface area). Given the large correlation between body surface area and age, increasing age was also associated with a higher hazard (HR, 1.15; 95% CI, 1.11–1.18). Allowing for a nonlinear relationship between body surface area (or age) and hazard improved the fit of the models.
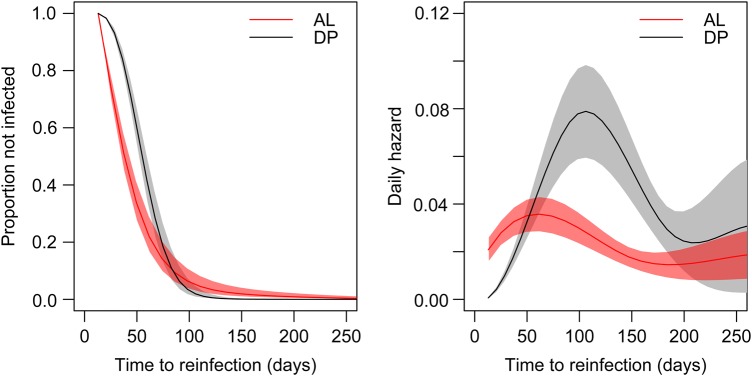
In this cohort, another key predictor of the hazard of infection was the antimalarial therapy to which children were randomly to receive for all malaria treatments. On average, treatment with AL was associated with a hazard of infection that was 1.3 times (95% CI, 1.2–1.4 times) that of treatment with DP. To characterize this relationship further, we fit models that allowed the hazard to vary as a function of time since the last treatment (Figure 2). As expected, the hazard of infection peaked earlier for children receiving AL as compared to those receiving DP (60 vs 110 days). However, the hazard peaked higher for children receiving DP (hazard, 0.079 [95% CI, .059–.098] vs 0.036 [95% CI, .027–.042]).
Figure 2.
Figure showing survival curves and daily hazards for children in the dihydroartemisinin-piperaquine (DP) and artemether-lumefantrine (AL) groups. The left panel shows the survival curves of the times to reinfections (in days) for both treatment arms. The right panel shows the average estimated daily hazards (average daily rates of infection) obtained by fitting models that allowed the hazard to vary as a function of time since the last treatment.
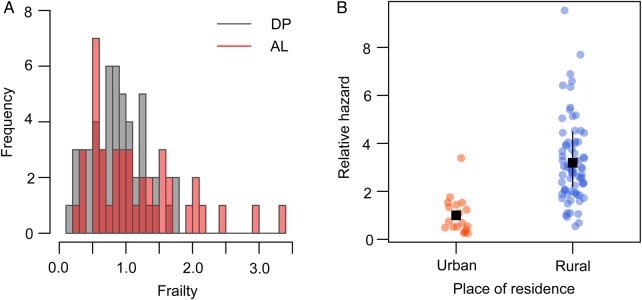
Models that included a multiplicative random effect (frailty) for the individual hazard of infection fit the data significantly better, consistent with a large heterogeneity in the hazards (relative risks of infection) experienced by individuals. In models that were adjusted for body surface area and place of residence, individual frailties ranged between 0.17 and 3.4 (Figure 3A), implying that the individual at highest risk of infection experience hazards that are approximately 20 times higher than that of the individual at lowest risk. Differences are even larger when comparing individuals who live in urban vs rural settings (Figure 3B).
Figure 3.
A, Histogram showing distribution of estimated individual frailties (relative hazards of infection as compared to the population average) after adjustment for body surface area and location of residence. B, Relative hazards of individuals living in urban versus rural households. The average relative hazard of individuals living in rural households versus urban households (reference group) is shown in black. Abbreviations: AL, artemether-lumefantrine; DP, dihydroartemisinin-piperaquine.
Overall, the best adjusted model of the time to infection included body surface area and location of residence (rural vs urban) and is shown in Figure 4A. Treatment arm did not improve the fit in models that allowed for an individual random effect, which was expected because treatment was randomly assigned at the individual level.
Figure 4.
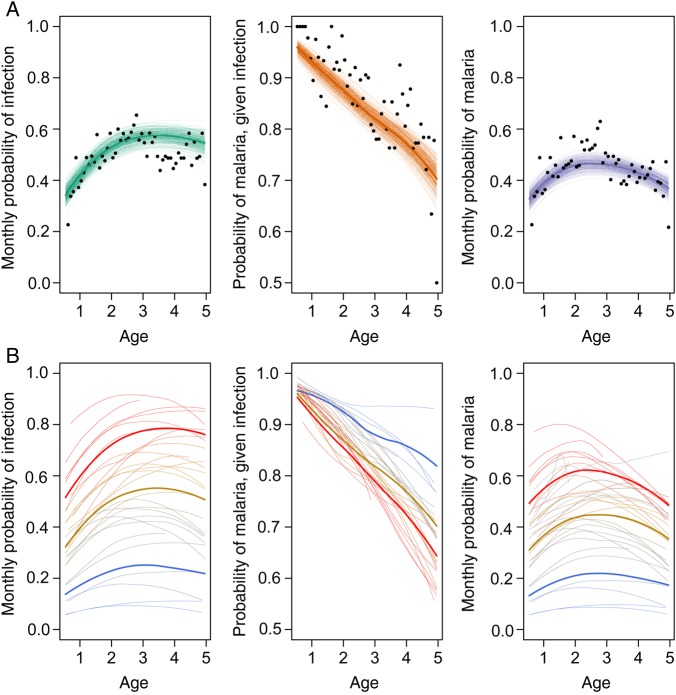
Data and fit of model to the mean monthly probability of infection (A), probability of malaria, given infection (B), and resulting monthly probability of malaria (C) as a function of age. Data are shown as dots. Thick lines represent mean fitted values, and thin lines represent draws from the posterior distribution (uncertainty around mean values). Whereas panel A shows the fit of the model to mean probabilities, panel B illustrates fitted individual probabilities across 3 tertiles of exposure (tertiles of estimated frailties). Thin lines represent specific trajectories predicted for a subset of children within each tertile and thus illustrate the large heterogeneity that exists in the sample. Thick lines represent the mean expected trajectory for each tertile of frailty values.
Factors Associated With the Probability of Clinical Malaria, Given Infection
To explore factors associated with the probability of developing clinical malaria upon infection, we then fit a series of models in which this probability was expressed as a function of covariates. Since we were particularly interested in quantifying the effects of age and prior number of infections on the probability of clinical disease, we fit several models evaluating these associations (Supplementary Text 1 and Supplementary Table 2).
Both age and the cumulative number of prior infections (symptomatic and asymptomatic) were negatively associated with the probability of clinical malaria upon infection (Table 2). In unadjusted analyses, the risk of developing clinical malaria upon infection decreased on average by 20% (95% CI, 7%–42%) for each additional year of age and by 3% (95% CI, 2%–3%) for each additional prior infection. Both associations remained significant when included simultaneously in the model. The risk of developing clinical malaria upon infection decreased on average by 6% (95% CI, 0%–12%) for each additional year of age and by 2% (95% CI, 1%–3%) for each additional prior infection.
Interestingly, our results suggest that immunity against clinical malaria may develop faster in individuals receiving AL for treatment of malaria (relative risk [RR], 0.76; 95% CI, .72–.8) than in those receiving DP (RR, 0.84; 95% CI, .81–.88; Supplementary Figure 1). This interaction between age and treatment allocation remained significant even after adjustment for the cumulative number of prior infections and in all sensitivity analyses (Supplementary Table 3). As expected, given the posttreatment prophylaxis of antimalarials used, malaria treatment in the last month decreased the risk of clinical malaria upon infection for the next infection following treatment both in participants treated with AL (RR, 0.61; 95% CI, .54–.69) and in those treated with DP (RR, 0.49, 95% CI, .39–.6).
The best multivariate model of the probability of clinical malaria upon infection included age, cumulative number of prior infections, and antimalarial treatment in the last month. The model also included the interaction between age and treatment arm and between recent treatment and treatment arm. Figure 4 illustrates the fit of the model to the data.
Overall, our analyses revealed great heterogeneity in the development of immunity against clinical malaria among children in this cohort and how it is related to an individual's level of exposure (Figure 4B). While individuals under higher infection hazards (ie, individuals with higher frailties) experienced a larger number of infections, they also tended to develop immunity faster and experienced a more rapid decline in the probability of presenting clinical malaria upon infection.
Quantifying Changes in the Probability of Malaria With age and Different Levels of Exposure
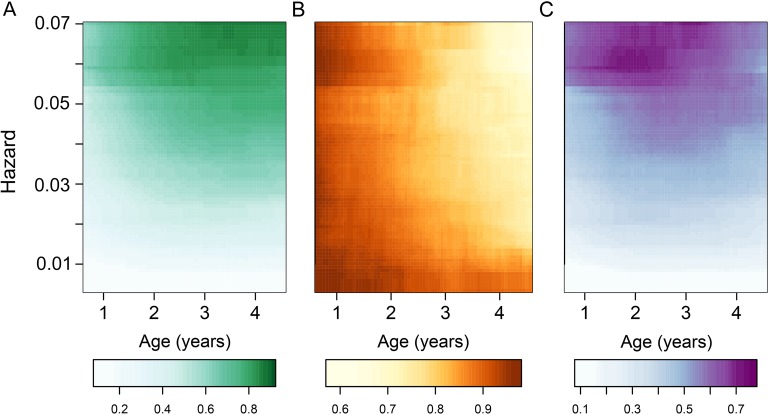
To further quantify the joint effects of exposure and age on malaria parasite infection and disease, we used the best-fitting model to predict the expected monthly probabilities of infection; probabilities of malaria, given infection; and resulting monthly probabilities of malaria across a range of hazards and ages represented in the data. Figure 5 shows the predictions for children in this cohort and illustrates how changes in the observed risk of malaria are the product of nonlinear changes in the risk of infection and changes in the probability of clinical disease.
Figure 5.
Predicted mean probabilities of infection and clinical malaria for different ages and exposures. A, Monthly probability of infection. B, Probability of malaria, given infection. C, Monthly probability of malaria.
DISCUSSION
Although it is well known that immunity to clinical malaria develops gradually in individuals living in malaria-endemic settings, factors driving its development are not fully understood [6–9, 11]. Here, we used very detailed longitudinal data from 93 children living in an area where the level of malaria transmission is known to be high to quantify the contributions of individual-level factors, including age and exposure, on the risk of infection and of clinical disease during early life. Our results are consistent with the idea that age-associated incidence patterns observed in malaria-endemic settings are shaped by multiple factors. While repeated infection leads to reductions in the probability of clinical disease upon reinfection, age appears to act independently in both directions, increasing the probability of infection (primarily through increases in the body surface area and the likelihood of being bitten) and reducing the probability of clinical disease upon infection. Independent effects of age and exposure have been extensively discussed before [22, 35–38], but their quantification is often elusive due to the inevitable collinearity that exists between the two in malaria-endemic settings (age is a proxy for cumulative exposure). Longitudinal data sets like this one, with detailed follow-up and sufficient heterogeneity in the exposed population, are required to isolate the two effects.
In the absence of reliable biomarkers of immunity, numerous studies have modeled epidemiological data in an attempt to characterize the development of immunity [22, 39–41]. These studies generally model age-associated incidence of clinical malaria or age-associated prevalence of parasitemia and often ignore the large heterogeneity in exposure between individuals living in most malaria-endemic settings. A major strength of this study is that it involved longitudinal data, allowing us to model heterogeneity in transmission explicitly. In addition, the study design included regular active and passive surveillance of infection and disease, therefore capturing both asymptomatic and symptomatic infections. This enabled us to separate the processes of infection and disease, modeling independently the factors driving both.
Surprisingly, our findings suggest that immunity against clinical malaria may develop faster in children regularly treated with AL as compared to those treated with DP. This might be explained in part by the fact that DP has been shown to protect against infection for longer periods and thus reduces the total exposure of the immune system to the parasite during that time [28, 42]. Our finding that recent treatment with DP is more protective against subsequent clinical malaria than treatment with AL is also consistent with this idea. While reducing the number of infections is certainly a desirable outcome, potential interference with development of clinical immunity needs to be considered when evaluating the overall impacts of such interventions.
Our findings also underscore the great heterogeneity that exists in the risk of infection between individuals and the resulting heterogeneity in the rate of development of immunity against clinical disease. Properly accounting for this heterogeneity is fundamental when modeling data on the age-associated incidence of malaria, to avoid misinterpretation of observed epidemiologic patterns. This is particularly true when comparing data from different sites where the extent of heterogeneity may also differ.
While the analysis presented here was based on a rich, longitudinal data set, a limitation is that parasitemia measured by microscopy was the only available metric of P. falciparum infection. Poor sensitivity for the detection of low-density parasitemia and consequent underestimation of the number of times children were infected by P. falciparum could explain some of the patterns in the monthly probabilities of infection in this data set. Further, since microscopy does not reveal whether infection comprises parasite clones from one or more infective mosquito bites, it may further underestimate the number of times a person might have been infected, thus leading to an underestimate of the true hazard of infection. Subsequently, our estimate of the impact of each additional episode on the probability of clinical malaria may be an overestimate. In any case, these potential biases should not invalidate our finding of independent effects of age and exposure on the development of immunity against clinical disease. Studies that include independent measures of transmission intensity or exposure (eg, household-level entomological inoculation rates and molecular forces of infection) will be needed to properly quantify the effects of exposure on the development of immunity against malaria. Finally, while our approach provides strong statistical evidence of the independent effects of age and exposure on the development of immunity against clinical malaria, it does not provide any mechanistic insight or distinguish between types of immunity (eg, immunity against parasite infection vs immunity against disease). Similarly, since severe malaria was extremely rare in this study, our model is not informative about the role of age and exposure in the development of immunity against severe disease.
Few studies have been able to quantify the independent effects of individual-level factors, including age and exposure, in the development of immunity against malaria, while accounting for heterogeneity in transmission. Careful analyses of longitudinal data sets, like this one, will be key in filling these gaps. Improved understanding of the factors driving antimalaria immunity will provide much needed information to properly evaluate malaria-control strategies, anticipate the impact of malaria-control interventions, and guide policy decisions.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank all of the parents and guardians, for kindly giving their consent; the study participants, for their cooperation; and all members of the study team, for their tireless effort and excellent work.
Financial support. This work was supported by the Centers for Disease Control and Prevention, (cooperative agreement U62P024421), the Doris Duke Charitable Foundation the National Institutes of Health (U19AI089674), the National Health and Medical Research Council Australia (early career fellowship to M. J. B.), the National Health and Medical Research Council Australia Infrastructure for Research Institutes Support Scheme (to the Burnet Institute), and Victorian State Government Operational Infrastructure Support (to the Burnet Institute).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization (WHO). World malaria report 2014; Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 2.Naghavi M, Wang H, Lozano R, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilama M, Smith DL, Hutchinson R et al. . Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J 2014; 13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gething PW, Patil AP, Smith DL et al. . A new world malaria map: Plasmodium falciparumendemicity in 2010. Malar J 2011; 10:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okello PE, Van Bortel W, Byaruhanga AM et al. . Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg 2006; 75:219–25. [PubMed] [Google Scholar]
- 6.Griffin JT, Hollingsworth TD, Reyburn H, Drakeley CJ, Riley EM, Ghani AC. Gradual acquisition of immunity to severe malaria with increasing exposure. Proc R Soc Lond [Biol] 2015; 282:20142657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyburn H, Mbatia R, Drakeley C et al. . Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 2005; 293:1461–70. [DOI] [PubMed] [Google Scholar]
- 8.Okiro EA, Al-Taiar A, Reyburn H, Idro R, Berkley JA, Snow RW. Age patterns of severe paediatric malaria and their relationship to Plasmodium falciparum transmission intensity. Malar J 2009; 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carneiro I, Roca-Feltrer A, Griffin JT et al. . Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS ONE 2010; 5:e8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idro R, Aloyo J, Mayende L, Bitarakwate E, John CC, Kivumbi GW. Severe malaria in children in areas with low, moderate and high transmission intensity in Uganda. Trop Med Int Health 2006; 11:115–24. [DOI] [PubMed] [Google Scholar]
- 11.Roca-Feltrer A, Carneiro I, Smith L, Schellenberg JR, Greenwood B, Schellenberg D. The age patterns of severe malaria syndromes in sub-Saharan Africa across a range of transmission intensities and seasonality settings. Malar J 2010; 9:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran TM, Li S, Doumbo S et al. . An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis 2013; 57:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DL, Guerra CA, Snow RW, Hay SI. Standardizing estimates of the Plasmodium falciparumparasite rate. Malar J 2007; 6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bejon P, Williams TN, Nyundo C et al. . A micro-epidemiological analysis of febrile malaria in Coastal Kenya showing hotspots within hotspots. Elife 2014; 3:e02130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 2005; 438:492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark TD, Greenhouse B, Njama-Meya D et al. . Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J Infect Dis 2008; 198:393–400. [DOI] [PubMed] [Google Scholar]
- 17.Kreuels B, Kobbe R, Adjei S et al. . Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. J Infect Dis 2008; 197:85–93. [DOI] [PubMed] [Google Scholar]
- 18.Oesterholt MJAM, Bousema JT, Mwerinde OK et al. . Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar J 2006; 5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagannathan P, Eccles-James I, Bowen K et al. . IFNγ/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog 2014; 10:e1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenhouse B, Ho B, Hubbard A et al. . Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J Infect Dis 2011; 204:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helb DA, Tetteh KKA, Felgner PL et al. . Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc Natl Acad Sci U S A 2015; 112:E4438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filipe JA, Riley EM, Drakeley CJ, Sutherland CJ, Ghani AC. Determination of the processes driving the acquisition of immunity to malaria using a mathematical transmission model. PLoS Comput Biol 2007; 3:e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith T, Ross A, Maire N, Rogier C, Trape JF, Molineaux L. An epidemiologic model of the incidence of acute illness in Plasmodium falciparum malaria. Am J Trop Med Hyg 2006; 75(2 suppl):56–62. [DOI] [PubMed] [Google Scholar]
- 24.Cameron E, Battle KE, Bhatt S et al. . Defining the relationship between infection prevalence and clinical incidence of Plasmodium falciparum malaria. Nat Commun 2015; 6:8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hviid L. Clinical disease, immunity and protection against Plasmodium falciparum malaria in populations living in endemic areas. Expert Rev Mol Med 1998; 1998:1–10. [DOI] [PubMed] [Google Scholar]
- 26.Sandison TG, Homsy J, Arinaitwe E et al. . Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ 2011; 342:d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagannathan P, Muhindo MK, Kakuru A et al. . Increasing incidence of malaria in children despite insecticide-treated bed nets and prompt anti-malarial therapy in Tororo, Uganda. Malar J 2012; 11:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arinaitwe E, Sandison TG, Wanzira H et al. . Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in Young Ugandan Children. Clin Infect Dis 2009; 49:1629–37. [DOI] [PubMed] [Google Scholar]
- 29.Stan development Team. Stan: A C++ Library for Probability and Sampling, Version 2.5.0. [Internet]. http://mc-stan.org/.
- 30.R: A language and environment for statistical computing. RFoundation for Statistical Computing. 2nd ed. Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- 31.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci 1992; 7:457–72. [Google Scholar]
- 32.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser A 2002; 64:583–639. [Google Scholar]
- 33.Wanzira H, Kakuru A, Arinaitwe E et al. . Longitudinal outcomes in a cohort of Ugandan children randomized to artemether-lumefantrine versus dihydroartemisinin-piperaquine for the treatment of malaria. Clin Infect Dis 2014; 59:509–16. [DOI] [PubMed] [Google Scholar]
- 34.Port GR, Boreham P, Bryan JH. The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera: Culicidae). Bull Entomol Res 1980; 70:133–44. [Google Scholar]
- 35.Struik SS, Riley EM. Does malaria suffer from lack of memory? Immunol Rev 2004; 201:268–90. [DOI] [PubMed] [Google Scholar]
- 36.Rogier C, Commenges D, Trape J. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am J Trop Med Hyg 1996; 54:613–9. [DOI] [PubMed] [Google Scholar]
- 37.Bødker R, Msangeni HA, Kisinza W, Lindsay SW. Relationship between the intensity of exposure to malaria parasites and infection in the Usambara Mountains, Tanzania. Am J Trop Med Hyg 2006; 74:716–23. [PubMed] [Google Scholar]
- 38.Baird JK. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today (Regul Ed) 1995; 11:105–11. [DOI] [PubMed] [Google Scholar]
- 39.Aron JL. Mathematical modelling of immunity to malaria. Math Biosci 1988; 90:385–96. [Google Scholar]
- 40.Maire N, Smith T, Ross A, Owusu-Agyei S, Dietz K, Molineaux L. A model for natural immunity to asexual blood stages of Plasmodium falciparum malaria in endemic areas. Am J Trop Med Hyg 2006; 75(2 suppl):19–31. [DOI] [PubMed] [Google Scholar]
- 41.Smith T, Killeen GF, Maire N et al. . Mathematical modeling of the impact of malaria vaccines on the clinical epidemiology and natural history of Plasmodium falciparum malaria: Overview. Am J Trop Med Hyg 2006; 75(2 suppl):1–10. [DOI] [PubMed] [Google Scholar]
- 42.White NJ. Intermittent presumptive treatment for malaria. PLoS Med 2005; 2:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.