Abstract
Plasmodium vivax, the most widely distributed human malaria parasite, is restricted to reticulocytes, limiting its asexual proliferation. In recent years, cases of severe and high-level P. vivax parasitemia have been reported, challenging the assumption that all isolates are equally restricted. In this article, we analyze the reticulocyte preference of a large number of Indian P. vivax isolates. Our results show that P. vivax isolates significantly vary in their level of reticulocyte preference. In addition, by carefully staging the parasites, we find that P. vivax schizonts are largely missing in peripheral blood, with the presence of schizonts in circulation correlating with a high reticulocyte preference.
Keywords: Plasmodium vivax, reticulocyte preference, asexual stage
Plasmodium vivax, one of the main Plasmodium species causing malaria in humans, is also the most widespread worldwide [1]. Despite its public health importance, progress in its research has been relatively slow as compared to that for P. falciparum, in large part because of the lack of a robust in vitro culture system. Recently, there has been renewed interest in its biology and epidemiology, with the development of short-term ex vivo assays and molecular tools to study P. vivax in various field settings [2]. These new studies have challenged several aspects of P. vivax biology and disease that had been generally accepted. Indeed, malaria severity and even associated death is no longer considered unique to P. falciparum, with cases of severe malaria due to P. vivax reported consistently in various regions [3]. Sequestration of developing parasites, another key feature in P. falciparum that appeared to be lacking for P. vivax, has been questioned, with a paucity of schizonts in circulation being observed with evidence of increased cytoadherence of later asexual stages to endothelial cells ex vivo [4]. This has led to the speculation that invasion may be happening in areas of high reticulocyte density to which P. vivax is restricted, such as the bone marrow [5]. Unlike these features, the huge preference of P. vivax to reticulocytes over normocytes has been an undisputed feature of P. vivax since its early observation [6] and has been attributed as the limiting factor for parasite density in vivo. Although recent studies have confirmed that P. vivax isolates from Thailand preferentially invade reticulocytes ex vivo [5], P. vivax is highly genetically divergent [7], and the assumed reticulocyte preference of P. vivax field isolates has not been systematically investigated. In this study, we determine the intrinsic red blood cell (RBC) preference of a large series of Indian P. vivax isolates. By making microscope slides within hours of blood sample collection from patients, we can analyze a snapshot of the parasite and the RBC compositions in peripheral blood. India is a particularly interesting geographical area to investigate because it contributes to the majority of malaria cases in South Asia [8] and because several studies on P. vivax severe malaria have been performed in India [3].
MATERIAL AND METHODS
Ethics Statement
All human subjects protocols have been approved by the ethics boards at Goa Medical College and Hospital, the University of Washington, the Division of Microbiology and Infectious Diseases of the National Institutes of Health, and the Government of India Health Ministry Screening Committee.
Sample Collection
Blood samples were collected from patients at Goa Medical College and Hospital with a diagnosis of P. vivax infection based on positive results of a rapid diagnostic test (FalciVax; Zephyr Biomedicals, Verna, Goa, India). This site is established by the Malaria Evolution in South Asia International Center of Excellence in Malaria Research and the University of Washington.
Parasite Slide Preparation
Blood samples were obtained from 113 patients. Thin smears were prepared with 1.5 μL of whole blood, which was fixed and subjected to Giemsa staining (Sigma Aldrich, St Louis, Missouri) for 20 minutes or Hemacolor Rapid staining (EMD Millipore Billerica, Massachusetts) following the manufacturer's protocol. For reticulocyte staining, 2–5 μL of each sample was incubated with an equal amount of New Methylene Blue Reticulocyte Stain (Sigma Aldrich). Another set of reticulocyte-stained smears was fixed with 100% methanol after incubation and then was double stained for parasites with Giemsa (Sigma Aldrich).
Stage Determination
The stage distribution of each sample was recorded for at least 100 parasitized cells according to the following criteria. Stage I corresponds to very young trophozoites, which have a small ring with a single bead of chromatin with cytoplasm. The cell may be slightly enlarged and the ring form can start to become amoeboid. Stage II corresponds to mid-trophozoite forms, which have a much bigger cytoplasm and a large vacuole that does not completely fill the RBC but noticeably deforms it. Stage III corresponds to an advanced trophozoite filling the cytoplasm, with 1 or 2 nuclear chromatin spots that are no longer prominent. Stage IV corresponds to schizont forms with >3 nuclei and defined nuclear segments. Mature gametocytes are denote by “G.”
Parasitemia and reticulocytemia counts were determined on thin smears. A minimum of 500 RBCs were counted and infected RBCs recorded using a 1:9 miller reticle, or the recently described NWF method, for greater accuracy at low frequencies [9]. Counts were performed by at least 2 experienced microscopists.
Relative Frequency of Reticulocyte Infection
Double-stained smears were analyzed for at least 50 parasitized cells for their RBC type (normocytes or reticulocytes). The relative frequency of reticulocyte infection was calculated as the ratio of the estimated proportion of reticulocytes that contain parasites versus the estimated proportion of normocytes that contain parasites, as described in Hegner et al [6]. For comparison, samples were first divided into 2 groups on the basis of median value of all relative frequencies of infection in reticulocytes (RFRs; 104.4), then further into 4 groups with comparable numbers of samples in each (<50, 50 to <100, 100 to <200, and ≥200).
Selectivity Index (SI) Determination
Samples were counted for RBCs containing >1 ring stage parasite in at least 100 infected RBCs. The SI, or ratio of the observed number of multiple-parasite-infected RBCs to the expected number, assuming a Poisson-distributed random invasion process, was calculated as previously described by Simpson et al [10].
Statistical Analysis
Kruskal-Wallis 1-way analysis of variance was used for comparison of groups. Correlation was determined using Spearman correlation. P values of <.05 were considered significant. All statistical analysis was done using GraphPad Prism 5.0 (GraphPad Software, La Jolla, California).
RESULTS
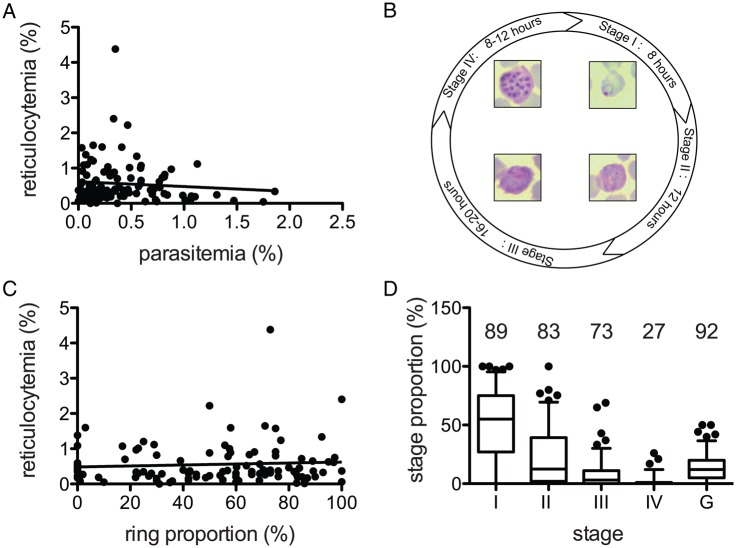
The range of parasitemia frequencies observed across 109 patients was highly variable among patients (0.0015%–1.86%), as was the range of reticulocytemia frequencies (0.0015%–4.38%). However, there was no correlation (positive or negative) between peripheral parasite and reticulocyte densities, indicating that parasitemia is not solely driven by a higher proportion of reticulocytes, the most susceptible RBC in circulation (Figure 1A). In these isolates, reticulocytemia was found to be independent of hemoglobin and hematocrit levels (data not shown).
Figure 1.
Parasitemia range, reticulocytemia range, and stage distribution of Plasmodium vivax isolates. A, P. vivax–infected patients exhibit a range of parasitemia and reticulocytemia percentages at admission, but these values are not correlated (Spearman correlation; P = .8892). B, Schematic asexual blood stage development time line. The parasitized cells were categorized into 5 stages of development (stages I–IV and the gametocyte stage [G]). C, The ring proportion (stage I) of each sample was plotted against the reticulocytemia percentage. There was no correlation between the 2 values (Spearman correlation; P = .7726). D, The proportions of each stage were calculated for all samples. The number above each stage corresponds to the percentage of samples that had at least 1 of the each stage in circulation (n = 113).
Recently, reticulocytes were shown to mature rapidly within ex vivo when infected ex vivo [5] and reticulocytemia could thus be affected by the distribution of different stages. We thus carefully determined the stage distribution of each sample (Figure 1B). We confirmed that the proportion of rings was not correlated with reticulocytemia (Figure 1C). However, we also saw that as the stages became more advanced, their proportions decreased, as previously reported with Brazilian isolates [4]. Gametocytes on the other hand were observed in the majority of samples, at varying degree of abundance (Figure 1D). During the 48 hours of the asexual life cycle, schizontemia only occurred within the last 8 hours (Figure 1B) [11]. Even considering the relatively short period they would be detectable, stage IV parasites (schizonts) were disproportionately missing from circulation. However, unlike P. falciparum, for which sequestration of developing trophozoite is well documented and robust, P. vivax schizonts in circulation are not uncommon. Indeed, circulating schizonts could be seen in 27% of all samples, generally at low numbers but in up to 26% of the total number of parasites.
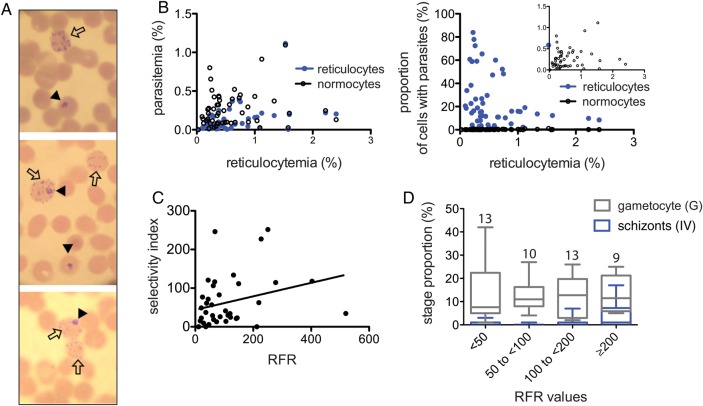
We also speculated that if maturation of infected reticulocyte in vivo happened at the same rate as described ex vivo [5], parasitized cells would no longer have the hallmark reticulocyte characteristics, such as reticular material. We thus stained samples with both reticulocyte and parasite stain, a method that reliably distinguishes parasites in normocytes from those in reticulocytes (Figure 2A). For every sample, we could always detect early stage parasites in reticulocytes (Figure 2B), suggesting that host RBC modification in circulation may not happen so rapidly in vivo.
Figure 2.
Reticulocyte preference measurement and correlation with stages. A, Blood samples were stained for both parasites and reticulocytes, allowing us to detect the red blood cell type the parasite is in. Black arrowheads point to parasitized cells, and the empty arrows point to reticulocytes. B, As the percentage of reticulocytes is much lower than that of normocytes, the parasitemia percentage among infected normocytes is generally higher than the percentage of infected reticulocytes (left panel). When compared as proportion of each cell type that is infected (that is, the proportion of all reticulocytes that contain a parasite), all samples have a higher proportion of reticulocytes (right panel). C, The ratio of each data point from the 2 graphs in panel (B) results in the relative frequency of infection in reticulocytes (RFR). RFRs have a wide range and correlate with their corresponding selectivity index (Spearman correlation; P = .0193). D, The samples were divided into 4 groups of increasing RFR (<50, 50 to <100, 100 to <200, and ≥200). The number of samples in each bin is indicated above each column. The proportions of the later stages (IV) and gametocyte (G) stage for each RFR bin were compared. There were significantly more schizonts in samples with high RFRs (≥200, indicating higher restriction and selectivity; P = .042, by the Kruskal-Wallis test), with comparable proportions of gametocytes (P = .1836).
To further determine whether reticulocytes are all equally preferred and invaded in every sample, we must account for the lower percentage of reticulocytes, compared with normocytes. We thus calculated the proportions of RBCs that contained a parasite for each cell type (normocyte or reticulocyte; Figure 2B) [6]. The ratio of the 2 proportions results in the RFR as compared to normocytes. A value of 1 would suggest a lack of preference for reticulocytes over normocytes [6]. We also measured the SI, the measure of the proportion of multiple-parasite-infected cells. The SI is also frequently used as an indicator of RBC susceptibility and has been associated with disease severity in P. falciparum [10]. SIs and RFRs were highly variable (range, 11–519 for the RFR and 0–251 for the SI), suggesting that some field strains are less restricted than others (Figure 2C). Previous studies using this method showed that P. falciparum also has a moderate preference for younger RBCs but that the range of RFRs reported for P. falciparum by use of the same method are much narrower (average, 1–4) [12], with the highest reported value being 14 [6].
We wondered differences in reticulocyte preference level, reflected by the RFR values, could explain the disparity in stage distribution. We thus compared the RFR values to the proportion of either late asexual stage schizonts (stage IV) or sexual gametocytes (G) to see whether reticulocyte preference could influence development into particular stages. Interestingly, the samples that indicated a greater reticulocyte preference (ie, a high RFR) had significantly more schizonts (stage IV) than those with lower RFRs (Figure 2D). Gametocyte proportions were comparable in isolates demonstrating high and low RFRs (Figure 2D).
DISCUSSION
The current study is the first to reveal large differences in reticulocyte preference among Indian P. vivax strains. The reticulocyte population is a small but heterogeneous subset of RBCs susceptible to P. vivax invasion, and our data indicate that Indian P. vivax isolates may differ in their level of restriction within the available reticulocyte pool. We have previously shown with Plasmodium knowlesi, a zoonotic malaria parasite of macaques, that a preference for young RBCs can greatly limit parasite density [13]. Mathematical modeling also suggested that, for the density to increase in vivo, an expansion of the pool of susceptible cells was optimal. The parasitemia frequencies observed in our data set frequently exceeded the number of peripheral reticulocytes, suggesting that reticulocyte availability is not the only factor for restricting parasite density. Of note, a previous transcriptional analysis study of P. vivax samples detected differences between isolates in their expression of various invasion ligands [11], including some that have been shown to mediate restriction to reticulocytes, providing a potential mechanism for variations in reticulocyte preference.
We further confirmed the low prevalence of circulating schizonts, as previously reported [4]. This rarity of schizonts in peripheral blood has led to the proposition that P. vivax may be sequestering in reticulocyte-rich zones such as the bone marrow [14]. Further studies investigating the reticulocyte and parasite burden in patient bone marrow would thus be highly informative.
We find an association between increased reticulocyte preference and schizont formation. This could indicate that there is successful development into schizonts when younger reticulocytes are invaded. Alternatively, but not mutually exclusively, there may be a greater conversion rate to sexual stages when invading older reticulocytes, limiting the number of schizonts one can detect. Robust ex vivo assays following asexual and sexual development will be needed to confirm this hypothesis.
This study suggests that P. vivax strains vary largely in their reticulocyte preference, suggesting the use of alternative invasion pathways, and this may determine the choice between asexual proliferation and sexual development in vivo. It is known that some P. vivax isolates demonstrate Duffy-independent invasion pathways [15]. A greater understanding of RBC heterogeneity and the molecular underpinnings of RBC preference by P. vivax isolates will be of great interest. This could have particular implications for the P. vivax burden and pathogenesis in vivo, as well as its transmissibility. In addition, it may inform strategies for the successful establishment of continuous in vitro culture for P. vivax, which is a fundamental challenge in the field.
Notes
Acknowledgments. We thank all the patients at Goa Medical College and Hospital who participated in this study and their family; and the clinical research assistants, for their help in enrolling patients for the study.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (agreement U19AI089688 to P. K. R., program director); and the Bill and Melinda Gates Foundation (OPP1023594).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization (WHO). World malaria report 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 2.Russell B, Suwanarusk R, Malleret B et al. . Human ex vivo studies on asexual Plasmodium vivax: The best way forward. Int J Parasitol 2012; 42:1063–70. [DOI] [PubMed] [Google Scholar]
- 3.Naing C, Whittaker MA, Nyunt Wai V, Mak JW. Is Plasmodium vivax malaria a severe malaria? A systematic review and meta-analysis. PLoS Negl Trop Dis 2014; 8:e3071–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes SCP, Albrecht L, Carvalho BO et al. . Paucity of Plasmodium vivax mature schizonts in peripheral blood is associated with their increased cytoadhesive potential. J Infect Dis 2014; 209:1403–7. [DOI] [PubMed] [Google Scholar]
- 5.Malleret B, Li A, Zhang R et al. . Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood 2015; 125:1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegner R. Relative frequency of ring-stage plasmodia in reticulocytes and mature erythrocytes in man and monkey. Am J Epidemiol 1938; 27:690–718. [Google Scholar]
- 7.Neafsey DE, Galinsky K, Jiang RHY et al. . The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nature 2012; 44:1046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg 2007; 77(6 suppl):69–78. [PubMed] [Google Scholar]
- 9.Lim C, Pereira L, Shardul P et al. . Improved light microscopy counting method for accurately counting Plasmodium parasitemia and reticulocytemia. Am J Hematol 2016; 91:852–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson JA, White NJ. Red cell selectivity in malaria : a study of multiple-infected erythrocytes. T Roy Soc Trop Med H 1999; 93:165–8. [DOI] [PubMed] [Google Scholar]
- 11.Bozdech Z, Mok S, Hu G et al. . The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci U S A 2008; 105:16290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitchen S. The infection of mature and immature erythrocytes by Plasmodium falciparum and Plasmodium malariae. Am J Trop Med Hyg 1939; 1:47–62. [Google Scholar]
- 13.Lim C, Hansen E, DeSimone TM et al. . Expansion of host cellular niche can drive adaptation of a zoonotic malaria parasite to humans. Nat Commun 2013; 4:1638–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayor A, Alano P. Bone marrow reticulocytes: a Plasmodium vivax affair? Blood 2015; 125:1203–5. [DOI] [PubMed] [Google Scholar]
- 15.Menard D, Barnadas C, Bouchier C et al. . Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A 2010; 107:5967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]