Abstract
Background. Each year dengue virus (DENV) infects 400 million human but causes symptomatic disease in only a subset of patients, suggesting that host genetic factors may play a role. HLA molecules that restrict T-cell responses are one of the most polymorphic host factors in humans.
Methods. Here we map HLA DRB1–restricted DENV-specific epitopes in individuals previously exposed to DENV, to identify the breadth and specificity of CD4+ T-cell responses. To investigate whether HLA-specific variations in the magnitude of response might predict associations between dengue outcomes and HLA-DRB1 alleles, we assembled samples from hospitalized patients with known severity of disease.
Results. The capsid protein followed by nonstructural protein 3 (NS3), NS2A, and NS5 were the most targeted proteins. We further noticed a wide variation in magnitude of T-cell responses as a function of the restricting DRB1 allele and found several HLA alleles that showed trends toward a lower risk of hospitalized disease were associated with a higher magnitude of T-cell responses.
Conclusions. Comprehensive identification of unique CD4+ T-cell epitopes across the 4 DENV serotypes allows the testing of T-cell responses by use of a simple, approachable technique and points to important implications for vaccine design.
Keywords: Dengue virus, CD4+ T cells, HLA, disease association
CD4+ HLA class II–restricted T cells are one of the pillars of adaptive immunity to microbes. CD4+ T-helper (Th) responses are key for the induction, maturation, and isotype switching of antibody responses and regulate the magnitude and quality of antiviral T-cell responses [1].
Some studies have suggested a potential contribution of DENV-specific CD4+ T-cell responses to disease pathogenesis, while other studies have illustrated a potential protective role for this type of adaptive response [2–4]. DENV-specific CD4+ T cells were associated with direct effector cytotoxic function and, thus, have the potential to directly contribute to viral clearance by clearing DENV-infected macrophages that contribute to disease pathogenesis through the effects of antibody dependent enhancement [5–7].
Despite their importance, CD4+ T cells have received less attention than their CD8+ counterparts [7–9]. Only 40 human CD4+ epitopes with known restriction are currently available in the Immune Epitope Database (available at: http://www.IEDB.org), reflecting an important knowledge gap in several respects. For major histocompatibility complex (MHC) class I–restricted responses, it has recently been shown that different allelic variants are associated with a differential magnitude of CD8+ T-cell responses and that HLA alleles known to be associated with an increased risk of severe dengue are associated with weaker CD8+ T-cell responses [8]. It is thus important to establish whether protection from severe disease is associated with alleles mediating more or less vigorous CD4+ T-cell responses [10, 11]. Second, the exact knowledge of which epitopes are recognized is necessary to clearly discriminate the relative role of responses directed against serotype-specific or conserved epitopes. The knowledge of dominant targets of CD4+ T-cell responses is also an important issue in the context of the development of vaccination strategies, since it is likely that optimal immunity would require induction of CD4+ T-cell responses against the same antigens recognized as dominant in natural infection. Finally, knowledge of epitopes and associated restricting elements is key in terms of the manufacturing of tetramer staining reagents and so-called epitope mega-pools that effectively and comprehensively enable studying the highly heterogeneous populations of patients and vaccinees from different ethnic origins and backgrounds [12].
Here, we have analyzed CD4+ T-cell responses and associated epitopes restricted by 16 common HLA-DRB1 alleles, revealing that human CD4+ T-cell responses in natural infection are largely restricted to cytoplasmic DENV antigens (ie, capsid [C], nonstructural protein 2A [NS2A], NS3, and NS5). Finally, the more dominant epitopes derived from all 4 serotypes were pooled in a single mega-pool that allows responses to be measured directly ex vivo, which will be of significant utility in measuring CD4+ T-cell responses in natural infection, severe disease, and vaccination settings.
MATERIALS AND METHODS
Human Blood Samples
A total of 150 peripheral blood samples were obtained from healthy adult blood donors from the National Blood Center, Ministry of Health, Colombo, Sri Lanka, in an anonymous fashion as previously described [8]. To compare HLA frequencies between the general population and hospitalized patients, we have collected 440 samples from patients with an initial diagnosis of clinically suspected dengue fever (DF). Diagnosis was later confirmed by detection of virus (by polymerase chain reaction [PCR]) and/or DENV-specific immunoglobulin M (IgM) and immunoglobulin G (IgG) in serum, as listed in Supplementary Table 1. Classification in Sri Lanka follows the 2011 World Health Organization guidelines for DF, dengue hemorrhagic fever (DHF), or dengue shock syndrome (DSS). The hospital cohort described in this study comprised 335 patients classified as having DF and 105 patients classified as having DHF. Presence of pleural effusion on chest radiographs and ultrasonography for the evidence of fluid in the abdominal cavity was used to determine evidence of plasma leakage. None of the patients showed signs indicating DSS or died. The institutional review boards of both La Jolla Institute for Allergy and Immunology and the Medical Faculty, University of Colombo (which served as a National Institutes of Health–approved institutional review board for Genetech), approved all protocols described in this study.
HLA Typing
Donors were HLA typed by an American Society for Histocompatibility and Immunogenetics-accredited laboratory at Murdoch University (Western Australia), using locus-specific PCR amplification on genomic DNA as previously described [13].
Serology
DENV seropositivity was determined by DENV-specific IgG enzyme-linked immunosorbent assay and flow cytometry–based neutralization assays as previously described [14, 15].
MHC Class II Binding Predictions and Peptide Selection
HLA-DRB1 binding predictions were performed using the consensus prediction method publicly available through the IEDB Analysis Resource (available at: http://www.iedb.org) [16, 17]. For each allele, predicted peptides present in >30% of the isolates were selected within the 2% consensus threshold, roughly corresponding to the top 10% of 15-mers overlapping by 10 residues. This resulted in the synthesis of 2046 peptides (Mimotopes, Victoria, Australia). Peptides were combined into pools of 20 individual peptides according to their HLA prediction and tested in 8–12 HLA-matched donors, with the exception of the relatively rare DRB1*0901 allele. This allele has been tested in the only 2 donors expressing this allele in our donor cohort but was included in this study because of its reported association with protection from severe disease [11].
In Vitro Expansion of DENV-Specific T Cells and Interferon γ (IFN-γ) Enzyme-Linked Immunospot Assay
CD4+ T cells were isolated by magnetic bead negative selection and cocultured with autologous antigen-presenting cells at a 2:1 ratio in Roswell Park Memorial Institute 1640 medium (Omega Scientific) supplemented with 5% human serum (Cellgro) at a density of 2 × 106 cells/mL in 24-well plates (BD Biosciences). Cells were stimulated with DENV-specific pools, and additional interleukin 2 (10 U/mL; eBioscience) was added every 4 days as previously described. After 14 days of in vitro expansion, 5 × 104 peripheral blood mononuclear cells (PBMCs) were incubated in triplicate and tested for IFNγ response against individual peptides (2 μg/mL) as previously described [7, 8].
Flow Cytometry
Detailed information of all monoclonal antibodies used in this study is listed in Supplementary Table 2. For intracellular cytokine staining, PBMCs were cultured in the presence of HLA-matched peptide pools (1 μg/mL) for 6 hours as previously described [7, 8].
RESULTS
Selection of a Set of HLA-DRB1 Alleles Affording High Coverage of the Sri Lanka Population
Consistent with previous reports, HLA typing in our cohort confirmed that the 7 allelic variants DRB1*0701, 1501, 1502, 1301, 1001, 0403, and 1404 were most common and encountered with phenotypic frequencies of ≥10% (Figure 1A) [10]. An additional 7 alleles (DRB1*0101, 0301, 0401, 0803, 1101, 1202, and 1302) were found with frequencies between 4% and 10%. DRB1*1302 was excluded because of the lack of a reliable predictive algorithm at the point of study initiation. In addition, DRB1*0802 and DRB1*0901 were included in light of reported association with differential clinical outcomes [10, 11]. These 15 DRB1 alleles allowed for coverage of at least one of the DRB1 genes expressed in 95% of individual cases and both DRB1 genes in 60% of the cases (Figure 1B).
Figure 1.
Phenotypic frequency of HLA-DRB1 allelic variants in Sri Lanka. The HLA phenotype of 307 donors from the general population of Sri Lanka was determined. A, Phenotypic frequencies for all HLA-DRB1 alleles detected are shown (white bars). Gray arrows indicated alleles present in >5% of the population or alleles for which previous disease associations have been reported in the literature. Worldwide phenotypic frequencies for all HLA-DRB1 alleles are shown in black bars. B, HLA allele coverage in the Sri Lankan cohort is shown. Bars represent the relative number of donors in whom the donor-specific HLA alleles have been exactly matched with the 15 alleles selected for our study.
The DRB1 phenotype frequencies found in the Sri Lankan samples were subsequently compared to corresponding frequencies in the worldwide population. While frequencies for many of the alleles were similar, DRB1*0803, *1101, and *1602 were underrepresented in the Sri Lankan cohort, while DRB1*0701, *1404, and *1501/02 were overrepresented in this population in comparison to their frequencies worldwide (Figure 1A). Overall, these alleles should provide wide coverage in the general worldwide population.
Identification of a Large Number of Novel DENV Epitopes
Comprehensive analysis all epitope identification studies were performed in healthy seropositive donors from whom large blood donations were available. Each peptide was tested in an average of 9 HLA-matched donors. Of the 2046 peptides that were predicted to bind the chosen DRB1 alleles, 867 gave a response, corresponding to approximately 35% of the peptides being positive in 1 or more of the donors tested. The immune epitope algorithms used in this study predict the capacity of a peptide to bind any given MHC molecule but do not predict whether the MHC:peptide ligand will be recognized by a T cell. Thus, this response rate is in line with MHC binding being necessary but not sufficient for T-cell immunogenicity [18–20]. For comparison, querying the Immune Epitope Database (available at: http://www.iedb.org) for DENV-derived human CD4+ epitopes retrieved 91 epitopes, of which only 40 were associated with any reported HLA restriction. Supplementary Table 4 lists all DENV epitopes previously described in the Immune Epitope Database. This finding highlights how the present study increased the number of DENV-derived human CD4+ epitopes by approximately 10-fold. A complete listing of all peptides tested, including response frequency and HLA restriction, has been submitted to the Immune Epitope Database (available at: http://www.iedb.org/submission/1000699).
CD4+ T Cells Dominantly Recognize the C Protein
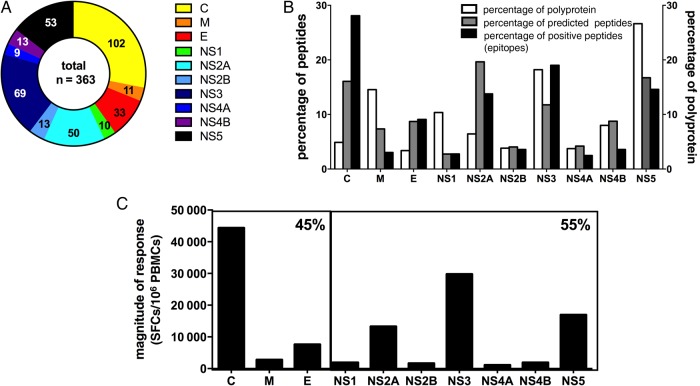
To ensure we had a broad but still robust representation, we picked all epitopes represented in ≥15% of all donors tested, resulting in a set of 457 epitopes. Next we eliminated redundancies (eg, variants derived from the same serotype that have been detected in the same donor), resulting in a set of 363 unique epitopes. Epitopes were derived from all 10 proteins (C, membrane, and envelope proteins and the 7 NS proteins [NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5]) albeit in very different proportions. The C protein was the most dominantly targeted CD4+ antigen, with nearly twice as many epitopes as any other antigen (Figure 2A and 2B). The same observation was made when responses were analyzed according to the infection history of the donors in whom they were identified. In both primary and secondary infections, the C protein was the most dominantly targeted protein, accounting for 40% and 26% of all epitopes identified in primary and secondary donors, respectively (data not shown). To examine whether larger antigen size might influence dominance by providing more immunogenic peptides, we calculated the percentage of all possible peptides derived from each protein, considering all of the possible 15-mers overlapping by 14 amino acids of DENV-1–4 consensus sequences. The average percentage for each protein is represented in Figure 2B, as is the number of peptides predicted to bind the various DR molecules.
Figure 2.
Protein location of epitopes identified. A total of 363 unique epitopes represented in ≥15% of HLA-matched donors were identified. A, The fraction of unique epitopes is plotted as a function of the protein they are derived from (capsid protein [C], yellow; membrane protein [M], orange; envelope protein [E], red; nonstructural protein 1 [NS1], green; NS2A, turquoise; NS2B, blue; NS3, dark blue; NS4A, purple; NS4B, pink; and NS5, black). Numbers represent the actual number of epitopes identified. B, Relative distribution of epitopes derived from any of the 3 structural proteins (C, M, and E) or 7 NS proteins (NS1–5) is shown (black bars). The white bars show the percentage of all possible peptides (percentage of the total polyprotein) accounted for by each antigen. Gray bars reflect relative numbers of peptides for each protein predicted to bind the various DR molecules. C, The magnitude of response defined as the average sum total of spot-forming cells (SFCs)/donor associated with each protein is shown. Boxes represent structural proteins (C, M, and E) and NS proteins. Percentages in the upper right corner reflect the relative response directed at either structural protein or NS protein responses.
We next introduced a correction for protein size by dividing the percentage of positive peptides by the number of all possible peptides (Table 1). For any given protein, a value of >1 indicates that more epitopes are derived from that protein than expected on the basis of its size. This analysis indicated that C and NS2A are intrinsically more immunogenic than expected on the basis of their size. Conversely, the large size of NS3 and NS5 explains the relative large number of epitopes contained within them.
Table 1.
Predicted and Immunogenic Peptides per Protein
| Variable | Peptides, %, by Protein |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | M | E | NS1 | NS2A | NS2B | NS3 | NS4A | NS4B | NS5 | |
| All possible peptides | 3.4 | 4.9 | 14.6 | 10.3 | 6.4 | 3.8 | 18.2 | 3.7 | 8.0 | 26.6 |
| Predicted peptides | 15.6 | 7.1 | 8.8 | 3.5 | 19.3 | 4.2 | 12.0 | 4.0 | 9.0 | 16.5 |
| Positive peptides | 29 | 3 | 8 | 3 | 14 | 4 | 21 | 2 | 4 | 13 |
| Corrected for size | 8.5 | 0.6 | 0.5 | 0.3 | 2.2 | 1.0 | 1.2 | 0.5 | 0.5 | 0.5 |
| Corrected for predicted peptides | 4.6 | 1.4 | 0.6 | 0.3 | 3.0 | 1.1 | 0.7 | 1.1 | 1.1 | 0.6 |
| Corrected for immunogenicity | 1.9 | 0.4 | 0.9 | 0.9 | 0.7 | 1.0 | 1.7 | 0.5 | 0.4 | 0.8 |
Abbreviations: C, capsid protein; E, envelope protein; M, membrane protein; NS, nonstructural protein.
Interestingly, DRB1-predicted binders were enriched in C and NS2A, thus providing a potential basis for their relative immunodominance (Table 1). However, peptides derived from the C protein, even after correcting for size and motif density, were still most immunogenic, as shown when we generated an immunogenicity ratio by dividing the percentage of epitope values by the percentage motif values (Table 1). In conclusion, the C antigen is dominant not only because it contains more HLA-binding peptides (as judged by the number of predicted binders relative to other regions of the DENV polyprotein), but also because these peptides are more frequently recognized than those derived by other antigens, as judged by calculating the fraction of positive peptides per tested peptides derived from the C antigen, as compared to the other antigens. When the magnitude of response is considered (defined as the average sum of spot-forming cells [SFC] per donor associated with each antigen), a similar pattern is observed, as shown in Figure 2C. In total, 55% of the response was associated with NS proteins, while 45% of the response was associated with structural proteins.
Development and Validation of a CD4+ Epitope Mega-pool
We have previously shown that a DENV specific CD8+ T-cell mega-pool can be used to detect CD8+ T-cell responses in a variety of different areas of endemicity [9]. Accordingly, we designed a CD4+ T-cell mega-pool containing 363 epitopes restricted by all 15 DRB1 alleles (Figure 3A) and covering serotype-specific epitopes derived from all 4 serotypes, as well as a large fraction of epitopes conserved between serotypes (Figure 3B). The CD4+ T-cell mega-pool was able to elicit ex vivo responses in 11 of 16 randomly selected donors with secondary DENV infection who had not been involved in the epitope identification studies (Figure 3C). Sequences of the 363 peptides and the corresponding DRB1 restrictions are provided in Supplementary Table 5.
Figure 3.
Composition and reactivity of a dengue virus (DENV)–specific CD4+ T-cell mega-pool (CD4+-MP). Epitopes included in the mega-pool are shown as a function of their HLA restriction (A) or as a function of the serotype they are derived from (DENV1–4; B). Epitopes that are shared between at least 2 serotypes (allowing 1–2 amino acid substitutions) have been considered conserved. C, Percentages of CD4+ T cells that produce interferon γ (IFN-γ) upon stimulation with the CD4+-MP in donors previously exposed to DENV from Sri Lanka (n = 16). IFN-γ production among CD4+ T cells stimulated with a CD8+ T-cell mega-pool (CD8+-MP) have been used as a control. The dotted line at 0.02% represents the cutoff for positivity. The average response (+standard error of the mean) for all cohorts is shown. Statistical significance was determined using a 2-tailed Mann–Whitney test. MHC, major histocompatibility complex.
Epitopes Are Highly Clustered in Relatively Few Dominant Regions
Mapping the epitopes identified to the exact location in the dengue polyprotein revealed that certain regions were more dominant and encompassed highly homologous peptides from different serotypes and/or largely overlapping peptides predicted to bind in the context of different alleles. To further examine positive responses, overlapping peptides were clustered into antigenic regions spanning 15–25 residues, which each identifying 92 epitope clusters (Supplementary Table 3). At least 1 of the top 25 clusters has been identified for the C protein (A; 8 clusters), membrane protein (B; 1 cluster), envelope protein (C; 1 cluster), NS2A (D; 5 clusters), NS3 (E; 5 clusters), NS4B (F; 1 cluster), and NS5 (G; 4 clusters) as shown in Supplementary Figure 1.
Magnitude of Responses Varies as a Function of HLA-DR Alleles
Previous studies had demonstrated that CD8+ T-cell responses restricted by various HLA alleles vary substantially in magnitude and that HLA alleles reported to be associated with disease resistance are associated with higher magnitudes of response [8]. Equally striking differences in the dominance of CD4+ T-cell responses (defined as the average reactivity per donor to all positive peptides predicted to bind a particular allele) were noted in function of HLA restriction (Figure 4). Certain HLA alleles, such as DRB1*0401, DRB1*0701, DRB1*0901, DRB1*1202, DRB1*1301, and DRB1*1501, were associated with responses of high magnitude (>10 000 SFCs) and relatively large breadth (>25–50 epitopes; Figure 3A). By contrast, other alleles, such as DRB1*0301, DRB1*0403, DRBB1*0802, DRB1*1101, and DRB1*1502, were found to be associated with responses of lower magnitude (<5000 SFCs).
Figure 4.
HLA-restricted CD4+ T-cell responses. Differential magnitude of HLA-restricted responses in dengue virus–seropositive donors. A, CD4+ T cells are cocultured with autologous antigen-presenting cells and peptides at a ratio of 2:1. Black bars represent the magnitude of T-cell responses as the total number of spot-forming cells (SFCs) per 106 recovered cells and are sorted according to their restriction element. The dotted line indicates the arbitrary threshold of 10 000 SFCs per 106 peripheral blood mononuclear cells (PBMCs). B, Dots represent the magnitude response of individual DRB*1506 donors after stimulation with DRB*1506 peptides (n = 8). The box represents the mean of responses. The dotted line indicates the arbitrary threshold of 10 000 SFCs per 106 PBMCs.
Of note, large differences in response magnitude were present in different subtypes of the same DR antigen. For example, DRB1*0401 was associated with higher numbers of epitopes and a higher magnitude of responses than the closely related *0403 allelic variant. Similarly, DRB1*1501 was associated with a higher number of epitopes and higher magnitude of responses than the closely related *1502 allelic variant.
Frequency of HLA-DRB1 Alleles in Acute Patients, Compared With the General Sri Lankan Population
Based on the results above we hypothesized that variations in T-cell response magnitude might predict associations between dengue outcomes and HLA-DRB1 alleles. A total of 440 samples were collected from hospitalized patients with confirmed severe dengue (105 of whom were associated with DHF). A total of 308 samples derived from DENV-seropositive healthy blood donors from the Colombo blood bank were used as controls representing the allele frequency of the general population. Odds ratios (ORs), which measure the association between a given HLA and a disease outcome, were calculated for each of the different DRB1 alleles. In this case, ORs of > 1 indicate that the given allele is associated with greater risk (susceptibility), while ORs of < 1 indicates a lower risk (protective effect).
Table 2 shows the results of this analysis and, for each of the control versus DF/DHF groups, shows the individuals either expressing or not expressing a given HLA-DRB1 allele, the total number of donors, and the OR and P values yielded when comparing the DF/DHF group to the control group. HLA types present in ≥1% of the study populations were included in this analysis.
Table 2.
Associations Between HLA-DRB1 Alleles and Disease Severity
| HLA Allele | Healthy Population, No. (%) | Hospitalized Population, No. (%) | Odds Ratio | P Value | Pcorrecteda |
|---|---|---|---|---|---|
| DRB1*01:01 | 19 (6.2) | 37 (8.4) | 1.4 | .263 | 1 |
| DRB1*03:01 | 26 (8.4) | 35 (8.0) | 0.9 | .892 | 1 |
| DRB1*04:01 | 19 (6.2) | 11 (2.5) | 0.4 | .014 | .28 |
| DRB1*04:03 | 30 (9.7) | 45 (10.2) | 1.1 | .902 | 1 |
| DRB1*04:04 | 1 (0.3) | 7 (1.6) | 5.0 | .150 | 1 |
| DRB1*04:05 | 0 (0.0) | 12 (2.7) | Infinity | .002 | .04 |
| DRB1*07:01 | 114 (37.0) | 162 (36.8) | 1.0 | 1.000 | 1 |
| DRB1*08:02 | 2 (0.6) | 6 (1.4) | 2.1 | .481 | 1 |
| DRB1*08:03 | 14 (4.5) | 18 (4.1) | 0.9 | .855 | 1 |
| DRB1*09:01 | 3 (1.0) | 4 (0.9) | 0.9 | 1.000 | 1 |
| DRB1*10:01 | 34 (11.0) | 70 (15.9) | 1.5 | .007 | .14 |
| DRB1*11:01 | 20 (6.5) | 24 (5.5) | 0.8 | .636 | 1 |
| DRB1*11:11 | 7 (2.3) | 3 (0.7) | 0.3 | .100 | 1 |
| DRB1*12:02 | 20 (6.5) | 41 (9.3) | 1.5 | .177 | 1 |
| DRB1*13:01 | 40 (13.0) | 35 (8.0) | 0.6 | .026 | .52 |
| DRB1*14:01 | 4 (1.3) | 5 (1.1) | 0.9 | 1.000 | 1 |
| DRB1*14:04 | 57 (18.5) | 78 (17.7) | 0.9 | .847 | 1 |
| DRB1*15:01 | 68 (22.1) | 93 (21.1) | 0.9 | .787 | 1 |
| DRB1*15:02 | 47 (15.3) | 62 (14.1) | 0.9 | .674 | 1 |
| DRB1*15:06 | 9 (2.9) | 4 (0.9) | 0.3 | .047 | .94 |
P values <.05 were considered statistically significant.
a Corrected by the Bonferroni inequality method.
Trends toward protective associations could be shown for 1 of 6 alleles associated with responses of >10 000 SFCs/donor, namely the DRB1*0401 (OR, 0.4; P = .014) and DRB1*1301 (OR, 0.6; P = .026) alleles. DRB1*0901, previously reported as being protective, was also associated with high responses [11]. No significant association was detected in our cohort, possibly because this allele is rare in the Sri Lanka population (frequency, about 1%). No significant protective effect was noted for the other 3 alleles associated with high CD4+ T-cell responses (DRB1*0701, DRB1*1202, and DRB1*1501) or for any of the other 9 DRB1 alleles with CD4+ T-cell responses of <10 000 SFCs/donor (Table 2). This corresponds to an overall significant, albeit weak, correlation between response magnitude and protection from severe disease (P = .022, by the Fisher exact test).
Differential OR Associations in Closely Related DRB1 Subtypes
The OR analysis showed a trend toward protection from severe disease of DRB1*0401, which generated high-magnitude T-cell responses (>10 000 SFCs; OR, 0.4; Table 2). No trend was detected for DRB1*0403, which generated only intermediate T-cell responses (OR, 1.1,). Interestingly, DRB1*0405, which is rare in the Sri Lanka cohort and, consequently, was not assessed for T-cell responses, was associated with significantly increased disease susceptibility (OR, infinity; P = .002). Likewise, the rare DRB1*0404 allele was also trending toward increased disease susceptibility (OR, 4.96). No trends were detected for the DRB1*1501 and DRB1*1502 subtypes, while DRB1*1506 was also associated with a trend toward protection from severe disease (OR, 0.3; Table 2).
DRB1*1506 was not studied initially because its frequency in the Sri Lankan population was <5%. To test the hypothesis that strong T-cell responses are associated with protection, DRB1*1506 peptides have been synthesized and screened for CD4+ T-cell reactivity in the 8 DRB1*1506 donors available (Figure 4B). Notably, the DRB1*1506 peptide set elicited CD4+ T-cell responses of >10 000 SFCs in all 8 donors, with an average response of >26 000 SFCs. These results confirm the predictive hypothesis that a protective HLA allele is associated with a strong CD4+ T-cell response.
DISCUSSION
Herein we present the most comprehensive characterization of DENV-specific HLA-restricted CD4+ epitopes to date, highlighting the extreme heterogeneity and complexity of human DENV responses in a population setting. Yet, it is likely that the present study still underestimates the complexity of MHC class II responses in 2 important aspects. First, HLA-predictive algorithms are bound to a certain false-negative rate, and not all HLA binders will be predicted [17, 21]. Second, the study has focused on the DRB1 molecules most frequent in the Sri Lanka population. Several alleles broadly expressed in the worldwide population, such as DRB1*1602, were underexpressed in Sri Lanka and thereby not analyzed in the present study. Third, other less common DR types, as well as DP and DQ molecules, will have to be addressed in future studies. Based on the available knowledge, predictions for the main DRB1 molecules will cover approximately 50% of the total response [22, 23]. We plan to perform additional epitope identification studies addressing other HLA class II molecules, continue to update our mega-pool, and provide the epitope sequences by submission to the Immune epitope database (available at: http://www.iedb.org) [24].
The large-scale epitope mapping allowed us to pinpoint immunodominant antigens and regions, gaining further insights in the mechanisms of immunodominance. In agreement with previous studies, we found that C, together with NS2A, NS3, and NS5, are immunodominant for CD4+ T-cell responses [25]. While NS3 and NS5 antigens are dominant for both CD4+ and CD8+ T-cell responses, C and NS2A are dominant for CD4+ but not CD8+ T-cell responses [8]. The immunodominance of the capsid is of particular interest in the light of recent studies showing that a capsid-based vaccine from DENV-2 was able to induce protective T-cell–mediated immunity in monkeys, without the contribution of neutralizing antibodies, and the fact that the DENV-derived C is not present in a chimeric dengue vaccine (CYD) [26, 27].
Different HLA class II molecules were associated with responses of different breadth and magnitude. While several significant associations were observed before Bonferroni correction, only 1 HLA allele remains significantly associated with protection. A Bonferroni correction is necessary if multiple comparisons are performed, to make the claim that a particular association is significant. However, a Bonferroni correction is not necessary if the P values are used to generate a hypothesis and test a generic correlation. In our case, we make the observation that alleles above or below a certain threshold (a P value of .05, before correction) are associated with responses above or below a certain magnitude (10 000 SFCs) and also verify this in a “blind prediction” for the DRB1*1506 allele. The results presented herein should therefore be interpreted with this caveat in mind, and confirmation with a larger patient data set will be required. Despite, these positive correlations, it is apparent that a weak CD4+ T-cell response does not predict disease susceptibility and that the converse can also be true, since *1202 is associated with strong CD4+ T-cell responses and disease susceptibility [10]. It is possible that interactions with CD8+ T cells or antibody responses might be responsible for this complex pattern. Alternatively, it is possible that, beyond response magnitude, the specific phenotype of the responding T cells might be a key factor in determining disease. Indeed, our recent studies suggest that both CD8+ and CD4+ DENV-specific T cells restricted by different HLA alleles are associated with specific phenotypes [7, 28]. Knowledge of CD4+ T-cell responses and associated epitopes restricted ex vivo will be of significant utility in measuring CD4+ T-cell responses in natural infection, severe disease, and vaccination settings.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Aravinda de Silva and his laboratory, for performing neutralization assays; and the National Blood Center, Ministry of Health, Colombo, Sri Lanka, for providing buffy coat samples used in this study.
Financial support. This work was supported by the National Institutes of Health (contracts HHSN272200900042C and HHSN27220140045C).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology 2010; 130:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goncalves AJ, Oliveira ER, Costa SM et al. . Cooperation between CD4+ T Cells and Humoral Immunity Is Critical for Protection against Dengue Using a DNA Vaccine Based on the NS1 Antigen. PLoS Negl Trop Dis 2015; 9:e0004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurane I, Matsutani T, Suzuki R et al. . T-cell responses to dengue virus in humans. Trop Med Health 2011; 39:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yauch LE, Prestwood TR, May MM et al. . CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J Immunol 2010; 185:5405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 2003; 60:421–67. [DOI] [PubMed] [Google Scholar]
- 6.Zellweger T, Chi K, Miyake H et al. . Enhanced radiation sensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin Cancer Res 2002; 8:3276–84. [PubMed] [Google Scholar]
- 7.Weiskopf D, Bangs DJ, Sidney J et al. . Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci USA 2015; 112:E4256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiskopf D, Angelo MA, de Azeredo EL et al. . Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci USA 2013; 110:E2046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiskopf D, Cerpas C, Angelo MA et al. . Human CD8+ T-Cell Responses Against the 4 Dengue Virus Serotypes Are Associated With Distinct Patterns of Protein Targets. J Infect Dis 2015; 212:1743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malavige GN, Rostron T, Rohanachandra LT et al. . HLA class I and class II associations in dengue viral infections in a Sri Lankan population. PLoS One 2011; 6:e20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TP, Kikuchi M, Vu TQ et al. . Protective and enhancing HLA alleles, HLA-DRB1*0901 and HLA-A*24, for severe forms of dengue virus infection, dengue hemorrhagic fever and dengue shock syndrome. PLoS Negl Trop Dis 2008; 2:e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrasco Pro S, Sidney J, Paul S et al. . Automatic Generation of Validated Specific Epitope Sets. J Immunol Res 2015; 2015:763461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham J, Oseroff C, Hinz D et al. . Sequence conservation predicts T cell reactivity against ragweed allergens. Clin Exp Allergy 2016; doi:10.1111/cea.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanakaratne N, Wahala WM, Messer WB et al. . Severe dengue epidemics in Sri Lanka, 2003–2006. Emerg Infect Dis 2009; 15:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraus AA, Messer W, Haymore LB, de Silva AM. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J Clin Microbiol 2007; 45:3777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol 2008; 4:e1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P, Sidney J, Kim Y et al. . Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics 2010; 11:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assarsson E, Sidney J, Oseroff C et al. . A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J Immunol 2007; 178:7890–01. [DOI] [PubMed] [Google Scholar]
- 19.Kotturi MF, Scott I, Wolfe T et al. . Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol 2008; 181:2124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol 1999; 17:51–88. [DOI] [PubMed] [Google Scholar]
- 21.Kotturi MF, Peters B, Buendia-Laysa F Jr et al. . The CD8+ T-cell response to lymphocytic choriomeningitis virus involves the L antigen: uncovering new tricks for an old virus. J Virol 2007; 81:4928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oseroff C, Sidney J, Vita R et al. . T cell responses to known allergen proteins are differently polarized and account for a variable fraction of total response to allergen extracts. J Immunol 2012; 189:1800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul S, Lindestam Arlehamn CS, Scriba TJ et al. . Development and validation of a broad scheme for prediction of HLA class II restricted T cell epitopes. J Immunol Methods 2015; 422:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vita R, Overton JA, Greenbaum JA et al. . The immune epitope database (IEDB) 3.0. Nucleic Acids Res 2015; 43:D405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivino L, Kumaran EA, Jovanovic V et al. . Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol 2013; 87:2693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil L, Izquierdo A, Lazo L et al. . Capsid protein: evidences about the partial protective role of neutralizing antibody-independent immunity against dengue in monkeys. Virology 2014; 456–457:70–6. [DOI] [PubMed] [Google Scholar]
- 27.Guy B, Briand O, Lang J, Saville M, Jackson N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine 2015; 33:7100–11. [DOI] [PubMed] [Google Scholar]
- 28.de Alwis R, Bangs DJ, Angelo MA et al. . Immunodominant dengue virus specific CD8+ T cells responses are associated with a memory PD-1+ phenotype. J Virol 2016; 90:4771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.