Abstract
The impact of human immunodeficiency virus (HIV) infection on innate and adaptive immune activation occurs in the context of host factors, which serve to augment or dampen the physiologic response to the virus. Independent of HIV infection, nutritional status, particularly body composition, affects innate immune activation through a variety of conditions, including reduced mucosal barrier defenses and microbiome dysbiosis in malnutrition and the proinflammatory contribution of adipocytes and stromal vascular cells in obesity. Similarly, T-cell activation, proliferation, and cytokine expression are reduced in the setting of malnutrition and increased in obesity, potentially due to adipokine regulatory mechanisms restraining energy-avid adaptive immunity in times of starvation and exerting a paradoxical effect in overnutrition. The response to HIV infection is situated within these complex interactions between host nutritional health and immunologic function, which contribute to the varied phenotypes of immune activation among HIV-infected patients across a spectrum from malnutrition to obesity.
Keywords: HIV, malnutrition, adipose tissue, obesity, inflammation, immune activation
Following the introduction of effective antiretroviral therapy (ART) in resource-rich, developed countries, the incidence of human immunodeficiency virus (HIV)–associated wasting in advanced disease has declined, while the proportion of overweight and obese HIV-infected individuals receiving long-term treatment has steadily risen [1, 2]. In contrast, due to the geographic overlap of high HIV prevalence and chronic food insecurity, new infections in many resource-limited countries frequently occur against a backdrop of chronically insufficient macronutrient intake (hereafter referred to as malnutrition) [3]. Host nutritional status affects innate immune activation through a variety of mechanisms, from altered mucosal barrier defenses and microbiome in malnutrition to proinflammatory cytokine expression by stromal vascular cells and hypertrophied adipocytes in obesity. Similarly, nutritional status modulates T-cell activation, proliferation, and function, in part via endocrine mechanisms thought to act on T-cell surface receptors. Here, we review the interaction of nutrition and the immune response to HIV across the spectrum of nutritional status, ranging from malnutrition to obesity (summarized in the Table 1 and Figure 1).
Table 1.
Summary Points on Nutrition and Immune Activation
| Enteropathy due to a confluence of environmental factors, nutrition deficits, and viral effects impairs mucosal barrier integrity and immune defenses and contributes to both innate and cellular immune activation in malnourished human immunodeficiency virus (HIV)–infected persons. |
| A gastrointestinal dysbiosis, characterized by increased Proteobacteria and reduced or altered Bacteroidetes and Firmicutes concentrations, is present in HIV-infected patients, and these changes are accompanied by increased mucosal and circulating T-cell activation and systemic inflammation. Similar phylum-level changes occur in malnutrition, but the microbiome consequences of comorbid HIV infection and malnutrition are unknown. |
| Malnutrition is associated with reduced T-cell proliferative responses, reduced T-cell expression of activation and memory surface markers, greater type 2 T-helper cell (TH2) polarization, and decreased TH1 cell interferon γ and interleukin 2 production, which compound HIV-related immunodeficiency and impair clearance or control of secondary infections. |
| Adipocytes constitutively express interleukin 6, tumor necrosis factor α, and other cytokines, and obese HIV-infected persons have substantially higher circulating levels of inflammation biomarkers. Because these cytokines derive from adipocytes as opposed to other tissues (eg, inflamed arterial vessels), obesity may confound previously reported associations between inflammation and health outcomes in HIV-infected persons. |
| A higher body mass index is associated with more robust CD4+ T-cell recovery during antiretroviral therapy, and obesity in is associated with higher circulating T-cell counts, increased T-cell activation, and CD4+ T-cell TH1 polarization in studies of HIV-negative individuals. |
| CD4+ T cells express a receptor for leptin, an adipokine produced by adipocytes, which may have an endocrine function modulating T-cell proliferation, activation, and T-helper cell polarization in states of both malnutrition and obesity. |
| Clinical trials of growth hormone–releasing hormone (GHRH) have shown a beneficial effect for reducing visceral and hepatic fat without the added insulin resistance observed in studies of recombinant growth hormone. However, the effect of GHRH on innate and cellular immune activation is still unclear. |
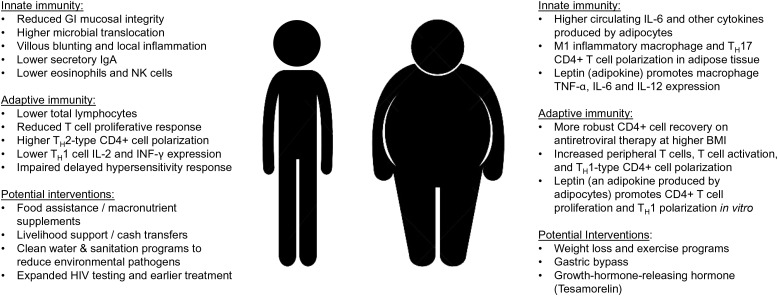
Figure 1.
Malnutrition and obesity-related factors potentially affecting chronic immune activation in human immunodeficiency virus (HIV) infection. Abbreviations: BMI, body mass index; GI, gastrointestinal; IFN-γ, interferon γ; IgA, immunoglobulin A; IL, interleukin; NK, natural killer; TNF-α, tumor necrosis factor α.
HIV AND MALNUTRITION IN RESOURCE-RICH AND RESOURCE-LIMITED CONTEXTS
The young, emaciated patient with advanced AIDS is an enduring image of the early HIV epidemic and can unfortunately still be found with alarming frequency in many resource-limited settings where HIV testing and treatment have not become universally available or accepted. However, a low body mass index (BMI, a marker of generalized malnutrition; calculated as the weight in kilograms divided by the height in meters squared) in the setting of HIV infection should be divided into 2 frequently overlapping phenotypes. The first, cachexia, is a wasting phenotype characterized by a dangerous cycle involving profound loss of adaptive immune system protection (ie, CD4+ T-cell depletion), increased basal metabolic rate (due in part to a persistent inflammatory response), and increased protein catabolism, which accelerates the loss of lean body mass [4–10]. The second phenotype arises from the simultaneous presence of clinical malnutrition due to insufficient caloric intake combined with concomitant HIV infection in varying stages of immunosuppression. Global surveys estimate that >800 million individuals have chronically insufficient caloric intake, with the highest prevalence in sub-Saharan Africa and southern Asia [11]. The prevalence of low BMI can be substantial in African HIV-infected patient populations; in a study of HIV-infected adults at clinics across Lusaka, the capitol of Zambia, one third were malnourished (BMI < 18.5) at the time of ART initiation [12]. Frequently these phenotypes overlap. In resource-rich settings, progressive weight loss with untreated HIV infection leads to low BMI and its nutrition-related organ system dysfunction and immune deficits, while in resource-limited settings the immune deficits accompanying a low BMI are exacerbated by the acquisition of HIV infection.
Malnutrition, Enteropathy, and Microbial Translocation
The combined effects of environmental factors, nutrient deficits, and HIV infection on gastrointestinal mucosal barrier defenses and microbiome composition (discussed below) contribute to increased translocation of microbes and microbial proteins into the bowel wall and circulation in malnourished HIV-infected individuals [13–16]. Microbial translocation, as measured by circulating lipopolysaccharide (LPS; a component of the bacterial cell wall), anti-endotoxin immunoglobulin M and immunoglobulin G antibodies, soluble CD14, and other biomarkers is associated with accelerated HIV disease progression and a higher risk of mortality in untreated HIV infection [17, 18], although the prognostic value of these biomarkers is less clear after ART initiation [19, 20]. The loss of barrier defenses against microbial translocation in HIV infection also has consequences for adaptive immune activation. In Italian HIV-infected patients, serum LPS levels predicted disease progression independently of age, CD4+ T-cell count, viral load, or duration of infection, and higher circulating LPS levels after ART initiation were associated with greater CD4+ and CD8+ T-cell activation and poor CD4+ T-cell recovery [17, 21].
Malnutrition enteropathy is characterized by bowel wall edema, reduced nutrient absorption and bowel transit time, reduced secretory immunoglobulin A production, and changes in mucosal surface morphology resulting in villous blunting, increased permeability, and local inflammation [22, 23]. Environmental enteropathy, thought to result from a combination of recurrent, transient infections with pathogenic bacteria and an altered intestinal microbiota, is common in tropical regions with poor sanitation and is also characterized by villous blunting, reduced nutrient absorption, and accelerated bowel transit [16, 24–26]. Last, HIV enteropathy is characterized by mucosal T-cell depletion in conjunction with impaired cellular tight junctions between epithelial cells [27–29]. The ensuing inflammatory response produces villous changes similar to malnutrition enteropathy, which reduces nutrient absorption [30, 31]. In resource-limited settings, the gastrointestinal system of malnourished HIV-infected individuals can be affected by all 3 conditions simultaneously, and treatment of one condition (eg, with ART initiation) may not reduce inflammation and microbial translocation due to concomitant conditions.
Impaired gastrointestinal mucosal integrity and microbial translocation do not appear to be present during acute HIV infection, and the temporal course of systemic inflammation attributed to microbial translocation does not correspond entirely to markers of mucosal integrity or damage [15, 32]. Despite the initiation of ART and plasma viral suppression, defects in junctional complex expression, the presence of bacterial products in the lamina propria, and reduced interleukin 17 (IL-17)– and (IL-22)–producing cells persist in treated HIV infection [28, 33], and even the early initiation of ART shortly after infection does not fully normalize gastrointestinal mucosal dysfunction markers [34]. These findings suggest that the changes in mucosal integrity accompanying HIV infection involve permanent changes in gastrointestinal cellular function, including the loss of IL-17– and IL-22–producing cells and altered epithelial gene expression, which require time to emerge. While most studies of microbial translocation and innate immune activation in HIV infection are from developed countries, similar findings are reported from resource-limited settings [13, 14].
Malnutrition, HIV, and the Microbiome
The centrality of the human gastrointestinal microbiome to the maintenance of host energy homeostasis and metabolism was recognized decades ago, but more-recent evidence points to an important role modulating mucosal and systemic immune activity [35–37]. The human microbiome is composed of an estimated 1014 microbes representing approximately 1000 species and including archaea, eukaryotes, and, predominantly, members of the 5 bacterial phyla of Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia [38]. Quantitation of the relative proportions of each phylum and more-specific taxonomic ranks have identified consistent phenotypes present in the setting of HIV infection, malnutrition, and states of persistent systemic inflammation and adaptive immune activation.
An altered gastrointestinal microbiome appears to occur early in the course of HIV-infection and may contribute to, or is at least correlated with, mucosal inflammatory activity, mucosal CD4+ T-cell depletion, and peripheral CD8+ T-cell activation [39–41]. The microbiome alterations and accompanying local and systemic immune effects persist following the early stages of infection and do not revert with ART, possibly because of a persistent presence of HIV at the mucosal surface or the lasting depletion of gastrointestinal CD4+ T cells and other immune effectors despite effective suppression of plasma viremia [42, 43].
In a study of rectosigmoid biopsy specimens from HIV-infected subjects not yet receiving ART, ART recipients, and HIV-negative controls, those with untreated HIV infection were found to have a marked dysbiosis of mucosal-adherent bacteria characterized by increased Proteobacteria levels and reduced Bacteroidetes levels, which was accompanied by increased mucosal CD4+ and CD8+ T-cell activation, increased circulating CD8+ T-cell activation, and, among ART recipients, increased circulating interleukin 6 (IL-6) levels [44]. In particular, the mucosal community was enriched for Proteobacteria genera, including Salmonella, Escherichia, Serratia, Shigella, and Klebsiella species, all of which can act as proinflammatory pathobionts. A similar shift in gastrointestinal microbiome was seen in a subsequent study of colon biopsy specimens from untreated HIV-infected persons, which found increased Proteobacteria, reduced Firmicutes, and alterations in the relative composition of the Bacteroidetes phylum, compared with HIV-negative controls. Furthermore, the HIV-associated changes in Bacteroidetes members, primarily an increase in Prevotella levels, were associated with both mucosal and circulating CD4+ and CD8+ T-cell activation [45]. Similar associations between microbiome composition and systemic immune activation were observed in the fecal microbiome of HIV-infected persons, including a potentially beneficial role for fecal Lactobacillales (phylum Firmicutes) in promoting circulating CD4+ T-cell recovery and lower CD8+ T-cell activation during ART [46, 47].
The preponderance of studies of HIV-negative, malnutrition-associated microbiome alterations enrolled children rather than adults, but despite this limitation the observed commonalities with HIV-associated gastrointestinal dysbiosis bear consideration. A link between kwashiorkor and a predominance of Staphylococcus aureus and coliform bacteria in gastric juice and rectal swab specimens was identified as early as 1958 [48]. Later studies of malnourished children and well-nourished controls in Bangladesh found that poor nutritional status was associated with enrichment of Proteobacteria, including a 174-fold and 9-fold increase in Klebsiella and Escherichia levels, respectively, and depletion of Bacteroidetes [49]. In Indian children, nutritional status was negatively correlated with the proportion of Proteobacteria (including Escherichia, Shigella, and Enterobacter) and positively correlated with the proportion of anaerobic Firmicutes (including Roseburia, Faecalibacterium, and Butyrivibrio) [50]. This pattern of enriched Proteobacteria and depleted Bacteroidetes and Firmicutes accompanying malnutrition has also been observed in other case-control pediatric studies [51, 52].
In summary, malnutrition is accompanied by gastrointestinal microbiome alterations at the phylum level similar to those observed in both untreated and ART-treated HIV-infected persons. While additional studies are needed to confirm whether the dysbiosis observed in underweight children is also present in malnourished adults, it seems reasonable to assume that adult malnutrition is accompanied by some degree of enrichment of Proteobacteria and a depletion of Bacteroidetes and Firmicutes. To explore this further, we propose 2 areas as research priorities: first, to investigate commonalities in mucosal immune dysfunction leading to similar dysbiosis phenotypes in HIV infection and malnutrition; and second, to determine the extent to which a high degree of persistent immune activation in malnourished HIV-infected individuals can be attributed to compounding or synergistic effects of HIV and nutritional factors on the gastrointestinal microbiome.
Food Insecurity
Food insecurity, or a lack of consistent access to a sufficient quantity of affordable, nutritious food, is associated with a higher likelihood of viral nonsuppression in HIV-infected persons, with resultant effects on disease progression and immune activation [53, 54]. In the United States and Europe, food insecurity is more common among HIV-infected persons with substance abuse, those with mental illness, and those living in poverty, while in resource-limited settings food insecurity is often endemic in areas with high HIV prevalence [55–57]. Food insecurity and the frequently attendant economic privations have adverse effects on clinic attendance, obtaining medication refills, and taking ART at the frequency and doses prescribed, all of which lead to loss of virologic suppression, increased inflammation and cellular immune activation, and higher likelihood of ART regimen failure and resistance [58–60]. Food assistance may have a role in incentivizing patients to attend clinic visits and collect medications as scheduled [57, 61, 62].
A second aspect of food insecurity and immune activation is dietary quality, particularly in resource-limited settings where HIV-infected individuals may be reliant on carbohydrate-rich staple foods (eg, ground maize) with a high glycemic index. A recent systematic review of glycemic index and glycemic load dietary intervention studies suggests that high-carbohydrate staple foods increase levels of IL-6, C-reactive protein (CRP), and other inflammation biomarkers [63], which may present an opportunity for properly constituted food assistance to reduce chronic immune activation, as well as improve clinic attendance and ART adherence.
Malnutrition and T-Cell Function
While there is a paucity of data from individuals with comorbid malnutrition and HIV-infection, malnutrition per se is associated with broad suppression of antigen-specific immunity, including reduced T-cell output, maturation, proliferation, and cytokine expression. The preponderance of these studies, by far, are in children or adolescents <18 years old and are summarized in a recent systematic review [64]; the findings should be extrapolated to adults with some caution. Compared with the well-nourished, malnutrition is associated with reduced T-cell proliferative responses, reduced T-cell expression of activation and memory surface markers [65, 66], and greater T-helper cell type 2 (TH2) polarization with concomitant decreased interferon γ (IFN-γ) and interleukin 2 production by TH1 cells [66, 67]. Malnutrition is also accompanied by a lower likelihood of skin test conversion after bacillus Calmette-Guérin vaccination and reduced dermal delayed type hypersensitivity responses to Candida, phytohemagglutinin, and other common recall antigens [68]. Last, while total immunoglobulin G and other antibody levels were comparable between malnourished and well-nourished subjects in most prior studies, reduced seroconversion rates or antibody titers were reported after typhoid, diphtheria, tetanus, hepatitis B, measles, and other vaccinations in individuals with severe malnutrition, although this does not appear to be as uniform of a finding for moderate and mild malnutrition [64]. While these deficits likely impair an efficient response to pathogens, it is important to note that the changes appear reversible and that the nutritional rehabilitation of malnourished individuals is associated with an improvement in adaptive lymphocyte proliferative responses, chemotaxis, and cytokine production [69].
ADIPOSE TISSUE AND IMMUNE ACTIVATION IN COMORBID HIV INFECTION AND OBESITY
Adipose tissue represents one of the largest organs in the body and comprises a range of cell types with diverse energy storage, metabolic regulation, neuroendocrine, and immunologic functions. HIV infection and ART cause alterations to adipose tissue distribution and biology, with broad effects on cytokine and hormone expression, lipid storage, and the composition of adipose-resident immune cell populations. The resultant changes have important consequences for innate and adaptive immune responses and chronic immune activation.
Obesity Prevalence in the HIV-Infected Population
The proportion of overweight and obese individuals in high- and middle-income countries has increased steadily over the past 3 decades, affecting all race/ethnicity, sex, and age groups to varying degrees, and more recently obesity rates have increased in low-income countries [70]. More than one third of adults in the United States are overweight (BMI, 25–29.9), and a similar proportion are obese (BMI, > 30) [71]. Obesity is also becoming more prevalent in the HIV-infected population. In an analysis of >14 000 HIV-infected persons in the United States and Canada, the percentage of patients who were obese at ART initiation increased from 9% to 18% between 1998 and 2010, and 22% of individuals with a normal BMI (18.5–25) at treatment initiation had become overweight after 3 years of ART, and 18% of those overweight at initiation had become obese. Compared with age-matched National Health and Nutrition Examination Survey controls from the general population, HIV-infected white women had a higher BMI after 3 years of ART than controls, while no difference in BMI after 3 years of ART was observed for HIV-infected white men, and nonwhite men and women, as compared to controls [2].
HIV Infection Alters Adipose Tissue Distribution and Metabolic Characteristics
Older ART agents, particularly the thymidine analogues zidovudine and stavudine, were associated with a high prevalence (up to 50% in some studies) of peripheral lipoatrophy of the limbs, face, and buttocks; lipohypertrophy of the visceral, cervical, and dorsocervical area (ie, the so-called buffalo hump); or a combination of these changes [72, 73]. The accumulation of ectopic adipose tissue in a variety of organs, particularly epicardial, hepatic, and muscle bundle fat infiltration, contributes to local inflammation and end-organ disease [74–76]. Subcutaneous fat biopsy specimens from individuals with HIV-associated lipoatrophy demonstrate reduced mitochondrial DNA and structural changes characterized by increased fibrosis, apoptosis, and formulation of lipogranulomas, while the adipocytes demonstrate reduced expression of several transcription factors necessary for cellular differentiation and fatty acid uptake but higher TNF-α and IL-6 expression [77–81]. Taken together, these findings indicate a shift to a proinflammatory, profibrotic, and dysregulated metabolic state within the fat tissue of HIV-infected patients. While the prevalence of lipodystrophy has declined with the introduction of newer ART agents, the presence of HIV viral particles and latently HIV-infected, adipose-resident CD4+ T cells within adipose tissue may still contribute to impaired lipid metabolism and storage [82, 83].
Obesity, HIV, and the Microbiome
As discussed above in the sections on malnutrition, HIV infection can be accompanied by a marked dysbiosis of fecal and mucosal-adherent bacteria characterized by increased Proteobacteria levels and reduced Bacteroidetes and Firmicutes levels, and these changes are associated with mucosal T-cell activation, circulating T-cell activation, and serum markers of innate immune activation [44, 45]. Independent of HIV infection, several studies found that obesity is accompanied by changes in the gastrointestinal microbiome characterized by lower levels of Bacteroidetes and proportionately higher levels of Firmicutes [84–86], which are postulated to enhance dietary nutrient absorption [87]. In animal models, stool characterized by this phylum-level shift was shown to “transmit” obesity when inoculated into lean animals [84], suggesting that alterations of the gastrointestinal microbiome by secondary conditions such as HIV infection may have consequences for energy uptake and the metabolic balance. Colon biopsy specimens from untreated HIV-infected persons show reduced Firmicutes levels but little change in Bacteroidetes levels at the phylum level as compared to HIV-negative controls (however, the relative composition of Bacteroidetes at the genus level did shift) [45]. Based on the microbiome profile reported in animal and human studies of excess adiposity, this alteration in the ratio of Bacteroidetes to Firmicutes levels in untreated HIV infection would appear to be protective against obesity. However, many HIV patients gain weight after ART initiation, particularly in the first 12 months, and the potential contribution of microbiome changes after ART initiation to weight gain is an important area for further study [2].
Obesity Is Associated With Increased Serum Inflammatory Markers in HIV-Infected Persons
As observed in the general population, serum levels of CRP are higher among HIV-infected adults with greater adiposity [88–91]. In the Fat Redistribution and Metabolic Change in HIV Infection cohort, each 2-fold increase in visceral adipose tissue was associated with a 17% higher serum CRP level, while a similar increase in subcutaneous adipose tissue was associated with 21% higher levels [88]. Circulating levels of IL-6, TNF-α receptor 1, and macrophage inflammatory protein 1α also rise in proportion to fat mass in HIV-infected persons, likely because of greater expression from stromal vascular cells and hypertrophied adipocytes [91, 92]. The enlargement of adipose tissue depots is primarily due to adipocyte hypertrophy, rather than hyperplasia, and increases in adipocyte size result in disproportionate increases in IL-6 and TNF-α expression [93–95]. It is estimated that adipose tissue–derived IL-6 constitutes up to 35% of circulating levels in obese individuals and is a substantial contributor to CRP production [96]. This raises the question of whether the reported association between CRP or IL-6 levels and adverse health outcomes in studies of predominantly nonobese HIV-infected individuals should be extrapolated to obese HIV-infected populations, because in the obese a higher proportion of these biomarkers may emanate from adipose tissue as opposed to other sites of inflammation [97–99].
Obesity and Adipose Tissue Immune Cell Profiles
Immune cell infiltration of adipose tissue accompanies progressive weight gain and contributes to both in situ and systemic inflammation. Adipose tissue from obese humans and animal models shows a striking increase in CD8+ T cells and TH1- and TH17-polarized CD4+ T cell counts, a decrease in T-regulatory cell counts, and an increase in the number of M1-phenotype proinflammatory macrophages (ie, those producing TNF-α, interleukin 12, and IL-23) [100–103]. CD8+ T-cell infiltration into adipose tissue is an early and necessary step preceding M1-phenotype macrophage recruitment in mice, and antibody-induced CD8+ T-cell depletion results in reduced infiltration of M1-phenotype macrophages into adipose tissue [100]. Adipocyte hypertrophy is associated with increased production of macrophage chemotactic protein 1 and macrophage inflammatory protein 1α, which promote macrophage infiltration, and increased production of interleukin 8, which promotes neutrophil chemotaxis [104–106].
Recent studies highlight an important role for TH17 cells, a subset of CD4+ effector T cells defined by their production of IL-17, in promoting adipose tissue inflammation and metabolic disease [107, 108]. TH17 cells are central contributors to the maintenance of mucosal barriers, pathogen clearance at the mucosal surface, and the defense against fungi and extracellular bacteria [109, 110], but loss or dysregulation of TH17 cells is also implicated in the pathogenesis of autoimmune and inflammatory conditions [111]. Adipose tissue CD4+ T cells in obese, insulin-resistant persons are skewed toward a TH17 phenotype, and the tissue microenvironment is characterized by high levels of TH17-promoting interleukin 1β (IL-1β) and IL-6, in addition to the TH17 markers RORC, IL-17, and IL-23R [107, 112]. M1-phenotype macrophage cytokine expression promotes a cycle of progressive TH17 polarization and inflammation, with IL-1β and IL-6 promoting the differentiation of TH17 cells, and IL-23 promoting their stabilization and expansion [113, 114]. While circulating IL-17 levels are frequently low or undetectable, in vitro IL-17 inhibits skeletal muscle glucose uptake and hepatocyte insulin sensitivity, suggesting local effects of IL-17 produced by TH17 cells residing in metabolically-active tissues may have an important role in insulin resistance [107]. A recent study describes the role of adenosine triphosphate (ATP) leakage into the extracellular space, a hallmark of pathologic cellular conditions such as apoptosis, inflammation, or ischemia, in promoting a TH17-polarizing milieu [115]. The addition of ATP to visceral adipose tissue from metabolically healthy lean subjects enriched the tissue microenvironment for IL-1β, IL-6, and IL-17 and yielded greater CD4+ T-cell expression of a characteristic TH17 cytokine signature [112]. These studies suggest a central role for TH17 CD4+ cells in propagating adipose tissue inflammation, and further studies are needed to understand whether HIV status alters the distribution and activity of adipose tissue TH17-polarized cells in obesity.
Adipose tissue also serves as a reservoir of CD4+ T cells harboring latent HIV infection. Recent studies found a higher percentage of activated CD4+ and CD8+ T cells in adipose tissue from HIV-infected subjects as compared to HIV-negative controls, in addition to the unique presence of latently HIV-infected memory CD4+ T cells [82, 116]. Furthermore, the median copy number of latent HIV DNA in subcutaneous adipose tissue CD4+ T cells was slightly higher than the median copy number in circulating CD4+ T cells, indicating that adipose tissue serves as a significant reservoir for latent HIV infection [116]. Similar findings regarding a higher proportion of activated CD8+ and CD4+ T cells and latently infected memory CD4+ T cells in both subcutaneous and visceral adipose tissue have been reported in simian immunodeficiency virus–infected macaques, compared with uninfected animals [116].
Obesity and Circulating T-Cell Profiles in HIV-Infected and HIV-Negative Persons
Studies from the pre-ART era found that a higher BMI was associated with slower disease progression and CD4+ T-cell decline [117–119]. However, it is unclear whether the delayed immunosuppression observed among HIV-infected individuals with a high BMI was due to an effect of greater adiposity or to other factors, such as fewer secondary infections or micronutrient deficiencies. Recent studies in the combination ART era found that a higher BMI may promote more-robust CD4+ T-cell recovery during treatment [120, 121]. An analysis of >14 000 HIV-infected adults in 13 multisite cohorts found that a higher time-updated BMI was significantly associated with greater CD4+ T-cell recovery during ART [122]. After 5 years of ART, the mean CD4+ T-cell count for a hypothetical patient with a BMI of 30 was 20% higher than that for patient with a BMI of 22 (524 vs 436 cells/µL) and 31% higher for a patient with a BMI of 40 than for a patient with a BMI of 22 (572 vs 436 cells/µL).
A minimum quantity of adipose tissue appears necessary to maintain normal-range lymphocyte subset counts, but assessing the relationship between adiposity and peripheral T-cell populations in the setting of HIV infection is confounded by CD4+ T-cell depletion, variations in immune recovery during ART, and the effects of HIV-related immune activation. Thus, studies of HIV-negative individuals may be revealing in this area. Overweight and obese HIV-negative women had higher CD4+ and total lymphocyte counts, compared with normal-weight women in one study [123]. In another study [124], the expression of activation marker CD25 on CD3+ T cells was 3-fold higher in obese subjects, compared with nonobese subjects, and the ratio of TH1 to TH2 CD4+ lymphocytes was also significantly higher. Similarly, an analysis of the European CODAM cohort of HIV-negative individuals found that greater waist circumference was associated with higher circulating markers of adaptive immune activation (neopterin and soluble CD25) [125]. Taken together, these data suggest that, irrespective of HIV infection, higher fat stores are associated with higher circulating CD4+ T-cell populations, greater TH1 polarization, and greater expression of surface markers of immune activation.
Adipose Tissue Hormones Alter Lymphocyte Function
Adipokines are hormones produced by adipocytes, which demonstrate a range of metabolic, neuroendocrine, and immunomodulatory properties. Leptin, an adipokine encoded by the ob gene and produced roughly in proportion to fat cell mass, was initially characterized as a regulator of appetite but also appears to have a range of local and potentially systemic immune effects [126–128]. Leptin independently induces expression of proinflammatory cytokines by macrophages and monocytes [129, 130] and acts directly on hepatocytes to promote CRP expression [131]. Mature CD4+ T cells express the long isoform of the leptin receptor [132, 133], and leptin stimulates T-cell proliferative responses in vitro, polarizes naive CD4+ T-cell proliferation toward the TH1 phenotype, and promotes a marked increase in levels of IFN-γ and other TH1-type cytokines [133–137]. Leptin also enhances in vitro expression of activation markers (CD69, CD25, and CD71) on both CD4+ and CD8+ T cells after antigen stimulation in a dose-dependent manner [136, 138]. While the administration of physiologic quantities of recombinant leptin to non–HIV-infected adults with acquired or congenital lipodystrophy increased peripheral CD4+ and CD8+ T-cell counts, 2 small trials involving HIV-infected individuals have not shown a benefit to CD4+ T-cell recovery during ART [139–142].
Therapeutic Trials to Reduce Adiposity and Immune Activation in HIV-Infected Individuals
Trials of exercise and lifestyle modification have reported a reduction in serum CRP level, weight loss, and improved cardiorespiratory fitness in HIV persons, although benefits for insulin sensitivity and fasting glucose are less clear [143–146]. In morbidly obese HIV-infected persons, bariatric surgery appears to be safe and does not affect viral suppression [147, 148].
The accumulation of visceral fat in HIV-infected individuals is accompanied by reductions in endogenous circulating and stimulated growth hormone (GH) levels, a finding also observed in HIV-negative persons with abdominal obesity and independent of age, BMI, and total body fat [149–151]. Inadequate GH levels are associated with reduced bone mineralization, dyslipidemia (characterized by elevated triglyceride levels and low high-density lipoprotein cholesterol levels), elevated blood pressure, reduced vascular health, higher circulating CRP levels, and a detrimental cycle of further accumulation of visceral adiposity with concomitant progressive reductions in GH secretion [152–155]. Among HIV-infected persons, lower peak levels of GH are associated with higher CRP levels, in addition to higher fasting glucose levels and triglyceride levels independent of waist circumference [156].
Studies of GH replacement in persons with hypopituitarism demonstrated reductions in visceral adiposity and inflammation and improved lipid parameters and markers of vascular health, which suggested possible benefits for HIV-infected persons with abdominal obesity [153, 157–159]. However, while trials of recombinant human growth hormone (rhGH) in obese HIV-infected persons have shown reductions in visceral adipose tissue and hepatic fat [160–162], these benefits must be weighed against the increased insulin resistance observed with rhGH treatment [161–164]. Furthermore, the beneficial effects of GH supplementation on innate immune activation in persons with hypopituitarism are not as evident in HIV-infected individuals. A multiarm study of rhGH, rosiglitazone, combination rhGH and rosiglitazone, and placebo found no significant difference in a range of serum inflammation biomarkers, including CRP, IL-1, IL-6, TNF-α, and IFN-γ, between study arms after 12 weeks of treatment [160].
Tesamorelin, a synthetic form of growth hormone–releasing hormone, is a Food and Drug Administration–approved treatment to reduce abdominal fat in HIV-infected patients with lipodystrophy. Trials of tesamorelin demonstrate visceral and hepatic fat reductions, gains in lean body mass, and improved lipid profiles, but without the increase in insulin resistance, which limited the clinical usefulness of rhGH [165–167]. However, despite substantial reductions in visceral fat with tesamorelin, it is notable that a 26-week randomized trial did not demonstrate a significant effect on CRP levels, and more studies are needed to characterize the effects of tesamorelin on innate and cellular immune activation [168].
CONCLUSION
Persistent, chronic innate and adaptive immune activation have been implicated in the pathogenesis of multiple comorbidities in HIV-infected patients and impaired immune recovery during ART. While the etiology of this heightened immune activation is multifactorial, the immunologic effects of HIV infection can be amplified and modulated by host nutritional factors. At the intersection of these nutritional and immunologic processes, an opportunity may be present for interventions to mitigate the adverse effects of both malnutrition and obesity on chronic immune activation and improve health outcomes in HIV-infected individuals.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Supplement sponsorship. This article appears as part of the supplement “Persistent Inflammation in Treated HIV Disease,” sponsored by Case Western Reserve University and supported by funds from the James B. Pendleton Charitable Trust.
Financial support. This work was supported by the National Institutes of Health (grant K23AI100700).
Potential conflicts of interests. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hasse B, Iff M, Ledergerber B et al. Obesity trends and body mass index changes after starting antiretroviral treatment: The Swiss HIV Cohort Study. Open Forum Infect Dis 2014; 1:ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koethe JR, Jenkins CA, Lau B et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koethe JR, Heimburger DC. Nutritional aspects of HIV-associated wasting in sub-Saharan Africa. Am J Clin Nutr 2010; 91:1138S–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotler DP, Tierney AR, Wang J, Pierson RN Jr. Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr 1989; 50:444–7. [DOI] [PubMed] [Google Scholar]
- 5.Macallan DC, Noble C, Baldwin C et al. Energy expenditure and wasting in human immunodeficiency virus infection. N Engl J Med 1995; 333:83–8. [DOI] [PubMed] [Google Scholar]
- 6.Grunfeld C, Pang M, Shimizu L, Shigenaga JK, Jensen P, Feingold KR. Resting energy expenditure, caloric intake, and short-term weight change in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Am J Clin Nutr 1992; 55:455–60. [DOI] [PubMed] [Google Scholar]
- 7.Shevitz AH, Knox TA, Spiegelman D, Roubenoff R, Gorbach SL, Skolnik PR. Elevated resting energy expenditure among HIV-seropositive persons receiving highly active antiretroviral therapy. AIDS 1999; 13:1351–7. [DOI] [PubMed] [Google Scholar]
- 8.Melchior JC, Raguin G, Boulier A et al. Resting energy expenditure in human immunodeficiency virus-infected patients: comparison between patients with and without secondary infections. Am J Clin Nutr 1993; 57:614–9. [DOI] [PubMed] [Google Scholar]
- 9.Yarasheski KE, Zachwieja JJ, Gischler J, Crowley J, Horgan MM, Powderly WG. Increased plasma gln and Leu Ra and inappropriately low muscle protein synthesis rate in AIDS wasting. Am J Physiol 1998; 275:E577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macallan DC, McNurlan MA, Milne E, Calder AG, Garlick PJ, Griffin GE. Whole-body protein turnover from leucine kinetics and the response to nutrition in human immunodeficiency virus infection. Am J Clin Nutr 1995; 61:818–26. [DOI] [PubMed] [Google Scholar]
- 11.Food and Agricultural Organzation of the United Nations. The State of Food Insecurity in the World 2014. Rome: FAO, 2014. http://www.fao.org/publications/sofi/2014/en/. Accessed 25 July 2106. [Google Scholar]
- 12.Koethe JR, Lukusa A, Giganti MJ et al. Association between weight gain and clinical outcomes among malnourished adults initiating antiretroviral therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr 2010; 53:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassol E, Malfeld S, Mahasha P et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis 2010; 202:723–33. [DOI] [PubMed] [Google Scholar]
- 14.Canipe A, Chidumayo T, Blevins M et al. A 12 week longitudinal study of microbial translocation and systemic inflammation in undernourished HIV-infected Zambians initiating antiretroviral therapy. BMC Infect Dis 2014; 14:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley JM, Price DA, Schacker TW et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 16.Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg 2012; 86:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti G, Cozzi-Lepri A, Merlini E et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS 2011; 25:1385–94. [DOI] [PubMed] [Google Scholar]
- 18.Leon A, Leal L, Torres B et al. Association of microbial translocation biomarkers with clinical outcome in controllers HIV-infected patients. AIDS 2015; 29:675–81. [DOI] [PubMed] [Google Scholar]
- 19.Sandler NG, Wand H, Roque A et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchetti G, Cozzi-Lepri A, Merlini E et al. Pre-cART pro-inflammatory milieu, microbial translocation (MT) and risk of disease progression in HIV-infected patients starting their first cART: data from the Icona Foundation Cohort Presented at: European AIDS Clinical Society, Barcelona, Spain, 21–24 October 2015. [Google Scholar]
- 21.Marchetti G, Bellistri GM, Borghi E et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS 2008; 22:2035–8. [DOI] [PubMed] [Google Scholar]
- 22.Elia M, Goren A, Behrens R, Barber RW, Neale G. Effect of total starvation and very low calorie diets on intestinal permeability in man. Clin Sci (Lond) 1987; 73:205–10. [DOI] [PubMed] [Google Scholar]
- 23.Welsh FK, Farmery SM, MacLennan K et al. Gut barrier function in malnourished patients. Gut 1998; 42:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly P, Menzies I, Crane R et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg 2004; 70:412–9. [PubMed] [Google Scholar]
- 25.Veitch AM, Kelly P, Zulu IS, Segal I, Farthing MJ. Tropical enteropathy: a T-cell-mediated crypt hyperplastic enteropathy. Eur J Gastroenterol Hepatol 2001; 13:1175–81. [DOI] [PubMed] [Google Scholar]
- 26.Dhaliwal W, Bajaj-Elliott M, Kelly P. Intestinal defensin gene expression in human populations. Mol Immunol 2003; 40:469–75. [DOI] [PubMed] [Google Scholar]
- 27.Brenchley JM, Paiardini M, Knox KS et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 2008; 112:2826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazli A, Chan O, Dobson-Belaire WN et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 2010; 6:e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sankaran S, George MD, Reay E et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol 2008; 82:538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keating J, Bjarnason I, Somasundaram S et al. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut 1995; 37:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapembwa MS, Fleming SC, Sewankambo N et al. Altered small-intestinal permeability associated with diarrhoea in human-immunodeficiency-virus-infected Caucasian and African subjects. Clin Sci (Lond) 1991; 81:327–34. [DOI] [PubMed] [Google Scholar]
- 32.Chevalier MF, Petitjean G, Dunyach-Remy C et al. The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLoS Pathog 2013; 9:e1003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AJ, Schacker TW, Reilly CS, Haase AT. A role for syndecan-1 and claudin-2 in microbial translocation during HIV-1 infection. J Acquir Immune Defic Syndr 2010; 55:306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenabian MA, El-Far M, Vyboh K et al. Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis 2015; 212:355–66. [DOI] [PubMed] [Google Scholar]
- 35.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 1977; 31:107–33. [DOI] [PubMed] [Google Scholar]
- 36.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005; 307:1915–20. [DOI] [PubMed] [Google Scholar]
- 37.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012; 336:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 2016; 14:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gori A, Tincati C, Rizzardini G et al. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol 2008; 46:757–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellis CL, Ma ZM, Mann SK et al. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr 2011; 57:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merlini E, Bai F, Bellistri GM, Tincati C, d'Arminio Monforte A, Marchetti G. Evidence for polymicrobic flora translocating in peripheral blood of HIV-infected patients with poor immune response to antiretroviral therapy. PLoS One 2011; 6:e18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mutlu EA, Keshavarzian A, Losurdo J et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog 2014; 10:e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voigt RM, Keshavarzian A, Losurdo J et al. HIV-associated mucosal gene expression: region-specific alterations. AIDS 2015; 29:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vujkovic-Cvijin I, Dunham RM, Iwai S et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillon SM, Lee EJ, Kotter CV et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 2014; 7:983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez-Santiago J, Gianella S, Massanella M et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS 2013; 27:1921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinh DM, Volpe GE, Duffalo C et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis 2015; 211:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smythe PM. Changes in intestinal bacterial flora and role of infection in Kwashiorkor. Lancet 1958; 2:724–7. [DOI] [PubMed] [Google Scholar]
- 49.Monira S, Nakamura S, Gotoh K et al. Gut microbiota of healthy and malnourished children in bangladesh. Front Microbiol 2011; 2:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh TS, Gupta SS, Bhattacharya T et al. Gut microbiomes of Indian children of varying nutritional status. PLoS One 2014; 9:e95547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51–168.These references are available in the Supplementary Appendix. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.