Abstract
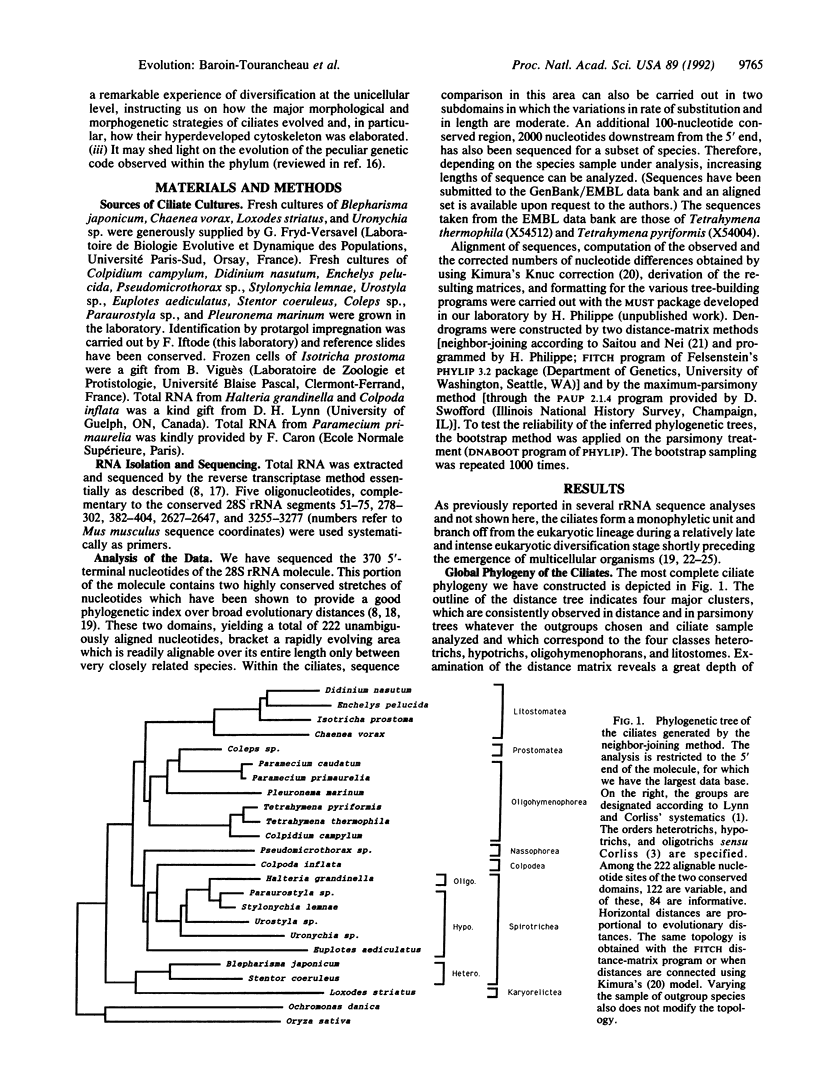
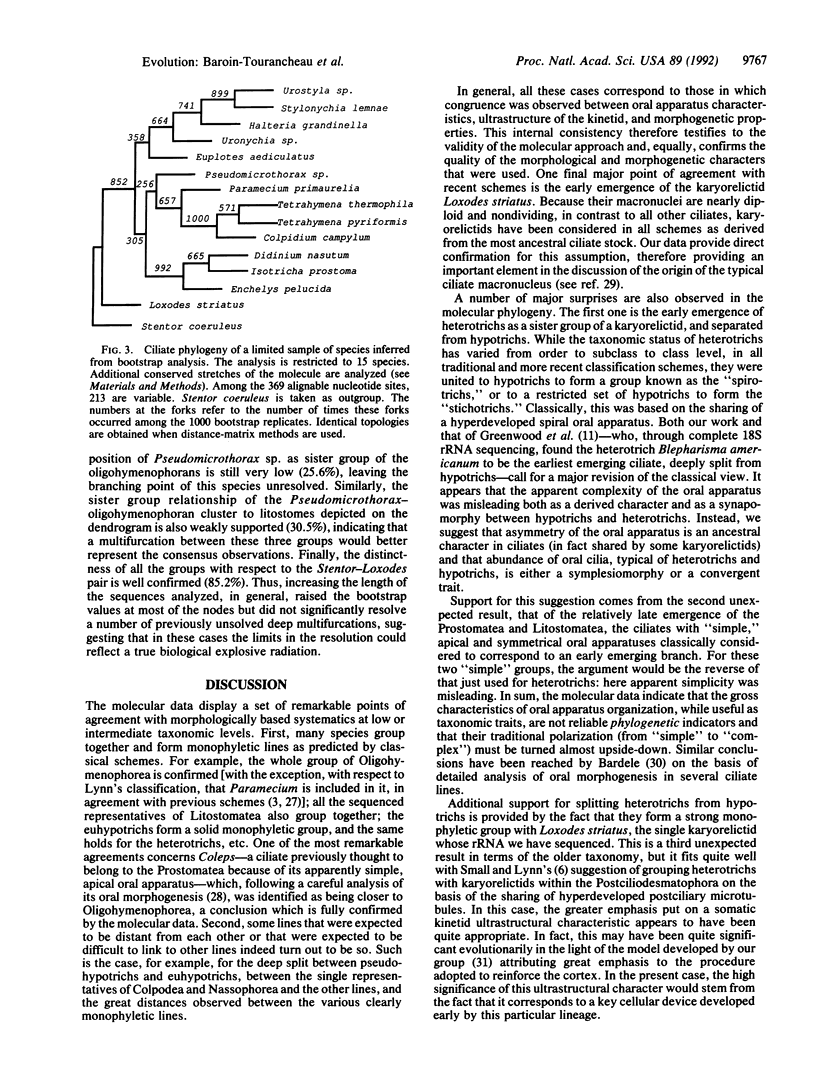
The cellular architecture of ciliates is one of the most complex known within eukaryotes. Detailed systematic schemes have thus been constructed through extensive comparative morphological and ultrastructural analysis of the ciliature and of its internal cytoskeletal derivatives (the infraciliature), as well as of the architecture of the oral apparatus. In recent years, a consensus was reached in which the phylum was divided in eight classes as defined by Lynn and Corliss [Lynn, D. H. & Corliss, J. O. (1991) in Microscopic Anatomy of Invertebrates: Protozoa (Wiley-Liss, New York), Vol. 1, pp. 333-467]. By comparing partial sequences of the large subunit rRNA molecule, and by using both distance-matrix and maximum-parsimony-tree construction methods (checked by boot-strapping), we examine the phylogenetic relationships of 22 species belonging to seven of these eight classes. At low taxonomic levels, the traditional grouping of the species is generally confirmed. At higher taxonomic levels, the branching pattern of these seven classes is resolved in several deeply separated major branches. Surprisingly, the first emerging one contains the heterotrichs and is strongly associated with a karyorelictid but deeply separated from hypotrichs. The litostomes, the oligohymenophorans, and the hypotrichs separate later in a bush-like topology hindering the resolution of their order of diversification. These results show a much more ancient origin of heterotrichs than was classically assumed, indicating that asymmetric, abundantly ciliated oral apparatuses do not correspond to "highly evolved" traits as previously thought. They also suggest the occurrence of a major radiative explosion in the evolutionary history of the ciliates, yielding five of the eight classes of the phylum. These classes appear to differ essentially according to the cytoskeletal architecture used to shape and sustain the cellular cortex (a process of essential adaptative and morphogenetic importance in ciliates).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Li C. I. Nucleotide sequence divergence among DNA fractions of different syngens of Tetrahymena pyriformis. Biochem Genet. 1974 Sep;12(3):213–233. doi: 10.1007/BF00486091. [DOI] [PubMed] [Google Scholar]
- Baroin A., Perasso R., Qu L. H., Brugerolle G., Bachellerie J. P., Adoutte A. Partial phylogeny of the unicellular eukaryotes based on rapid sequencing of a portion of 28S ribosomal RNA. Proc Natl Acad Sci U S A. 1988 May;85(10):3474–3478. doi: 10.1073/pnas.85.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron F. Eucaryotic codes. Experientia. 1990 Dec 1;46(11-12):1106–1117. doi: 10.1007/BF01936920. [DOI] [PubMed] [Google Scholar]
- Christen R., Ratto A., Baroin A., Perasso R., Grell K. G., Adoutte A. An analysis of the origin of metazoans, using comparisons of partial sequences of the 28S RNA, reveals an early emergence of triploblasts. EMBO J. 1991 Mar;10(3):499–503. doi: 10.1002/j.1460-2075.1991.tb07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood H. J., Olsen G. J., Sogin M. L. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol Biol Evol. 1985 Sep;2(5):399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- Fleury A. The use of correlated ultrastructural and morphogenetic characters in evolutionary taxonomy of hypotrich ciliates. Biosystems. 1988;21(3-4):309–316. doi: 10.1016/0303-2647(88)90027-5. [DOI] [PubMed] [Google Scholar]
- Greenwood S. J., Schlegel M., Sogin M. L., Lynn D. H. Phylogenetic relationships of Blepharisma americanum and Colpoda inflata within the phylum ciliophora inferred from complete small subunit rRNA gene sequences. J Protozool. 1991 Jan-Feb;38(1):1–6. doi: 10.1111/j.1550-7408.1991.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Greenwood S. J., Sogin M. L., Lynn D. H. Phylogenetic relationships within the class Oligohymenophorea, phylum Ciliophora, inferred from the complete small subunit rRNA gene sequences of Colpidium campylum, Glaucoma chattoni, and Opisthonecta henneguyi. J Mol Evol. 1991 Aug;33(2):163–174. doi: 10.1007/BF02193631. [DOI] [PubMed] [Google Scholar]
- Gunderson J. H., Elwood H., Ingold A., Kindle K., Sogin M. L. Phylogenetic relationships between chlorophytes, chrysophytes, and oomycetes. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5823–5827. doi: 10.1073/pnas.84.16.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Lenaers G., Maroteaux L., Michot B., Herzog M. Dinoflagellates in evolution. A molecular phylogenetic analysis of large subunit ribosomal RNA. J Mol Evol. 1989 Jul;29(1):40–51. doi: 10.1007/BF02106180. [DOI] [PubMed] [Google Scholar]
- Lynn D. H., Sogin M. L. Assessment of phylogenetic relationships among ciliated protists using partial ribosomal RNA sequences derived from reverse transcripts. Biosystems. 1988;21(3-4):249–254. doi: 10.1016/0303-2647(88)90020-2. [DOI] [PubMed] [Google Scholar]
- Miceli C., La Terza A., Melli M. Isolation and structural characterization of cDNA clones encoding the mating pheromone Er-1 secreted by the ciliate Euplotes raikovi. Proc Natl Acad Sci U S A. 1989 May;86(9):3016–3020. doi: 10.1073/pnas.86.9.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. C. Burgess shale faunas and the cambrian explosion. Science. 1989 Oct 20;246(4928):339–346. doi: 10.1126/science.246.4928.339. [DOI] [PubMed] [Google Scholar]
- Nanney D. L., Meyer E. B., Simon E. M., Preparata R. M. Comparison of ribosomal and isozymic phylogenies of tetrahymenine ciliates. J Protozool. 1989 Jan-Feb;36(1):1–8. doi: 10.1111/j.1550-7408.1989.tb02661.x. [DOI] [PubMed] [Google Scholar]
- Orias E. On the evolution of the karyorelict ciliate life cycle: heterophasic ciliates and the origin of ciliate binary fission. Biosystems. 1991;25(1-2):67–73. doi: 10.1016/0303-2647(91)90013-b. [DOI] [PubMed] [Google Scholar]
- Osawa S., Jukes T. H. Codon reassignment (codon capture) in evolution. J Mol Evol. 1989 Apr;28(4):271–278. doi: 10.1007/BF02103422. [DOI] [PubMed] [Google Scholar]
- Perasso R., Baroin A., Qu L. H., Bachellerie J. P., Adoutte A. Origin of the algae. Nature. 1989 May 11;339(6220):142–144. doi: 10.1038/339142a0. [DOI] [PubMed] [Google Scholar]
- Preparata R. M., Meyer E. B., Preparata F. P., Simon E. M., Vossbrinck C. R., Nanney D. L. Ciliate evolution: the ribosomal phylogenies of the tetrahymenine ciliates. J Mol Evol. 1989 May;28(5):427–441. doi: 10.1007/BF02603078. [DOI] [PubMed] [Google Scholar]
- Qu H. L., Michot B., Bachellerie J. P. Improved methods for structure probing in large RNAs: a rapid 'heterologous' sequencing approach is coupled to the direct mapping of nuclease accessible sites. Application to the 5' terminal domain of eukaryotic 28S rRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5903–5920. doi: 10.1093/nar/11.17.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L. H., Nicoloso M., Bachellerie J. P. Phylogenetic calibration of the 5' terminal domain of large rRNA achieved by determining twenty eucaryotic sequences. J Mol Evol. 1988 Dec;28(1-2):113–124. doi: 10.1007/BF02143502. [DOI] [PubMed] [Google Scholar]
- Raikov I. B. Primitive never-dividing macronuclei of some lower ciliates. Int Rev Cytol. 1985;95:267–325. doi: 10.1016/s0074-7696(08)60584-7. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]



