Abstract
We have reported that B6.CCR5−/− mice reject renal allografts with high serum donor-specific antibody (DSA) titers and marked C4d deposition in grafts, features consistent with AMR. B6.huCD20/CCR5−/− mice, where human CD20 expression is restricted to B cells, rejected A/J renal allografts by day 26 post-transplant with DSA first detected in serum on day 5 post-transplant and increased thereafter. Recipient treatment with anti-huCD20 mAb prior to the transplant and weekly up to 7 weeks post-transplant promoted long-term allograft survival (> 100 days) with low DSA titers. To investigate the effect of B cell depletion at the time serum DSA was first detected, recipients were treated with anti-huCD20 mAb on days 5, 8 and 12 post-transplant. This regimen significantly reduced DSA titers and graft inflammation on day 15 post-transplant and prolonged allograft survival > 60 days. However, DSA returned to the titers observed in control treated recipients by day 30 post-transplant and histological analyses on day 60 post-transplant indicated severe interstitial fibrosis. These results indicate that anti-huCD20 mAb had the greatest effect as a prophylactic treatment and that the distinct kinetics of DSA responses accounts for acute renal allograft failure versus the development of fibrosis.
Introduction
Current immunosuppressive strategies have markedly decreased the incidence of T cell mediated acute rejection of organ transplants. In contrast, the incidence of antibody-mediated graft rejection in organ grafts continues to increase. Acute antibody-mediated rejection (AMR) occurs in almost 7% of renal transplant patients and is also observed in cardiac and lung grafts (1–4). Donor-specific antibody (DSA) binding to the graft endothelium activates complement, which enhances antibody-mediated tissue injury by stimulating capillary endothelial cells to produce many inflammatory mediators including adhesion molecules, growth factors, cytokines, and chemokines (5–11). These factors direct the characteristic neutrophil and macrophage infiltration and their activation to express functions mediating graft tissue injury (1, 4, 6).
Preformed or de novo DSA mediates acute and/or chronic renal graft injury, each with distinct histopathology. Acute AMR is characterized by graft dysfunction manifested over several days and by high DSA titers that mediate peritubular capillaritis and glomerularitis (12). Biopsies from grafts experiencing acute AMR often show endothelial cell swelling, neutrophil infiltration of glomeruli and peritubular capillaries, fibrin thrombi, interstitial edema, and hemorrhage (13). Antibody-mediated chronic allograft injury is characteristically observed as transplant glomerulopathy (TG) with interstitial fibrosis/tubular atrophy, and/or intimal thickening of arteries in the kidney biopsy (14). It is likely that such antibody-mediated chronic injury is a major cause of the late kidney graft loss that has undermined successful long-term graft survival (15–17).
Despite the recognized importance of AMR as a leading factor in early and late renal allograft loss, few animal models recapitulate the de novo induction of DSA and the subsequent histopathologic features of both acute and chronic antibody-mediated injury. We previously reported marked de novo increases in DSA in B6.CCR5−/− recipients of complete MHC-disparate heart and kidney allografts (18–20). These dysregulated antibody responses appear more quickly and have 15–50-fold higher titers in B6.CCR5−/− recipients than those observed in wild type C57BL/6 recipients. The consequence of this increased antibody response is acute AMR accompanied by intense C4d/C3d deposition in the large vessels and capillaries of the allograft, peritubular capillaritis and glomerularitis. The current studies were conducted to investigate the efficacy of strategies utilizing anti-CD20 mAb to deplete B cells and attenuate DSA and renal allograft injury in this murine model of AMR. The results indicate that prophylactic depletion of recipient B cells promotes long-term renal allograft survival and more importantly that different antibody-mediated pathologies are induced in the allografts when B cells are depleted at different stages of the DSA response.
Materials and Methods
Mice
C57BL/6 (H-2b) and A/J (H-2a) mice were obtained from the National Cancer Institute (Frederick, MD) and B6.CCR5−/− (B6.129P2-Ccr5tm1Kuz/J) mice that were backcrossed 11 times to the C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, ME). Human CD20 transgenic mice were obtained from Genentech (San Francisco, CA). B6.CCR5−/− and B6.huCD20Tg mice were crossed to generate the B6.huCD20/CCR5−/− mice expressing hCD20 only on B cells. All experiments used 8- to 12-week-old male mice, and all animal use procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Kidney Transplantation
Murine kidney transplantation was performed as described by Zhang and colleagues (21). Briefly, the kidney with vascular supply and ureter were harvested en bloc and the donor artery and vein were anastomosed to the recipient abdominal aorta and inferior vena cava. Following successful anastomosis, the kidney graft perfused instantly. Urinary reconstruction was performed as described by Han and colleagues (22). The remaining native kidney was nephrectomized at the time of the transplant so that recipient survival was dependent on the kidney graft. Kidney graft survival was assessed by daily examination of overall animal health and measurement of serum creatinine levels. Renal allograft rejection was diagnosed when the mouse showed signs of illness and the creatinine level was elevated to 70 ~ 100 μmol/l at which time grafts were harvested for histopathology analysis. Allograft recipients were treated intraperitoneally with 250 μg anti-human CD20 mAb (Rituximab/Rituxan, Genentech, San Francisco, CA) or control rat IgG (Sigma-Aldrich, St. Louis, MO).
Creatinine Determination
Quantitative whole blood creatinine levels were determined using an I-Stat portable clinical analyzer (Heska Corp., Fort Collins, CO). Conventional units (mg/dl) were converted to SI units by multiplying the conventional units by 88.4. The concentration of creatinine is expressed in μmol/l.
Cell preparation and immunofluorescence analysis
Single-cell leukocyte suspensions from bone marrow and spleens were generated and peripheral blood mononuclear cells (PBMC) prepared using Lymphocyte Separation Medium (LSM, MP Biomedicals, Solon, OH). Cell aliquots were incubated with anti-CD16/CD32 Fc receptor mAb to block nonspecific antibody binding and each sample was stained for common phenotypic surface markers (CD45, B220, CD20, CD19) using standard methods and commercially available antibodies (BD Bioscience, San Jose, CA; eBioscience, San Diego, CA). Cells were analyzed by three-color immunofluorescence on a FACSCalibur (BD Biosciences) and FlowJo analysis software (Tree Star Inc., Ashland, OR).
Donor-specific antibody detection and quantitation by flow cytometry
Flow cytometry to detect and quantitate donor-specific antibodies in non-recipient and renal graft recipient serum was performed as previously reported (18–20). Briefly, aliquots of donor strain thymocyte suspensions were incubated with serial dilutions of recipient sera taken pretransplant and at several time points between transplantation and rejection. After 30 min, the cells were washed and stained with FITC-conjugated goat anti-mouse IgG, Fcγ fragment specific mAb (Jackson Immunoresearch, West Grove, PA). The mean channel fluorescence (MCF) of each dilution of each serum sample was determined and the dilution that returned the MCF to the level observed when A/J thymocytes were stained with a 1:4 dilution of normal wild-type serum was divided by two and reported as the titer.
Immunohistochemistry
Frozen or paraffin-embedded/methanol and acetic acid fixed sections of spleens or cross sections of the center of kidney grafts were prepared. Sections were stained with hematoxylin and eosin (H&E), Masson Trichrome, anti-B220 mAb and anti-CD8 mAb (BD Bioscience), anti-CD3 mAb (AbD Serotec, Raleigh, NC), anti-Mac2 mAb (Cedarlane, Burlington, NC) and rabbit anti- mouse C4d Ab (23). Sections were counter-stained with hematoxylin (Richard Allan Scientific, MI), dehydrated, cleared in Clear Rite3 (Richard Allan Scientific) and mounted. Representative images were captured and analyzed with NIS-Elements BR (Nikon, Melville, NY).
ELISPOT assays
Antibody-secreting B cells (plasma cells) were assessed by B cell ELISPOT assay according to the manufacture’s protocol (MabTech, Nacka Strand, Sweden). Briefly, ELISPOT plates were coated with capture anti-IgG or anti-IgM antibody (BD Pharmingen), and then spleen and bone marrow cell suspensions in 5% FBS/complete RPMI were added to each well and incubated for 24 h at 37°C in 5% CO2. Plates were extensively washed to remove the cells and biotinylated anti-IgG or anti-IgM antibody was added followed by Streptavidin-ALP and substrate (BCIP/NBT; MabTech). After color development, total spots per well were quantified using an ImmunoSpot Series 2 Analyzer (Cellular Technology Ltd., Shaker Heights, OH). Priming of donor-reactive T cells to IFN-γ–producing cells was quantified by ELISPOT assay as previously described (19, 20).
RNA purification and qRT-PCR
Snap-frozen grafts were crushed, homogenized, and RNA was isolated using RNeasy Mini Kits (QIAGEN, Valencia, CA). RNA was reverse transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Reverse transcription and Real-Time PCR were performed using commercially available reagents, probes and a 7500 Fast Real-Time Thermocycler (Applied Biosystems). For relative quantification of message expression (ΔΔCT Method), target gene expression was normalized to Mrpl32 gene expression and RNA isolated from a naïve A/J kidney sample was used as a calibrator.
Statistics
All data were analyzed using GraphPad Prism Pro (GraphPad Software Inc, San Diego, CA). Kaplan-Meier analysis was performed for graft survival. Log-rank testing was performed to determine differences in survival data and Mann-Whitney nonparametric test was used to determine significance throughout as indicated with P < 0.05 being considered a significant difference.
Results
B cell depletion in huCD20/CCR5−/− mice with anti-huCD20 mAb
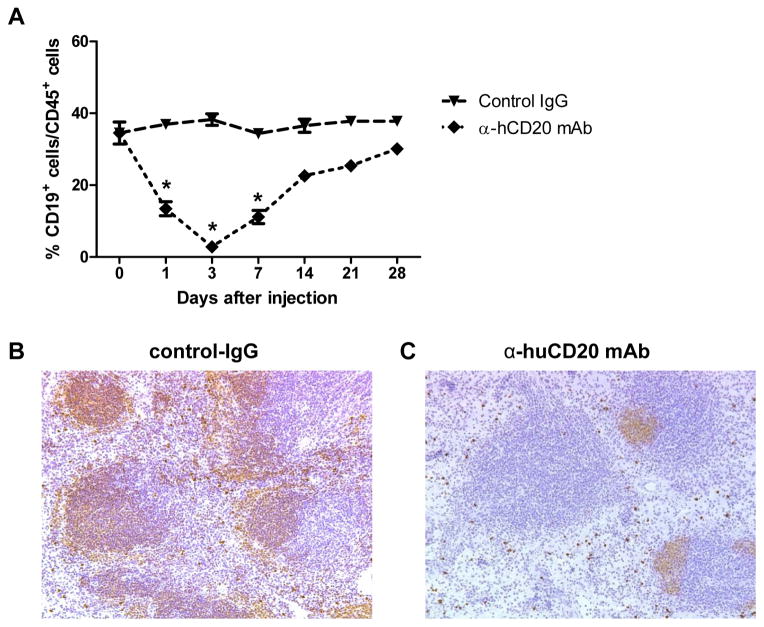
To assess the efficacy of B cell depletion by treating B6.huCD20/CCR5−/− mice with anti-human CD20 mAb, groups were treated with a single dose of control IgG or anti-huCD20 mAb and flow cytometry analyses of anti-CD45 and anti-B220 mAb stained PBMC was performed to detect B cells (Figure 1A). The control IgG had no discernible effect on B cells in the peripheral blood of human CD20 transgenic mice whereas the single dose of anti-huCD20 mAb resulted in rapid depletion of B cells within a day. B cell depletion reached nadir 3 days after mAb administration followed by a gradual recovery, nearing the percentages observed in the control IgG treated mice 28 days after the anti-huCD20 mAb administration. Sections of spleens prepared 7 days after control IgG and anti-huCD20 mAb treatment were stained with B220-specific antibody (Figure 1B). In contrast to spleens from control IgG-treated mice, spleens from anti-CD20 mAb-treated mice had many follicles that were either completely devoid or severely depleted of B cells and marked decreases of B cells in the marginal zone.
Figure 1. B cell depletion by treating B6.huCD20/CCR5−/− mice with a single dose of anti-huCD20 mAb.
(A) Groups of B6.huCD20/CCR5−/− mice were treated with a single dose of 250 μg control IgG or anti-huCD20 mAb i.p. On the indicated days, peripheral blood mononuclear cells were stained with anti-CD45 and anti-B220 mAb and analyzed by flow cytometry to determine the percent B cells in the CD45+ population (n = 4, each time point). *P < 0.05. On day 7 post-treatment, spleens were harvested from B6.huCD20/CCR5−/− mice treated with the single dose of (B) control IgG or (C) anti-huCD20 mAb. Methanol-fixed sections were stained with CD19-specific antibody for immunohistochemical analysis. Images shown are representative of > 5 sections examined from three to four spleens in each group. Magnification, 200X.
Prophylactic treatment of B6.huCD20/CCR5−/− renal allograft recipients with anti-huCD20 mAb results in long-term allograft survival
Anti-human CD20 mAb was used to test if depletion of B cells prior to the transplant prevented AMR of renal allografts. B6.huCD20/CCR5−/− mice received complete MHC-mismatched A/J renal grafts and underwent nephrectomy of the remaining native kidney. Groups of allograft recipients were treated with control IgG or anti-huCD20 mAb prior to the transplant (i.e. day -1) and then weekly up to 7 weeks post-transplant. More than 60% of B6.huCD20/CCR5−/− recipients treated with anti-huCD20 mAb accepted their allografts long-term (> 100 days) while recipients treated with control IgG rejected their allografts between days 18 and 26 post-transplant (P < 0.01, Figure 2A). C57BL/6 kidney isografts survived more than 100 days in B6.huCD20/CCR5−/− recipients (data not shown). Compared to control IgG-treated huCD20/CCR5−/− renal allograft recipients, peri-transplant treatment with anti-huCD20 mAb did not decrease donor-reactive T cell priming to IFN-γ-producing cells when assessed on day 8 post-transplant (Figure 2C) and did not decrease the numbers of CD4 and CD8 T cells infiltrating the allografts on day 8 post-transplant (data not shown). Recipients treated with control IgG had rapid rises in serum creatinine and DSA within a week of transplant and these levels continued to increase until rejection (Figure 2B and 2D). In contrast, recipients treated with peri-transplant anti-huCD20 mAb and weekly thereafter had low creatinine levels and DSA titers. When assessed at or beyond day 100 post-transplant, DSA titers of long-term surviving renal allograft recipients remained under 103 (data not shown).
Figure 2. Prophylactic treatment with anti-huCD20 mAb promotes long-term renal allograft survival in B6.huCD20/CCR5−/− recipients.
(A) B6.huCD20/CCR5−/− mice received complete MHC-mismatched A/J kidneys and groups were treated with 250 μg/day control IgG (-▼-: n = 12) or anti-huCD20 mAb (-◆-: n = 8) on the day prior to transplant (i.e. day -1) and then weekly thereafter through day 49 post-transplant. A third group of recipients was treated with anti-huCD20 mAb starting on day 7 post-transplant and then weekly until rejection (-●-: n = 4). (B) Kidney graft survival was assessed by daily examination of overall animal health and measurement of serum creatinine levels. Whole blood was collected from recipients treated with control IgG or anti-huCD20 mAb starting from day -1 and weekly up to 7 weeks. Renal allograft rejection was diagnosed when the mouse showed signs of illness and serum creatinine was elevated to 70 ~ 100 μmol/L. (C) On day 7 post-transplant, whole spleen cell suspensions were prepared from naïve B6.huCD20/CCR5−/− mice and from B6.huCD20/CCR5−/− renal allograft recipients treated with control IgG or with anti-human CD20 mAb on the day before the transplant. Aliquots of 106 spleen cells were co-cultured with donor (A/J) stimulator cells for 24 h to enumerate T cells producing IFN-γ by ELISPOT assay. Data are representative of three independent experiments and the data indicate mean number of donor-reactive cells producing IFN-γ from individual mice in groups of 3 ± SEM. Culture of the spleen cells with syngeneic stimulators always resulted in minimal numbers of spots (5–7/culture). The numbers of donor-reactive cells producing IFN-γ between the two allograft recipient groups was not significantly different; P = 0.0812. (D) Serum from B6.huCD20/CCR5−/− recipients was tested for titers of donor-reactive antibody. *P < 0.05; **P < 0.01.
Renal C57BL/6 isografts from wild type C57BL/6 recipients showed no evidence of inflammatory cell infiltration and normal kidney structure on day 100 post-transplant (Figure 3A and D). Rejecting allografts from control IgG treated recipients had intense leukocyte infiltration, including CD8 T cells, edema and glomerular fibrin thrombi (Figure 3 B and E), whereas allografts from recipients treated with peri-transplant anti-huCD20 mAb had low CD8 T cell infiltration but normal glomeruli and kidney structure when assessed on day 100 (Figure 3C and F).
Figure 3. Histological analysis of renal allografts in anti-huCD20 mAb treated B6.huCD20/CCR5−/− recipients.
The histopathology of renal allografts from recipients treated with control IgG or anti-huCD20 mAb starting from day -1 and weekly up to 7 weeks was analyzed on day 21 and day 100, respectively. As a control, C57BL/6 kidney isografts were harvested from C57BL/6 recipients on day 100. (A–C) Paraffin-embedded sections were prepared and stained with hematoxylin and eosin. (D–F) Frozen sections were stained with anti-CD8 mAb. Magnification, 400X.
The impact of administering anti-huCD20 mAb at the time when DSA levels had achieved high titers was tested on renal allograft outcome. Donor-reactive antibodies are first detected on day 5 post-transplant in B6.huCD20/CCR5−/− recipients and titers increase rapidly thereafter (Figure 2D). Therefore, recipient treatment with anti-human CD20 mAb was initiated on day 7 post-transplant and continued weekly from that time. Renal allograft survival in recipients treated with this regimen was similar to that observed in recipients treated with control IgG (Figure 2A), indicating that the B cell depleting therapy was ineffective once high titers of DSA were achieved.
Effect of anti-huCD20 mAb treatment initiated upon first detection of DSA
In practice, anti-CD20 mAb would be given when DSA and increases in serum creatinine are detected in renal graft recipients. Therefore, anti-human CD20 mAb treatment was initiated on day 5 post-transplant, when increased creatinine and DSA were first detected in the B6.huCD20/CCR5−/− recipients and doses were also given on days 8 and 12. The kinetics of B cell depletion and reappearance in the peripheral blood of B6.huCD20/CCR5−/− renal allograft recipients treated with anti-huCD20 mAb on days 5, 8 and 12 was virtually identical with those observed following treatment of B6.huCD20/CCR5−/− mice with a single dose of the mAb as a nadir of B cell depletion was quickly achieved and then slowly rebounded to control levels within 25 days (Figure 4A). Treatment with anti-huCD20 mAb on days 5, 8 and 12 post-transplant resulted in significant decreases in DSA titers when assessed on days 15 and 20 post-transplant, but between days 30 and 40 the titers neared those observed in control IgG-treated recipients that rejected their allografts by day 25 (Figure 4B). Treatment of huCD20/CCR5−/− renal allograft recipients with anti-huCD20 mAb on days 5, 8 and 12 post-transplant did not decrease donor-reactive T cell priming to IFN-γ-producing cells in the spleen when assessed on day 15 post-transplant (Figure 4C).
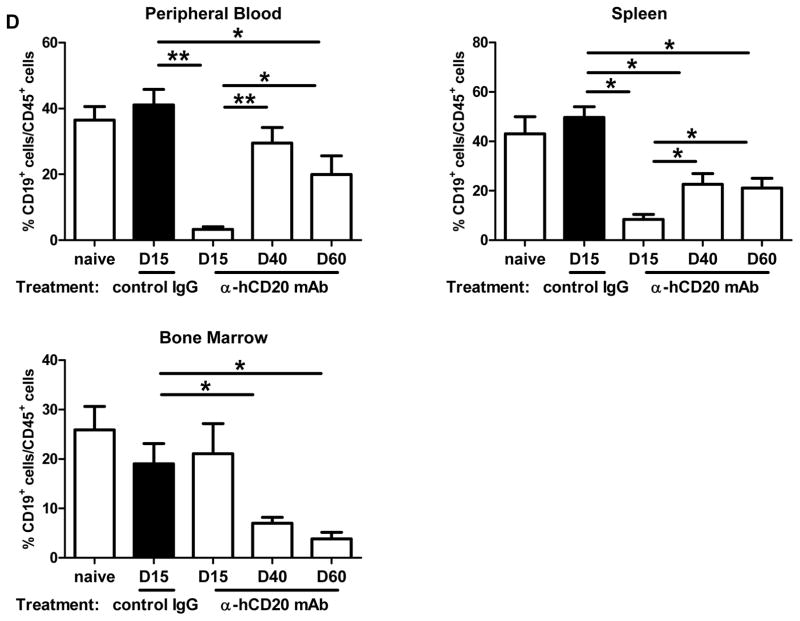
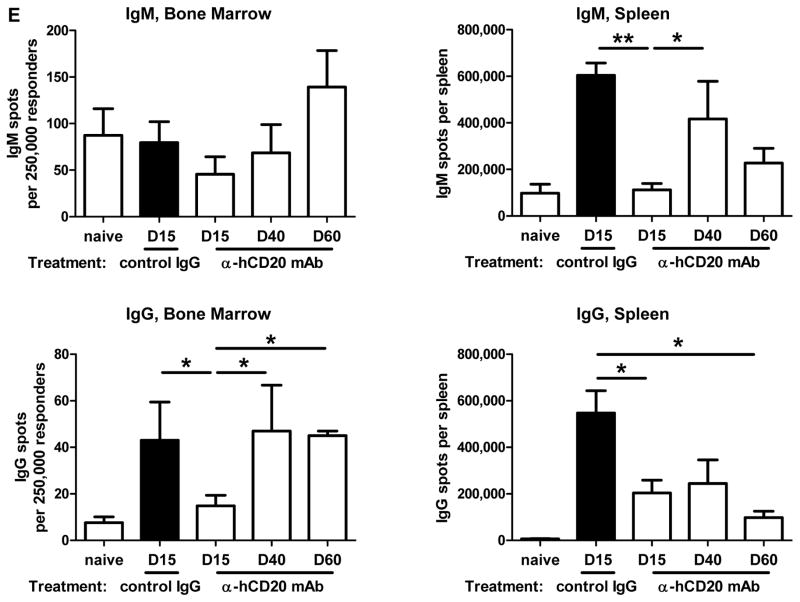
Figure 4. Initiation of anti-huCD20 mAb on day 5 post-transplant depletes B cells from B6.huCD20/CCR5−/− renal allograft recipients and delays achievement of peak donor-specific antibody titers.
Groups of B6.huCD20/CCR5−/− mice received complete MHC-mismatched A/J kidneys and were treated with 250 μg/day control IgG or anti-huCD20 mAb on days 5, 8 and 12 post-transplant. (A) On the indicated days, peripheral blood mononuclear cells were stained with anti-CD45 and anti-B220 mAb and analyzed by flow cytometry to determine the percent B cells in the CD45+ population (n = 3 per group). *P < 0.05; **P < 0.01. (B) Serum samples collected from B6.huCD20/CCR5−/− recipients of A/J allografts treated with control IgG (-▼-) or anti-huCD20 mAb (-◆-) on days 5, 8 and 12 post-transplant were tested for DSA titers. The mean titer for 3–5 recipients per group ± SEM is shown. *P < 0.05. (C) On day 15 post-transplant, whole spleen cell suspensions were prepared from naïve B6.huCD20/CCR5−/− mice and from B6.huCD20/CCR5−/− renal allograft recipients treated with control IgG or with anti-human CD20 mAb on days 5, 8 and 12 post-transplant. Aliquots of 106 spleen cells were co-cultured with donor (A/J) stimulator cells for 24 h to enumerate T cells producing IFN-γ by ELISPOT assay. The data indicate mean number of donor-reactive cells producing IFN-γ from individual mice in groups of 3 ± SEM. There was no significant difference in the numbers between the two groups of B6.huCD20/CCR5−/− renal allograft recipients. (D) Peripheral blood cells, spleen and bone marrow were collected from a naïve B6.huCD20/CCR5−/− mouse as a control and from the B6.huCD20/CCR5−/− renal allograft recipients on days 15, 40, and 60 post-transplant and cells were stained with anti-CD45 and anti-CD19 mAb and analyzed by flow cytometry to determine the percent B cells in the CD45+ population. Data indicate the mean percent B cells in the CD45+ population ± SEM for 6–8 mice per group. (E) Numbers of cells producing IgM and IgG were measured in the bone marrow and spleen by ELISPOT. Data indicate mean number of IgM- and IgG-secreting cells ± SEM for 6–8 mice per group.
To determine the impact of the delayed anti-huCD20 mAb treatment on the allograft recipient B cells, PBMC, spleens and bone marrow were collected on days 15, 40, and 60 post-transplant from naïve/untransplanted B6.huCD20/CCR5−/− and B6.huCD20/CCR5−/− recipients of A/J allografts treated with control IgG or with anti-huCD20 mAb on days 5, 8 and 12 post-transplant. First, flow cytometry analyses of anti-CD45 and anti-CD19 mAb stained cells were performed to detect B cells (Figure 4D). Consistent with the kinetic studies, anti-huCD20 mAb significantly depleted B cells from the peripheral blood and spleen when assessed on day 15 post-transplant. B cell numbers gradually increased to control levels by day 40 post-transplant but then slightly decreased in comparison to the numbers observed in naïve and in control IgG treated allograft recipients when assessed on day 60 post-transplant. The three doses of anti-huCD20 mAb did not deplete bone marrow B cells on day 15 post-transplant, but these numbers were markedly reduced versus those from control IgG-treated recipients when assessed on days 40 and 60 post-transplant.
The bone marrow and spleen of naïve/non-recipient and control IgG- and anti-CD20 mAb-treated renal allograft recipients were also tested for numbers of IgM- and IgG-secreting plasma cells by ELISPOT assay (Figure 4E). Anti-huCD20 mAb reduced IgM- and IgG-secreting cells in the bone marrow when assessed on day 15 post-transplant but these numbers rebounded by day 40 in the bone marrow. In the spleen anti-huCD20 mAb treatment reduced the numbers of IgM-producing cells to naïve levels by day 15 with a rebound on day 40 although there was a modest decrease again when assessed on day 60 post-transplant. Numbers of IgG-secreting plasma cells in the spleens of anti-human CD20 mAb treated renal allograft recipients were significantly decreased on day 15 and remained low versus control IgG treated recipients.
Renal allograft outcome in recipients treated with anti-human CD20 mAb at the time of initial DSA detection
Recipients treated with three doses of anti-huCD20 mAb beginning on day 5 post-transplant had significantly improved allograft survival versus control IgG-treated recipients (mean survival time: day 69 vs. 20, respectively, P < 0.01), but all rejected their allografts by day 75 post-transplant (Figure 5A). Treatment with anti-huCD20 mAb on days 5, 8 and 12 post-transplant significantly reduced donor-specific Ab titers on day 15 post-transplant to the titers observed in wild type C57BL/6 recipients that do not reject the A/J allografts (Figure 5B).
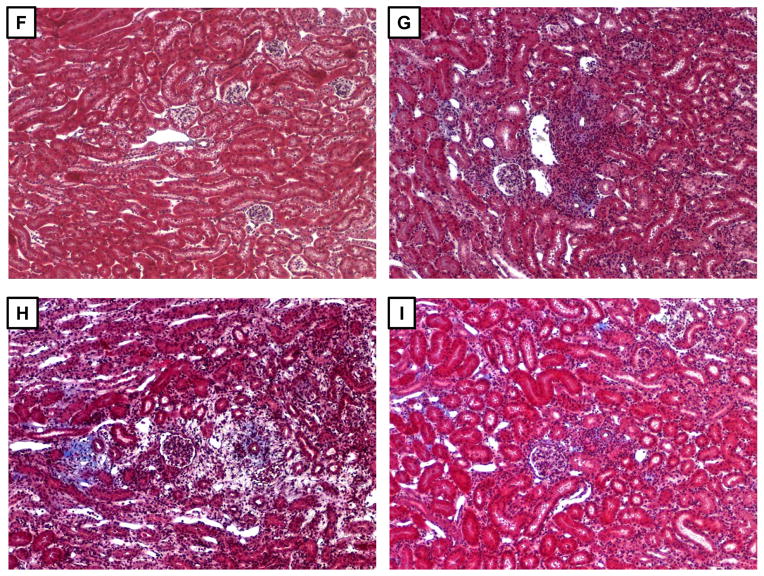
Figure 5. Initiation of anti-huCD20 mAb treatment on day 5 post-transplant in B6.huCD20/CCR5−/− renal allograft recipients prolongs allograft survival.
Groups of B6.huCD20/CCR5−/− mice received complete MHC-mismatched A/J kidneys and were treated with 250 μg/day control IgG (-▼-: n = 12) or anti-huCD20 mAb (-●-: n = 7) on days 5, 8 and 12 post-transplant. (A) Kidney allograft survival was assessed by daily examination of overall animal health and measurement of serum creatinine levels. **P < 0.01. (B) Serum from wild type C57BL/6 recipients of renal allografts and from the B6.huCD20/CCR5−/− renal allograft recipients treated with control IgG or anti-huCD20 mAb on days 5, 8 and 12 was collected at day 15 post-transplant and tested for DSA titers. The mean titer for 3–6 recipients per group is shown. **P < 0.01. (C–E) On day 15 post-transplant, renal grafts were harvested from: (C) wild type C57BL/6 recipients of A/J allografts, (D) B6.huCD20/CCR5−/− renal allograft recipients treated with control IgG, and (E) B6.huCD20/CCR5−/− renal allograft recipients treated with anti-huCD20 mAb. Paraffin-embedded sections were stained to detect C4d deposition in the graft. (F–I) On day 15 post-transplant, renal grafts were harvested from: (F) wild type C57BL/6 recipients of C57BL/6 renal isografts, (G) wild type C57BL/6 recipients of A/J allografts, (H) B6.huCD20/CCR5−/− renal allograft recipients treated with control IgG, and (I) B6.huCD20/CCR5−/− renal allograft recipients treated with anti-huCD20 mAb. Paraffin-embedded sections were stained with Masson Trichrome to detect collagen deposition in the graft. Representative images from 4 grafts per group are shown. Magnification, 200X.
Renal allograft sections were prepared from each recipient group on day 15 post-transplant and stained to detect C4d deposition and leukocyte infiltration into the allografts. Allografts from control IgG-treated B6.huCD20/CCR5−/− recipients showed strong and diffuse C4d deposition in the peritubular capillaries and glomeruli (Figure 5D) when compared to allografts from wild type C57BL/6 recipients (Figure 5C). In allografts from recipients treated with anti-huCD20 mAb on days 5, 8 and 12 post-transplant C4d deposition was clearly present but decreased in intensity (Figure 5E). A/J allografts from wild type C57BL/6 recipients had prominent peri-arterial mononuclear infiltrates compared to isografts (Figure 5G vs. F). A/J allografts from control IgG-treated B6.huCD20/CCR5−/− recipients had marked edema and the initiation of fibrosis with fewer mononuclear infiltrates when compared to allografts from wild type C57BL/6 recipients (Figure 5H vs. G), consistent with antibody-mediated injury. Allografts from anti-huCD20 mAb-treated B6.huCD20/CCR5−/− recipients showed mild scattered infiltrates and no edema on day 15 post-transplant with no indication of fibrosis (Figure 5I).
Renal allografts were also harvested on days 15/16 and 60 post-transplant from B6.huCD20/CCR5−/− renal allograft recipients treated with control IgG and anti-huCD20 mAb on days 5, 8 and 12 and prepared paraffin-embedded sections were stained to detect graft infiltrating T cells and macrophages. Control treated allografts had intense and diffuse CD3+ and Mac2+ cell infiltrates on day 15 post-transplant that included severe tubulitis and many cells of each type in the glomeruli (Figure 6A and B). Allografts harvested on day 16 post-transplant from recipients treated with anti-huCD20 mAb had similar histopathologies with respect to the diffuse CD3+ and Mac2+ cell infiltrates into the tubules and glomeruli (Figure 6C and D). In contrast, allografts harvested on day 60 post-transplant from recipients treated with anti-huCD20 mAb had decreased but scattered CD3+ and Mac2+ cell infiltrates with almost no detectable CD3+ T cells but numerous Mac2+ cells within the glomeruli (Figure 6E and F).
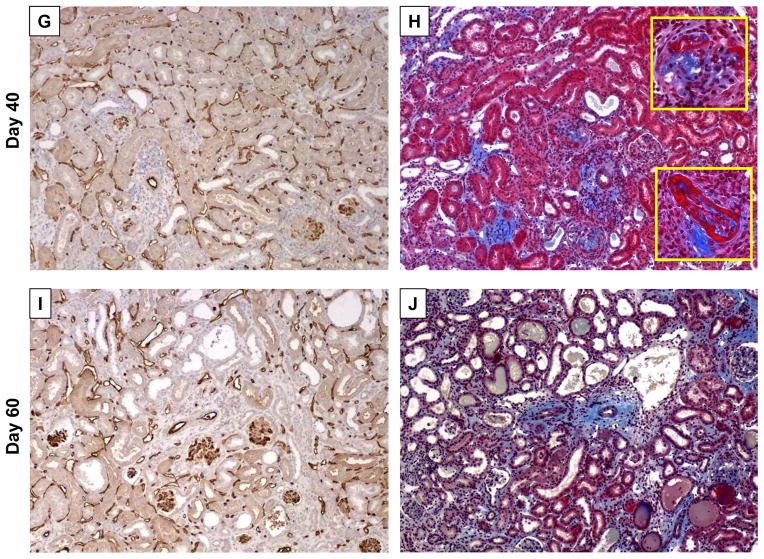
Figure 6. Development of severe interstitial fibrosis and diffuse C4d deposition in renal allografts from B6.huCD20/CCR5−/− recipients treated with anti-huCD20 mAb treatment on days 5, 8 and 12 post-transplant.
Groups of B6.huCD20/CCR5−/− mice received complete MHC-mismatched A/J kidneys and were treated with 250 μg/day control IgG or anti-huCD20 mAb on days 5, 8 and 12 post-transplant (n = 4–5 for each day of analysis). (A–F) Renal allografts from huCD20/CCR5−/− recipients treated with control IgG were harvested on day 15 (A and B) or from huCD20/CCR5−/− recipients treated with anti-huCD20 mAb were harvested on day 16 (C and D) or day 60 (E and F). Paraffin-embedded sections were prepared and stained with anti-CD3 (A, C and E) or with anti-Mac2 (B, D and F) antibody. Magnification, 100X for large panels with 400X inserts. (G–J) On days 40 and 60 post-transplant, allografts were harvested from B6.huCD20/CCR5−/− recipients treated with anti-huCD20 mAb on days 5, 8 and 12 post-transplant. Paraffin-embedded sections were stained with anti-mouse C4d mAb to detect deposition of C4d (G and I) or Masson Trichrome to detect collagen deposition (H and J). Representative images from 4 grafts per group are shown. Magnification, 200X for large panels and 400X for inserts in panel H.
Sections of renal allografts harvested on days 40 and 60 post-transplant from B6.huCD20/CCR5−/− recipients treated with anti-huCD20 mAb on days 5, 8 and 12 post-transplant were also stained to detect C4d deposition and with Masson Trichrome. In parallel with the development of DSA (Figure 4B), strong diffuse C4d deposition was observed in the tubular capillaries and glomeruli of the allografts (Figure 6G and I). Masson Trichrome staining also revealed pathologic characteristics of chronic AMR on day 40 post-transplant, including development of periarterial fibrosis, fibrinoid necrosis of arteries and glomeruli, thickened glomerular basement membrane and increased mesangial matrix as well as segmental and global glomerulosclerosis (Figure 6H) and this pathology increased to marked glomerular and periarterial fibrosis with tubular atrophy and peritubular fibrosis by day 60 post-transplant (Figure 6J).
Development of the fibrotic gene signature in long-term surviving renal allografts experiencing antibody-mediated injury
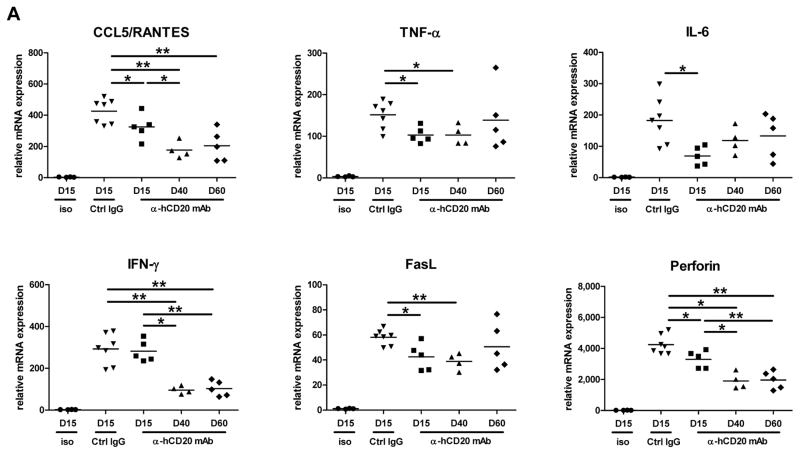
A/J renal allografts from B6.huCD20/CCR5−/− recipients treated with control IgG or anti-human CD20 mAb on days 5, 8 and 12 were harvested at day 15 and/or later time points post-transplant. The expression of genes associated with acute antibody- and/or cell-mediated rejection and with the development of interstitial fibrosis was assessed by preparing whole cell RNA and performing qPCR (Figure 7). Expression of CCL5, FasL and perforin as well as TNF-α, IL-6 was elevated during acute antibody-mediated injury on day 15 post-transplant, but not in allografts from recipients treated with anti-huCD20 mAb and remained low through day 60 (Figure 7A).
Figure 7. Expression of pro-inflammatory/pro-fibrogenic mRNA in allografts from B6.huCD20/CCR5−/− recipients treated with control IgG or anti-huCD20 mAb on days 5, 8 and 12 post-transplant.
Groups of B6.huCD20/CCR5−/− mice received complete MHC-mismatched A/J kidneys and were treated with 250 μg/day control IgG (n = 7) or anti-huCD20 mAb on days 5, 8 and 12 post-transplant (n = 4–5 for each day of analysis). Wild type C57BL/6 mice received syngeneic kidney grafts as a negative control (n = 4). On the indicated day, kidneys were harvested and whole cell RNA was prepared and analyzed by qRT/PCR for expression of the indicated (A) acute inflammatory and (B) fibrogenesis genes. Data indicate expression levels of test gene in allografts from individual recipients with the bars indicating the mean expression levels for each group on the indicated day. *P < 0.05; **P < 0.01.
On day 15 post-transplant the expression of genes associated with the development of fibrosis (CTGF, VCAM-1, PCAM-1, P-selectin, E-cadherin, and N-cadherin) were expressed at high levels in renal allografts during the acute antibody-mediated injury observed from control Ig treated B6.huCD20/CCR5−/− recipients when compared to isografts (Figure 7B). The expression levels of these genes significantly decreased in allografts from recipients treated with anti-huCD20 mAb on day 15 post-transplant, but then increased to high levels during the development of the interstitial allograft fibrosis on days 40 and 60.
Discussion
The increased incidence of antibody-mediated graft rejection observed in clinical transplantation as well as evidence implicating DSA in chronic graft injury has prompted the need for appropriate models to test mechanisms of antibody mediated tissue injury. Acute rejection of kidney allografts in CCR5−/− recipients is dependent on DSA and exhibits the pathological features of AMR in clinical renal transplantation, including complement activation in the peritubular capillaries, margination of neutrophils and macrophages in the peritubular capillaries and glomerulopathy that precipitate graft failure (1, 2, 18). This model is useful in studying the development of acute antibody-mediated graft tissue injury from the time of the transplant when there is no detectable DSA to the time when high serum titers of DSA are achieved. This model, however, has not allowed us to test the impact of interrupting an ongoing DSA response on graft outcome as is observed in clinical transplants or to achieve development of the interstitial fibrosis and/or nephropathy observed in chronic graft injury. In this study, we crossed transgenic mice expressing human CD20 on B cells with the CCR5−/− mice to generate huCD20/CCR5−/− mice for use as renal allograft recipients.
Whereas control-treated huCD20/CCR5−/− recipients rejected complete MHC-mismatched renal allografts by day 26, prophylactic treatment with anti-huCD20 mAb prior to the transplant and weekly up to 7 weeks post-transplant promoted long-term allograft survival (> 100 days) with low creatinine levels and DSA titers. These results suggested that prophylactic treatment of huCD20/CCR5−/− renal allograft recipients not only depletes recipient B cells, but also decreases B cell activation to make DSA following their recovery in the treated recipients. Whether the low/absent DSA in these treated recipients at later times post-transplant is due to deletion of alloantigen-reactive B cells, their inactivation, or in modulation of the T helper components required for antibody generation is unknown at this time. Possibilities that the anti-CD20 mAb provokes changes in the B cell populations producing allograft reactive antibodies or in their specificity or pathogenicity also need to be considered and are under investigation. Nevertheless, the absence of overt graft tissue injury in long-term surviving grafts from recipients prophylactically treated with anti-CD20 mAb is consistent with recent reports indicating that B cell depletion by pre-transplant Rituximab induction might play a role in preventing the onset of chronic AMR (24, 25).
In contrast to prophylactic pre-transplant B cell depletion, administration of anti-CD20 mAb at the time when DSA was first detected in recipient serum (i.e. day 5 post-transplant) yielded a different allograft outcome. The anti-huCD20 mAb significantly reduced donor-reactive antibody titers and graft inflammation on day 15 post-transplant and prolonged allograft survival > 60 days. However, the DSA returned to the titers observed in control treated recipients by day 30 post-transplant. Although the allografts survived up to day 75 post-transplant, they developed a progressive and fulminant interstitial fibrosis, reminiscent of that observed during late failure of clinical renal grafts (26). This chronic allograft injury was also observed when treatment was continued weekly after the day 12 dose up to day 50, suggesting that the low titers of DSA generated at the time of anti-CD20 mAb administration were sufficient to initiate the development of the chronic injury (data not shown). The return of the DSA to high titers in the anti-CD20 mAb-treated recipients without quickly provoking acute rejection raises several questions about differences in DSA mediating acute vs. chronic renal allograft injury in this model. We have considered 3 potential mechanisms that are not mutually exclusive. First, DSA mediating the acute vs. chronic injury might have differences in specificity and/or isotype that would confer different functions in mediating inflammation upon binding to antigens in the graft. An obvious second possibility is that the time at which peak DSA titers are achieved (e.g. rapid vs. gradual) impacts the type of inflammation induced by antibody binding to alloantigens in the graft that leads to acute injury vs. the development of fibrosis. A third consideration is that during the course of the low titers of DSA first observed in the anti-CD20 mAb-treated recipients, the graft is induced to express protective mechanisms that disrupt progression of acute injury but fails to cease the initiation of an injury program leading to progressive interstitial fibrosis. We have considered a fourth possibility that the short administration of the humanized anti-human CD20 mAb results in the production of anti-human IgG antibodies that neutralize the remaining therapeutic mAb to accelerate B cell recovery and possibly change the B cell populations present and/or those that return during reconstitution of the B cell compartment. However, we have tested sera taken at 40–60 days post-transplant from renal allograft recipients treated with the delayed regimen of anti-human CD20 mAb and have not detected the presence of anti-human IgG by ELISAs that include positive anti-human IgG antibody controls (data not shown). Each of these potential mechanisms warrants more rigorous investigation to obtain further insights into mechanisms underlying antibody-mediated acute vs. chronic injury of renal allografts.
Consistent with the second mechanism, analysis of molecular markers in the kidney allografts during antibody mediated acute and chronic injury revealed distinct differences. Antibody-mediated acute injury induced high expression of mRNA encoding inflammatory mediators, including TNFα, IL-6, CCL5, perforin and FasL. Expression of these genes was significantly lower during the progression of chronic injury/interstitial fibrosis than the levels observed during acute injury and remained low until the time of fulminant fibrosis and graft failure. These decreases indicate attenuated inflammatory mediator expression in renal allografts as early as day 15 post-transplant in recipients treated with anti-human CD20 mAb on days 5, 8 and 12 post-transplant. However, histological examination of the allografts at this time indicated no obvious decrease in T cell or macrophage graft infiltration (Figure 6), suggesting DSA and/or B cells may play key roles in provoking T cell and/or macrophage inflammatory function that contributes to graft failure. It is important to note that complete recovery of DSA and B cells is not observed until day 30–40 post-transplant (Figure 4). Recent studies have implicated IL-6 producing B cells as playing an important role in T cell-mediated autoimmunity of the central nervous system (27) and raise the possibility that anti-human CD20 mAb-mediated depletion of B cells during the initiation of acute antibody-mediated rejection removes a source of IL-6 that is an important factor in positively modulating T cell and/or macrophage activation with the renal allograft.
The expression of genes associated with the development of fibrosis, including CTGF, PCAM-1, E- and N-cadherin and P-selectin, were evident at high levels during acute AMR and lower in grafts that went on to develop interstitial fibrosis/chronic injury. However, expression of these genes progressively increased as the DSA titers rose following recovery from the anti-CD20 mAb treatment. These results indicate distinct expression patterns in genes associated with acute vs. chronic injury in that expression of acute injury genes is low and remains low throughout the development of the chronic injury, even when DSA titers are similar to those observed during acute antibody-mediated injury. These different gene expression patterns suggest that within the context of fibrogenesis within the renal allograft, slowly rising titers of DSA induce expression of the pro-fibrotic but not the acute injury genes.
In summary, we have developed a novel model of AMR that can be manipulated to achieve long-term renal allograft survival, acute antibody-mediated rejection, or the development of the indolent antibody-mediated chronic injury that is proposed to account for the majority of late loss of clinical kidney transplants. Potential limitations of this model are the very rapid appearance of high titers of de novo DSA in response to the renal allograft, the absence of inherent mechanisms regulating this antibody response, and the absent use of any other forms of immunosuppression that is likely to influence the prolongation of B cell depletion following anti-human CD20 mAb therapy and the function of the B cells that reconstitute the treated graft recipients. The development of the acute vs. chronic renal allograft injury in this mouse model is characterized by marked differences in the kinetics of the DSA response that is likely to underlie the differences in the injury observed. The development of acute vs. chronic graft injury in this model is also associated with distinct patterns of gene expression, primarily the low expression of genes associated with acute injury during the development of interstitial fibrosis. In conjunction with serum DSA levels, these gene expression profiles are likely to be useful in monitoring recipients with long-term surviving renal grafts for development of chronic injury. The results of this study suggest several potential mechanisms that may provoke acute vs. chronic renal allograft injury and further investigation of these mechanisms should provide novel clues for the diagnosis and treatment of AMR in transplant patients.
Acknowledgments
The authors thank the staff of the Cleveland Clinic Biological Resources Unit for excellent care of the animals used in this study and Genentech for providing the human CD20 transgenic mice. The authors also thank members of the Fairchild and Valujskikh lab for helpful comments during the course of this work.
This work was supported by grant NIH PO1 AI087506 from the NIAID/NIH to AV, WMB, and RLF.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–17. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 2.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria-an addition ot the Banff ‘97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–14. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 3.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033–41. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 4.Uber WE, Self SE, van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart trasnplantation. Am J Transplant. 2007;7:2064–74. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, et al. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol. 2004;164:849–59. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gloor J, Cosio F, Lager DJ, Stegall MD. The spectrum of antibody-mediated renal allograft injury: implications for treatment. Am J Transplant. 2008;8:1367–73. doi: 10.1111/j.1600-6143.2008.02262.x. [DOI] [PubMed] [Google Scholar]
- 7.Saadl S, Holzknecht RA, Patte CP, Platt JL. Endothelial cell activation by pore-forming structures: pivotal role for interleukin-1a. Circulation. 2000;101:1867–73. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- 8.Saadl S, Platt JL. Humoral rejection and endothelial cell activation. Xenotransplantation. 2002;9:239–41. doi: 10.1034/j.1399-3089.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 9.Taflin C, Charron D, Glotz D, Mooney N. Immunological function of the endothelial cell within the setting of organ transplantation. Immunol Lett. 2011;139:1–6. doi: 10.1016/j.imlet.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Reed EF. Effect of antibodies on endothelium. Am J Transplant. 2009;9:2459–65. doi: 10.1111/j.1600-6143.2009.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation by complement C3a and C5aq anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003–14. doi: 10.1096/fj.02-0737com. [DOI] [PubMed] [Google Scholar]
- 12.Terasaki P, Mizutani K. Antibody mediated rejection: update 2006. Clin J Am Soc Nephrol. 2006;1:400–3. doi: 10.2215/CJN.02311205. [DOI] [PubMed] [Google Scholar]
- 13.Trpkov K, Campbell P, Pazderka F, Cockfield S, Solez K, Halloran PF. Pathologic features of acute renal allograft rejection associated with donor-specific antibody. Analysis using the Banff grading schema. Transplantation. 1996;61:1586–92. doi: 10.1097/00007890-199606150-00007. [DOI] [PubMed] [Google Scholar]
- 14.Regele H, Bohmig GA, Habicht A, Gollogwitzer D, Schillinger M, Rockenshaub S, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371–80. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- 15.Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8:348–57. doi: 10.1038/nrneph.2012.81. [DOI] [PubMed] [Google Scholar]
- 16.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, et al. Antibody-mediated microcirculation injury is the major casue of late kidny transplant failure. Am J Transplant. 2009;9:2520–31. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 17.Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:389–99. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 18.Bickerstaff A, Nozaki T, Wang J-J, Pelletier R, Hadley G, Nadasdy G, et al. Acute humoral rejection of renal allografts in CCR5−/− recipients. Am J Transplant. 2008;8:557–66. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 19.Hattori Y, Bucy RP, Kubota Y, Baldwin WM, III, Fairchild RL. Antibody-mediated rejection of single class I MHC-disparate cardiac allografts. Am J Transplant. 2012;12:2017–28. doi: 10.1111/j.1600-6143.2012.04073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nozaki T, Amano H, Bickerstaff A, Orosz CG, Novick AC, Tanabe K, et al. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. J Immunol. 2007;179:5238–45. doi: 10.4049/jimmunol.179.8.5238. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16:103–9. doi: 10.1002/micr.1920160212. [DOI] [PubMed] [Google Scholar]
- 22.Han WR, Murray-Segal LJ, Mottram PL. Modified technique for kidney transplantatiion in mice. Microsurgery. 1999;19:272–4. doi: 10.1002/(sici)1098-2752(1999)19:6<272::aid-micr3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Murata K, Fox-Talbot K, Qian Z, Takahashi K, Stahl GL, Baldwin WM, III, et al. Synergistic dpeosition of C4d by complement-activating and non-activating antibodies in cardiac transplants. Am J Tranasplant. 2007;7:2605–2614. doi: 10.1111/j.1600-6143.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 24.Loupy A, Suberbielle-Boissel C, Zuber J, DA, Timsit MO, Martinez F, et al. Combined posttransplant prophylactic IVIg/anti-CD20/plasmaphoresis in kidney recipients with preformed donor-specific antibodies: a pilot study. Transplantation. 2010;89:1403–10. doi: 10.1097/TP.0b013e3181da1cc3. [DOI] [PubMed] [Google Scholar]
- 25.Kohei N, Hirai T, Omoto K, Ishida K, Tanabe K. Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. Am J Transplant. 2012;12:469–76. doi: 10.1111/j.1600-6143.2011.03830.x. [DOI] [PubMed] [Google Scholar]
- 26.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, et al. Banff ‘05 Meeting report: Differential diagnosis of chronic allograft injury ad elimination of chronic allograft nephropathy (“CAN”) Am J Transplant. 2007;7:518–26. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 27.Barr TA, Shen P, Brown S, Lanpropoulou V, Roch T, Lawrie S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]