Abstract
Background
The lower-expressing (S′) alleles of the serotonin transporter (5-HTT) gene promoter polymorphism (5-HTTLPR) are linked to mood and anxiety related psychopathology. However, the specific neural mechanism through which these alleles may influence emotional and cognitive processing remains unknown. We examined the relationship between both 5-HTTLPR genotype and in vivo 5-HTT binding quantified via PET with amygdala reactivity to emotionally negative stimuli. We hypothesized that 5-HTT binding in both raphe nuclei (RN) and amygdala would be inversely correlated with amygdala reactivity, and that number of S′ alleles would correlate positively with amygdala reactivity.
Methods
In medication-free patients with current major depressive disorder (MDD; N = 21), we determined 5-HTTLPR genotype, employed functional magnetic resonance imaging (fMRI) to examine amygdala responses to negative emotional stimuli, and used positron emission tomography with [11C]DASB to examine 5-HTT binding.
Results
[11C]DASB binding in RN and amygdala was inversely correlated with amygdala response to negative stimuli. 5-HTTLPR S′ alleles were not associated with amygdala response to negative emotional stimuli.
Limitations
Primary limitations are small sample size and lack of control group.
Conclusions
Consistent with findings in healthy volunteers, 5-HTT binding is associated with amygdala reactivity to emotional stimuli in MDD. 5-HTT binding may be a stronger predictor of emotional processing in MDD as compared with 5-HTTLPR genotype.
Keywords: Depression, Genetics, Functional MRI, Biological Markers, Brain Imaging
1. Introduction
Triallelic variation of the upstream, human serotonin transporter-promotor polymorphic region (5-HTTLPR) comprises one short (S) and two long (LG and LA) variants, of which the S and LG variants (together designated as S′) have comparably lower transcriptional efficiency in vitro compared with LG (designated L′) (Heils et al., 1996; Hu et al., 2006; Lesch et al., 1996; Mortensen et al., 1999). Low-expressing alleles are associated with elevated amygdala reactivity to threat (Hariri et al., 2002) and other negative stimuli (see Murphy et al. (2012) for a review), and are a risk factor for psychopathology such as major depressive disorder (MDD) in context of life stress (Caspi et al., 2003). However, the mechanism by which genotype at this locus confers risk for psychopathology remains unknown.
One hypothesis is that low-expressing 5-HTTLPR polymorphisms alter intra-synaptic serotonin levels, leading to increased amygdala activity (Fisher et al., 2009, 2006; Hariri and Holmes, 2006). Although 5-HTTLPR genotype affects 5-HTT mRNA expression in vitro (Heils et al., 1996; Hu et al., 2006; Lesch et al., 1996; Mortensen et al., 1999), in vivo studies using positron emission tomography (PET) imaging report both null findings (Guzey et al., 2012; Kobiella et al., 2011; Miller et al., 2013; Murthy et al., 2010; Oquendo et al., 2007; Parsey et al., 2006a; Rhodes et al., 2007; Shioe et al., 2003) and decreased 5-HTT binding associated with the S′ allele (Kalbitzer et al., 2009; Praschak-Rieder et al., 2007; Reimold et al., 2007).
Although S′ alleles are associated with greater amygdala responses to negative stimuli (reviewed in (Murphy et al., 2012)), less is known about the relationship between 5-HTT protein levels and amygdala reactivity. 5-HTT binding in amygdala is inversely correlated with amygdala responses to negative stimuli in healthy controls and depressed subjects (Rhodes et al., 2007; Ruhe et al., 2014). However, the same studies disagree regarding the relationship between raphe nuclei (RN) 5-HTT binding and amygdala activity. These discrepant findings justify further study of regional 5-HTT binding with respect to amygdala reactivity.
We sought to clarify relationships between 5-HTTLPR genotype, regional 5-HTT binding, and amygdala responses to negative stimuli in currently depressed MDD subjects. Based on the regulatory role of serotonergic projections to the amygdala (Jasinska et al., 2012; Jiang et al., 2009; Rainnie, 1999), we hypothesized that 5-HTT binding in both RN and amygdala would be inversely correlated with amygdala reactivity. Given that the S′ allele is associated with lower transcriptional efficiency of 5-HTT in vitro, we predicted that S′ allele number would be positively correlated with amygdala reactivity. This is the first study, to our knowledge, that combines these genetic and neuroimaging approaches in a study of MDD.
2. Methods
2.1. Sample
We studied a convenience sample of depressed subjects who had undergone a multimodal fMRI/PET imaging study including: (1) the negative emotional faces fMRI task employed in previous studies examining amygdala reactivity (Fisher et al., 2009, 2006; Hariri et al., 2002); (2) quantification of 5-HTT binding using PET with [11C]DASB; and (3) genotyping of the 5-HTTLPR. A subset of these subjects had also undergone PET scanning with [11C]WAY-100635 to quantify 5HT1A receptor binding, allowing for accurate localization of raphe nuclei. The average time between MRI and PET scan was M = 7, SD ± 6 days.
Subjects (n = 21) with current MDD based on the Structured Clinical Interview for DSM-IV, Axis I (SCID-I) (Spitzer et al., 1992) enrolled in this study. Complete eligibility criteria are enumerated in S1.
2.2. Genotyping
Genotype classification followed the method described in Parsey et al. (2006a). S or LG alleles were classified as an S′ allele and the LA allele (which is higher-expressing in vitro (Hu et al., 2005)) was classified as the L′ allele. Subjects were classified into 3 functional genotypes: L′L′, L′S′, S′S′. Correlations between S′ allele and demographic variables were calculated in SPSS (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc) using Pearson and Spearman correlations for normally and non-normally distributed variables.
2.3. PET protocol
Details of the PET protocol have been described elsewhere (Ogden et al., 2007) and are included in Supplemental information.
2.4. MRI image acquisition
MRI scans were acquired on a 3T SignaHDx scanner (General Electric Medical Systems, Milwaukee, WI). Full acquisition parameters are described in S1. EPI acquisition was acquired with a TR = 2000 msec and voxel dimension = 3.75 × 3.75 × 5 mm3.
2.5. fMRI task
The task was modeled after the task reported in Hariri et al. (2002). In this blocked ABAB design, subjects viewed a screen with three pictures of either angry/fearful faces or shapes in a triangular arrangement. They were instructed to determine which of the top two stimuli matches the bottom one. Stimuli were presented for four seconds, with inter-stimulus interval of two to six seconds. Shape stimuli were presented four times per block and face stimuli six times. Five face and four shapes blocks were presented.
2.6. MRI image processing
MRI Image processing is described in S1.
2.7. PET analysis
PET image processing is described further in S1. Because no brain region is devoid of specific binding with [11C]DASB (Parsey et al., 2006b), we used an outcome measure that does not rely on a reference region: VT/fP where VT = volume of distribution in the region of interest and fP is the plasma free-fraction (Chin et al., 2011; Esterlis et al., 2010; Fujita et al., 2012; Ichise, 2009; Mukhin et al., 2008). [11C]DASB regional VT values were derived using likelihood estimation in the graphical approach (LEGA) (Ogden, 2003; Parsey et al., 2003). Brain activity was corrected for the contribution of plasma activity assuming a 5% blood volume in regions of interest (Mintun et al., 1984). To facilitate comparison to other [11C]DASB studies using different outcome measures, the following outcome measures were also estimated: BPF* ((VT(ROI) − VT(REF))/fP); BPP* (VT(ROI) − VT(REF)); and BPND* ((VT(ROI) − VT(REF))/VT(REF)), using cerebellar gray matter as reference region. Asterisks are added to consensus terminology (Innis et al., 2007) to emphasize that the “reference” region does have measureable specific binding (Parsey et al., 2006b), which violates the assumption underlying estimation of these alternative outcome measures. Time activity curves were generated by plotting measured activity within ROIs over the course of PET acquisition.
2.8. fMRI analyses
BOLD data were analyzed using the general linear model to identify voxel-wise parameter estimates to a faces-greater-than-shapes regressor, convolved with a double gamma hemodynamic response function. Activity during the instruction period was covaried as a nuisance regressor. Relative frame displacement for all subjects was less than half the width of a single voxel. Motion parameters were included as nuisance variables.
For all of the following analyses, the minimum Z-score required for significance was set at z≥2.3 (cluster p < 0.05).
2.9. Genotype and 5-HTT
To identify the relationship of 5-HTTLPR genotype to BOLD responses to angry/fearful faces in amygdala, we regressed parameter estimates of task-related activity in the amygdala ROI onto the number of S′ alleles. We selected this approach given in vitro (Hu et al., 2006) and in vivo (Neumeister et al., 2006) evidence that number of triallelic HTTLPR LA alleles exert a dose response on 5-HTT mRNA expression and downstream effects. Amygdala activity was regressed independently onto VT/fP in RN and amygdala. Mean response to faces across the whole brain was calculated.
3. Results
3.1. Sample
Subjects were moderately depressed, with a mean HDRS score of 16.2 ± 6.7 at the time of brain imaging, and a median of 2 previous major depressive episodes. The sample included 5 L′L′ homozygotes, 10 L′S′ heterozygotes, and 6 S′S′ homozygotes. Sample demographics are summarized in Table 1. Number of S′ alleles did not correlate with current depression severity (r = −0.08, p = 0.72) or number of lifetime depressive episodes (rho = 0.37, p = 0.09).
Table 1.
Sample characteristics.
| Mean ± S.D. | |
|---|---|
| HDRS-17 | 16.19 ± 6.65 |
| Age | 41 ± 14.24 |
| Education | 15.9(3.12) |
| Median(range) | |
| Previous Episodes | 2(0–109) |
| Weeks off antidepressant medications | 24(0–364) |
| Frequency (%) | |
| Female | 12(57.1) |
| Ethnicity | |
| White | 13(61.9) |
| American Indian | 1(4.7) |
| Black | 5(23.8) |
| Multi-racial | 2(9.1) |
| Medication Naïve | 9(42.8) |
| First episode depression | 8(38.1) |
| Smoking | 3(14.2) |
| Co-morbid Anxiety | 8(38.3) |
| Suicide attempt | 4(19.1) |
3.2. Amygdala responses to negative stimuli: relationship to 5-HTT binding and 5-HTTLPR genotype
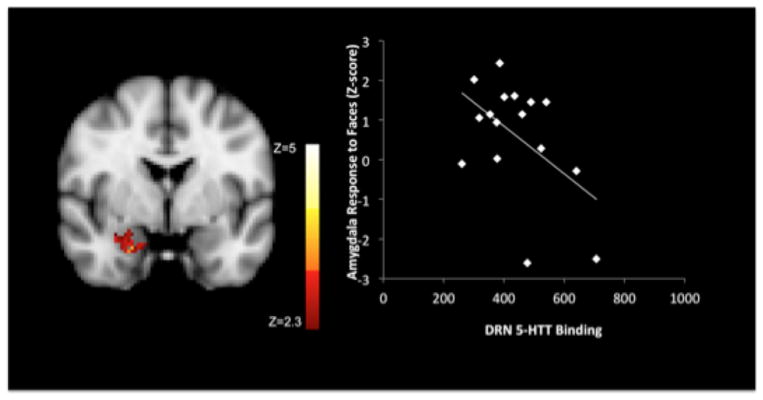
Higher 5-HTT VT/fP in the RN was associated with lower right (not left) amygdala responses to angry and fearful faces (Fig. 1, Cluster size 138 voxels, mean z-score −2.7). Higher 5-HTT VT/fP in amygdala bilaterally was associated with lower right amygdala response to faces (Cluster size 85 voxels, mean z-score −2.6). The mean neural response to faces across subjects revealed significant clusters in amygdala, midbrain (close to raphe nuclei), occipital and temporal fusiform gyrii, hippocampus, posterior and anterior cingulate (data not shown). A repeated main analyses using alternate PET outcome measures, revealed a predicted inverse relationship between RN BPF* and bilateral amygdala BOLD fMRI responses to faces, consistent with VT/fP findings. In contrast, RN BPP* and BPND* were not correlated with amygdala responses to faces.
Fig. 1.
Cluster of voxels in right amygdala whose response to faces is negatively associated with 5-HTT binding in the RN. Scatterplot shows correlation of average z-score in amygdala cluster with RN 5-HTT binding.
There were no clusters where 5-HTTLPR genotype was associated with response to faces in either the full 21-subject sample or the 16 subjects with multimodal PET and fMRI data.
4. Discussion
Consistent with our hypotheses, less 5-HTT binding in RN and amygdala was associated with greater amygdala reactivity assessed by fMRI. In contrast, 5-HTTLPR genotype was not associated with amygdala reactivity.
4.1. 5-HTT and neural response to negative stimuli
Our findings indicate that 5-HTT binding levels are related to emotion processing. 5-HTT levels may index serotonergic fiber density or intra-synaptic serotonin concentrations (Descarries et al., 1995; Soucy et al., 1994). Low RN 5-HTT binding may therefore indicate diminished RN serotonergic modulation of GABAergic neurotransmission in the amygdala (Bauman and Amaral, 2005; Jiang et al., 2009; O’Rourke and Fudge, 2006; Rainnie, 1999; Stutzmann and LeDoux, 1999). These data partially replicate and extend previous findings correlating regional [11C]DASB binding to amygdala responses in healthy volunteers. In one study, a negative relationship was observed between right amygdala BOLD fMRI activity and midbrain [11C]DASB BPND (Kobiella et al., 2011). In another study, a negative relationship was reported between amygdala reactivity and [11C]DASB BPP in the amygdala, but not in RN (Rhodes et al., 2007). Our study is notable for using within-subject PET images of the 5-HT1A somatodendritic receptor to identify the RN individually, and for its examination of this relationship in currently depressed medication-free subjects with MDD.
4.2. 5-HTTLPR and neural response to negative stimuli
5-HTTLPR was not associated with activity in amygdala in response to angry and fearful faces in our study. Our results diverge from some earlier studies in healthy controls that found the S′ allele associated with amygdala responses to negative stimuli (Costafreda et al., 2013; Hariri et al., 2002; Morey et al., 2011; von dem Hagen et al., 2011). Some studies have reported that the relationship between S′ allele and amygdala reactivity is specific to healthy volunteers (Friedel et al., 2009; Gillihan et al., 2011; Rao et al., 2007) while others have observed this effect in MDD (Dannlowski et al., 2007). The lack of observed genetic effect on amygdala responses may also be related to power limitations given the modest sample size in this study.
The association between RN 5-HTT binding and amygdala activity was limited to the right amygdala. Lateralized amygdala findings may be related to phase encoding direction artifact (Mathiak et al., 2012). However, other analyses in this study showed bilateral amygdala activity (group mean response to faces, and correlations with 5-HTT BPF*). It is therefore less likely that phase encoding artifact explains left lateralization for one analysis only. Most studies of the amygdala report lateralized findings, which may reflect actual biological differences (Baas et al., 2004).
4.3. Limitations
The absence of genotype associations with amygdala response to negative emotional stimuli may be due to the modest sample size. Statistical power likewise was insufficient for analysis of potential gene-environment interactions that might be related to amygdala activity. While we conducted rigorous correction for multiple comparisons within whole-brain voxelwise analyses, we did not correct across analyses. These analyses should be regarded as hypothesis-generating, and require replication in larger samples. The lack of a healthy volunteer group precluded determination of depression-specific effects.
4.4. Conclusion
We found greater right amygdala reactivity to be associated with less RN 5-HTT binding but not 5-HTTLPR genotype. Future studies could employ more specific tasks and include a healthy volunteer group to further delineate the neural correlates of 5-HTT and 5-HTTLPR linked to depression.
Supplementary Material
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jad.2015.10.047.
References
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res: Brain Res Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Amaral DG. The distribution of serotonergic fibers in the macaque monkey amygdala: an immunohistochemical study using antisera to 5-hydroxytryptamine. Neuroscience. 2005;136:193–203. doi: 10.1016/j.neuroscience.2005.07.040. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chin CL, Carr RA, Llano DA, Barret O, Xu H, Batis J, Koren AO, Seibyl JP, Marsh KC, Tamagnan G, Decker MW, Day M, Fox GB. Pharmacokinetic modeling and [123]5-IA-85380 single photon emission computed tomography imaging in baboons: optimization of dosing regimen for ABT-089. J Pharmacol Exp Ther. 2011;336:716–723. doi: 10.1124/jpet.110.173609. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, McCann P, Saker P, Cole JH, Cohen-Woods S, Farmer AE, Aitchison KJ, McGuffin P, Fu CH. Modulation of amygdala response and connectivity in depression by serotonin transporter polymorphism and diagnosis. J Affect Disord. 2013;150:96–103. doi: 10.1016/j.jad.2013.02.028. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, Kersting A, Arolt V, Heindel W, Deckert J, Suslow T. Serotonergic genes modulate amygdala activity in major depression. Genes Brain Behav. 2007;6:672–676. doi: 10.1111/j.1601-183X.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- Descarries L, Soucy JP, Lafaille F, Mrini A, Tanguay R. Evaluation of three transporter ligands as quantitative markers of serotonin innervation density in rat brain. Synapse. 1995;21:131–139. doi: 10.1002/syn.890210206. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Cosgrove KP, Batis JC, Bois F, Stiklus SM, Perkins E, Seibyl JP, Carson RE, Staley JK. Quantification of smoking-induced occupancy of beta2-nicotinic acetylcholine receptors: estimation of nondisplaceable binding. J Nucl Med: Off Publ Soc Nucl Med. 2010;51:1226–1233. doi: 10.2967/jnumed.109.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, Moses-Kolko EL, Berga SL, Hariri AR. Medial prefrontal cortex 5-HT(2A) density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex. 2009;19:2499–2507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Friedel E, Schlagenhauf F, Sterzer P, Park SQ, Bermpohl F, Strohle A, Stoy M, Puls I, Hagele C, Wrase J, Buchel C, Heinz A. 5-HTT genotype effect on prefrontal-amygdala coupling differs between major depression and controls. Psychopharmacology. 2009;205:261–271. doi: 10.1007/s00213-009-1536-1. [DOI] [PubMed] [Google Scholar]
- Fujita M, Hines CS, Zoghbi SS, Mallinger AG, Dickstein LP, Liow JS, Zhang Y, Pike VW, Drevets WC, Innis RB, Zarate CA., Jr Downregulation of brain phosphodiesterase type IV Measured with 11C-(R)-rolipram positron emission tomography in major depressive disorder. Biol Psychiatry. 2012;72:548–554. doi: 10.1016/j.biopsych.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillihan SJ, Rao H, Brennan L, Wang DJ, Detre JA, Sankoorikal GM, Brodkin ES, Farah MJ. Serotonin transporter genotype modulates the association between depressive symptoms and amygdala activity among psychiatrically healthy adults. Psychiatry Res. 2011;193:161–167. doi: 10.1016/j.pscychresns.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzey C, Allard P, Brannstrom T, Spigset O. Radioligand binding to brain dopamine and serotonin receptors and transporters in Parkinson disease: relation to gene polymorphisms. Int J Neurosci. 2012;122:124–132. doi: 10.3109/00207454.2011.631716. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M. Neuroreceptor Imaging and Kinetic Modeling. In: Van Heertum RL, Tikofsky RS, Ichise M, editors. Functional Cerebral SPECT and PET Imaging. 4. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. p. 44. [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Lowry CA, Burmeister M. Serotonin transporter gene, stress and raphe-raphe interactions: a molecular mechanism of depression. Trends Neurosci. 2012;35:395–402. doi: 10.1016/j.tins.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology. 2009;34:410–423. doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- Kalbitzer J, Frokjaer VG, Erritzoe D, Svarer C, Cumming P, Nielsen FA, Hashemi SH, Baare WF, Madsen J, Hasselbalch SG, Kringelbach ML, Mortensen EL, Knudsen GM. The personality trait openness is related to cerebral 5-HTT levels. Neuroimage. 2009;45:280–285. doi: 10.1016/j.neuroimage.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Kobiella A, Reimold M, Ulshofer DE, Ikonomidou VN, Vollmert C, Vollstadt-Klein S, Rietschel M, Reischl G, Heinz A, Smolka MN. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Transl Psychiatry. 2011;1:e37. doi: 10.1038/tp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Mathiak KA, Zvyagintsev M, Ackermann H, Mathiak K. Lateralization of amygdala activation in fMRI may depend on phase-encoding polarity. MAGMA. 2012;25:177–182. doi: 10.1007/s10334-011-0285-4. [DOI] [PubMed] [Google Scholar]
- Miller JM, Hesselgrave N, Ogden RT, Sullivan GM, Oquendo MA, Mann JJ, Parsey RV. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biol Psychiatry. 2013;74:287–295. doi: 10.1016/j.biopsych.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- Morey RA, Hariri AR, Gold AL, Hauser MA, Munger HJ, Dolcos F, McCarthy G. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry. 2011;11:76. doi: 10.1186/1471-244X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen OV, Thomassen M, Larsen MB, Whittemore SR, Wiborg O. Functional analysis of a novel human serotonin transporter gene promoter in immortalized raphe cells. Brain Res: Mol Brain Res. 1999;68:141–148. doi: 10.1016/s0169-328x(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F FA-85380. J Nucl Med: Off Publ Soc Nucl Med. 2008;49:1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, Godlewska BR, Cowen PJ, Mannie ZM, Harmer CJ, Munafo MR. The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Mol Psychiatry. 2012;18:512–520. doi: 10.1038/mp.2012.19. [DOI] [PubMed] [Google Scholar]
- Murthy NV, Selvaraj S, Cowen PJ, Bhagwagar Z, Riedel WJ, Peers P, Kennedy JL, Sahakian BJ, Laruelle MA, Rabiner EA, Grasby PM. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5-HTT to [11C]DASB binding in the living human brain. Neuroimage. 2010;52:50–54. doi: 10.1016/j.neuroimage.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, Herscovitch P, Goldman D, Drevets WC, Charney DS. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry. 2006;63:978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- O’Rourke H, Fudge JL. Distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol Psychiatry. 2006;60:479–490. doi: 10.1016/j.biopsych.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden RT. On estimation of kinetic parameters in graphical analysis of PET imaging data. Stat Med. 2003;22:3557–3568. doi: 10.1002/sim.1562. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Ojha A, Erlandsson K, Oquendo MA, Mann JJ, Parsey RV. In vivo quantification of serotonin transporters using [11C] DASB and positron emission tomography in humans: modeling considerations. J Cereb Blood Flow Metab. 2007;27:205–217. doi: 10.1038/sj.jcbfm.9600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, Goldman D, Arango V, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 2007;64:201–208. doi: 10.1001/archpsyc.64.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006a;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, Arango V, Mann JJ. Acute occupancy of brain serotonin transporter by sertraline as measured by [11C]DASB and positron emission tomography. Biol Psychiatry. 2006b;59:821–828. doi: 10.1016/j.biopsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Mann JJ. Determination of volume of distribution using likelihood estimation in graphical analysis: elimination of estimation bias. J Cereb Blood Flow Metab. 2003;23:1471–1478. doi: 10.1097/01.WCB.0000099460.85708.E1. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Ginovart N, Tharmalingam S, Masellis M, Houle S, Meyer JH. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [11C] DASB positron emission tomography study. Biol Psychiatry. 2007;62:327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GM, Kaercher KA, Brodkin ES, Detre JA, Farah MJ. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry. 2007;62:600–606. doi: 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Schumann G, Zimmer A, Wrase J, Mann K, Hu XZ, Goldman D, Reischl G, Solbach C, Machulla HJ, Bares R, Heinz A. Midbrain serotonin transporter binding potential measured with [11C]DASB is affected by serotonin transporter genotype. J Neural Transm. 2007;114:635–639. doi: 10.1007/s00702-006-0609-0. [DOI] [PubMed] [Google Scholar]
- Rhodes RA, Murthy NV, Dresner MA, Selvaraj S, Stavrakakis N, Babar S, Cowen PJ, Grasby PM. Human 5-HT transporter availability predicts amygdala reactivity in vivo. J Neurosci. 2007;27:9233–9237. doi: 10.1523/JNEUROSCI.1175-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe HG, Koster M, Booij J, van Herk M, Veltman DJ, Schene AH. Occupancy of serotonin transporters in the amygdala by paroxetine in association with attenuation of left amygdala activation by negative faces in major depressive disorder. Psychiatry Res. 2014;221:155–161. doi: 10.1016/j.pscychresns.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, Hirano M, Shinohara M, Kagami M, Okubo Y, Nankai M, Kanba S. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48:184–188. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- Soucy JP, Lafaille F, Lemoine P, Mrini A, Descarries L. Validation of the transporter ligand cyanoimipramine as a marker of serotonin innervation density in brain. J Nucl Med. 1994;35:1822–1830. [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19:RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EA, Passamonti L, Nutland S, Sambrook J, Calder AJ. The serotonin transporter gene polymorphism and the effect of baseline on amygdala response to emotional faces. Neuropsychologia. 2011;49:674–680. doi: 10.1016/j.neuropsychologia.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.