Abstract
Several derivatives of aminobenzoboroxole have been prepared starting from 2-boronobenzaldehyde. All of these derivatives have been evaluated for their anti-mycobacterial activity on Mycobacterium smegmatis and cytotoxicity on breast cancer cell line MCF7. Based on these studies, all the tested molecules have been found to be generally non-toxic and benzoboroxoles with unsubstituted (primary) amines have been found to exhibit good anti-mycobacterial activity. Some of the key compounds have been evaluated for their anti-tubercular activity on Mycobacterium tuberculosis H37Rv using 7H9 and GAST media. 7-Bromo-6-aminobenzoboroxole 4 has been identified as the lead candidate compound for further development.
Keywords: Benzoxaboroles, nitrobenzoxaborole, aminobenzoxaborole, bromoaminobenzoxaborole, anti-tuberculosis agents
Graphical Abstract

Introduction
Tuberculosis, caused by Mycobacterium tuberculosis (M. tuberculosis) is a highly contagious chronic bacterial infection.1 Nearly one third of world’s population has been affected by latent tuberculosis.1a This infection spreads through the air and causes millions of deaths every year. The standard treatment of tuberculosis includes a four-drug regimen of isoniazid, rifampicin, ethambutol, and pyrazinamide for six to nine months. The emergence of multi-drug resistant (MDR-TB) and extensively drug resistant strains of this bacterium is a cause of great concern worldwide (eg. WHO estimates indicate that nearly half million people globally have developed MDR-TB).1a Hence, new and highly effective therapeutic strategies are urgently required for the better treatment of this epidemic. Owing to the global importance of this disease, there has been an explosive growth of publications in the area of tuberculosis research.2 Despite this progress, discovery of biologically active small molecules with novel chemical entities is extremely critical to combat this disease in future.
Benzoboroxoles are cyclic boronic acids that are highly stable under strongly acidic and basic conditions.3 These compounds have been found to have valuable applications in materials4 and medicinal3,5 chemistry. While this work was in progress, a patent application has been recently published on the usage of tricyclic benzoboroxoles as anti-mycobacterial agents.6 Our long-standing interest7 in developing novel functionalized boronic acids and benzoboroxoles as therapeutic agents prompted us to explore the utility of these scaffolds as potential anti-tubercular agents.
Results and Discussion
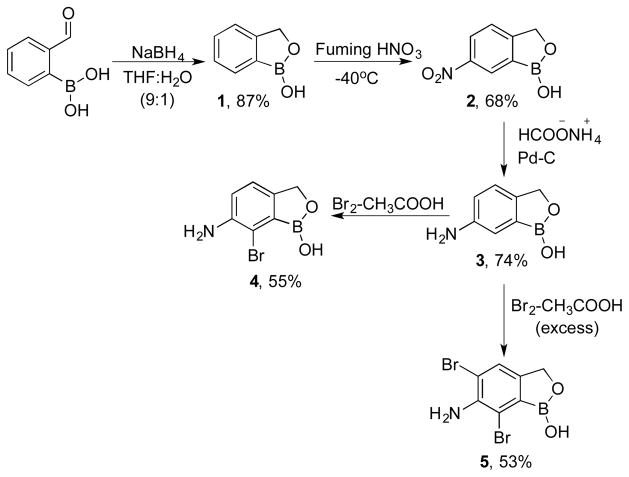
Based on the literature reports as well as our previous experience, it was found that the biological activity of benzoboroxoles greatly diminished upon substitution at the benzylic carbon in the oxaborole ring. Hence, we envisioned the preparation of aromatic ring-substituted benzoboroxoles while leaving the oxaborole methylene group unsubstituted. In this regard, we chose 6-aminobenzoboroxole 3 as the common synthon for the preparation of various functionalized derivatives. The amine 3 was prepared in three steps starting from 2-boronobenzaldehyde (Scheme 1).7a,8
Scheme 1.
Preparation of brominated aminobenzoboroxoles
The reaction of 2-boronobenzaldehyde with sodium borohydride in THF and water provided benzoboroxoles 1 in 90% yield. Nitration8a of 1 with fuming nitric acid followed by reduction of nitro group with ammonium formate in the presence of palladium carbon resulted in the formation of 6-aminobenzoboroxole 3. Electrophilic aromatic bromination of 3 with bromine in acetic acid provided 7-bromo-6- aminobenzoboroxole 4 in 55% yield along with ~20% of 5,7-dibromo-6-aminobenzoboroxole 5. Pure 4 could be readily obtained upon simple silica gel column chromatography using hexanes and ethyl acetate as eluents. Addition of excess bromine (2.5 eq) to 3 yielded 53% of 5 (Scheme 1).
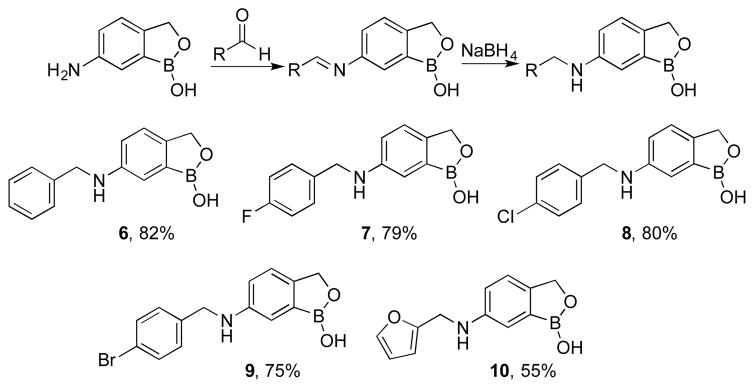
The 6-aminobenzoboroxole 3 was then subjected to reductive amination to prepare monosubstituted derivatives of the amino group.7a 6-Aminobenzoboroxole was treated with benzaldehyde, 4-flourobenzaldehyde, 4-chlorobenzaldehyde, 4-bromobenzaldehyde, and 2-furaldehyde in methanol to form the intermediate imines, followed by reduction of the imines with sodium borohydride to obtain N-monoalkylated 6-aminobenzo boroxoles 6–10 respectively (Scheme 2).
Scheme 2.
Reductive amination of 6-aminobenzoboroxoles
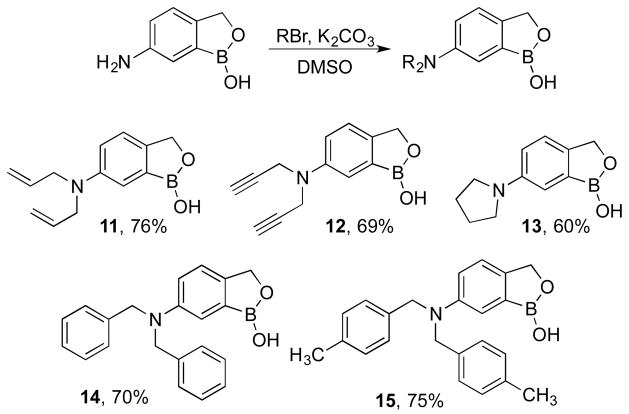
The 6-aminobenzoboroxole 3 was further derivatized via alkylation of the amine with alkyl and benzyl halides. Amine 3 was treated with allyl bromide, propargyl bromide, 1,4-dibromobutane, benzyl bromide9 and p-xylyl bromide in the presence of potassium carbonate and DMSO to obtain the N,N-dialkylated aminobenzoboroxoles 11–15 respectively (Scheme 3).
Scheme 3.
Dialkylation of 6-aminobenzoboroxoles
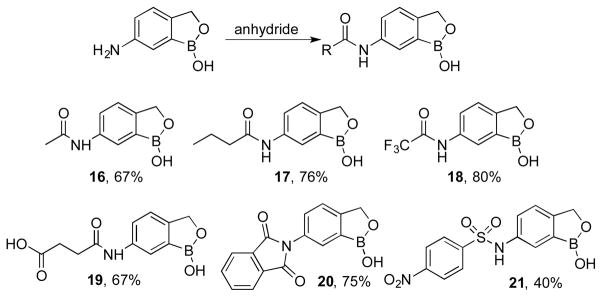
The 6-aminobenzoboroxole 3 was also functionalized as the corresponding amides 16–20 upon reaction with acetic anhydride10, butyric anhydride, trifloroacetic anhydride, succinic anhydride11, and phthalic anhydride respectively (Scheme 4). The amide with phthalic anhydride cyclized intramolecularly to provide the cylic imide 20. Similarly, a sulfonamide derivative 21 was also prepared by treating the amine 3 with 4-nitrobenzenesulfonyl chloride.12
Scheme 4.
Synthesis of 6-amidobenzoboroxoles
After synthesizing various functionalized benzoboroxoles, these molecules 1–21 were evaluated for their antimycobacterial activity. Before testing their antimycobacterial potential, all the molecules were evaluated for their general cytotoxicity against breast cancer cell line MCF-7. All of the tested compounds did not exhibit any significant cytotoxicity even at 50μM. Encouraged by their lack of cytotoxicity, we screened them for their biological activity against Mycobacterium smegmatis (M. smegmatis). This strain is especially useful in screening large chemical libraries because of the microbe’s speed of growth and its non-pathogenic nature that allows its use in biosafety level II hoods. Several laboratories have reported on the effectiveness of M. smegmatis as a surrogate screen for compounds that inhibit the growth of M. tuberculosis.13 We employed Kirby-Bauer disk method for the identification of lead compounds. The compounds that exhibited >3 cm zone of inhibition were further tested for their efficacy against M. tuberculosis. Unfortunately, most of the amine substituted derivatives exhibited weak or no inhibitory properties in the Kirby-Bauer disk method. Of all the derivatives, the 6-aminobenzoxaborole 3, 7-bromo-6-aminobenzoboroxole 4 and 5,7-dibromo-6-aminobenzoboroxole 5 were found to be the most active with zone of inhibition values >5 cm. However, 5 was not selected for further studies due to its high bromine content which may preclude its suitability as a pharmaceutical agent. Consequently, the two aminobenzoboroxoles 3 and 4 were further subjected to detailed biological evaluation against M. tuberculosis.
Initially, parent benzoboroxole 1, amines 3 and 4 were evaluated for their minimum inhibitory concentration (MIC99) under aerobic conditions. Isoniazid was used as the positive control. Compared to 1, the amine derivatives 3 and 4 exhibited superior inhibitory properties. The amine 3 was found to be ~2–4 times more potent than 1 whereas the bromo derivative 4 was found to be ~8–16 times more potent than 1 in 7H9 medium. The MIC99 values of 1, 3 and 4 were also determined in more sensitive glycerol-alanine-salts-tween (GAST) medium.14 In this study, 3 was found to be ~4 times more potent than 1 and 4 was found to be ~30 times more potent than 1 (Table 1).
Table 1.
Minimum Inhibitory Concentration
| Compound | MIC99 * (7H9) | MIC99* (GAST) | |
|---|---|---|---|
| Week 1 | Week 2 | Week 2 | |
| 1 | 62.5 | 62.5–125 | 62.5 |
| 3 | 15.6 | 31.25 | 15.6 |
| 4 | 3.9 | 7.8 | 1.9 |
| Isoniazid | 0.7 | 0.7 | 0.15 |
MIC99 expressed in μM
We then carried out the minimum bactericidal concentration of 4 under aerobic conditions to evaluate whether these compounds were bacteriostatic or bactericidal. In this case, rifampicin was used as the positive control and DMSO was used as the negative control. We also evaluated the effectiveness of the hydrochloride salt of 4 under these conditions. This compound as well as its hydrochloride salt were found to be bacteriostatic at lower concentrations but at higher concentration of >40μM, they were found to exhibit good bactericidal properties (Table 2).
Table 2.
Minimum Bactericidal Concentration
| Compound | Conc. | CFU*/mL | Conc. | CFU*/mL |
|---|---|---|---|---|
| DMSO | - | 3.8 × 108 | - | 1.06 × 108 |
| Rifampicin | 0.1 μM | 290 | 1.0 μM | 165 |
| 4 | 4 μM | 4.59 × 105 | 40 μM | 750 |
| 4** | 4 μM | 5.51 × 104 | 40 μM | 1475 |
CFU = colony forming units
Hydrochloride salt of bromo-aminobenzoboroxole 4 was used.
We then carried out the minimum anaerobicidal concentration study to determine if the candidate compounds have activity under conditions of dormancy. There is very little metabolic activity under these conditions hence most compounds including clinically used isoniazid fail to provide therapeutic benefit. Metronidazole was used as the positive control and DMSO was used as the negative control in this study. Unfortunately, aminobenzoboroxole 4 and its salt did not exhibit any significant activity at lower or higher concentrations (10 and 100μM) under these conditions (Table 3).
Table 3.
Minimum Anaerobicidal Concentration
| Compound | Conc. (μM) | CFU*/mL | Conc. (μM) | CFU*/mL |
|---|---|---|---|---|
| DMSO | 1.32 × 107 | - | - | |
| Metronidazole | 100 | 1.73 ×105 | - | - |
| Isoniazid | 10 | 1.36 ×107 | - | - |
| 4 | 10 | 7.13 × 106 | 100 | 4.81 × 106 |
| 4** | 10 | 6.55 × 106 | 100 | 4.30 × 106 |
CFU = colony forming units
Hydrochloride salt of 7-bromo-6-aminobenzoboroxole 4 was used.
Finally, we also carried out the intracellular macrophage-killing assay, which provides information about the ability of the test compounds to kill in macrophages, which are the natural hosts of Mtb. If a compound shows killing of Mtb in macrophages then it has better chances of showing efficacy in an animal model of disease. The candidate benzoboroxole 4 and its salt showed some cidality in the macrophages but not as much as the positive control INH (Table 4).
Table 4.
Intracellular Macrophage Killing Assay
| Compound | Conc. (μM) | Day 1 CFU*/mL | Conc. (μM) | Day 6 CFU*/mL |
|---|---|---|---|---|
| Untreated | - | 2.16 × 105 | - | 1.80 × 105 |
| Isoniazid | 4 | 1.34 ×103 | - | - |
| 4 | 4 | 4.31 ×104 | 40 | 2.28 × 104 |
| 4** | 4 | 6.09 × 104 | 40 | 2.44 × 104 |
CFU = colony forming units,
Hydrochloride salt of 7-bromo-6-aminobenzoboroxole 4 was used.
Based on the results, complete loss in biological activity was observed upon structural changes on the 6-position primary amino group in benzoboroxole. We observed that the conversion of primary amine to secondary amines (eg. compounds 6–10, Scheme 2), tertiary amines (eg. compounds 11–15, Scheme 3), as well as amides and sulphonamides (eg. compounds 16–21, Scheme 4) resulted in significant loss in biological acvitiy. However, some of the compounds such as 6-aminobenzoboroxole 3, 7-bromo-6-aminobenzoboroxole 4, and 5,7-dibromo-6-aminobenzoboroxole 5 exhibit good biological activity, and efforts are on going towards their development as anti-tuberculosis agents.
Conclusion
In conclusion, we have synthesized several derivatives of 6- aminobenzoboroxole starting from 2-boronobenzaldehyde. Some of the protocols employed in the synthesis include electrophilic aromatic bromination, reductive amination, dialkylation, and amide formation. All the synthesized derivatives have been evaluated for their zone of inhibition against M. smegmatis and cytotoxic properties against MCF7 cell line. From this study, two lead derivatives 3 and 4 have been identified for further anti-mycobacterial activity against M. tuberculosis H37Rv using 7H9 and GAST media. 3 and 4 have been found to be several times more potent than their parent benzoboroxole 1 in both these assays. Some of the other biology studies on 3 and 4 include minimum bactericidal concentration under aerobic and anaerobic conditions. Based on all these studies, 7-bromo-6- aminobenzoboroxole 4 has been identified as the lead candidate compound for further structure-activity studies to identify a derivative that exhibits much superior properties than the existing clinically used drugs.
Experimental Section
General Methods
All operations were carried out under an inert atmosphere of nitrogen. Glassware for all reactions was oven dried at 125 ºC and cooled under nitrogen prior to use. Liquid reagents and solvents were introduced by oven-dried syringes or cannulas through septa sealed flasks under a nitrogen atmosphere. THF was distilled from sodium benzophenone ketyl. All other solvents and reagents were purchased and used without further purification. The 1H and 13C NMR spectra were plotted on a Bruker-500 spectrometer fitted with a Quad probe. Elemental analysis was performed on Perkin Elmer PE2400 CHN analyzer.
6-amino-7-bromobenzo[c][1,2]oxaborol-1(3H)-ol (4)
To a stirred solution of aminobenzoboroxole 3 (1mmol) in acetic acid (4 mL) was added bromine (1 mmol) at 0°C and stirred for 5h. Upon completion via thin layer chromatography (TLC), reaction mixture was quenched by the addition of saturated NaHCO3 followed by extraction with ethyl acetate (3×15mL). The combined organic layers were dried over anhydrous MgSO4, concentrated under vacuum and purified via silica gel column chromatography (hexanes: ethyl acetate) to obtain pure compound 4 (55%). 1H NMR (500 MHz, DMSO-d6): δ 8.83 (s, 1H), 7.07 (d, J = 8.0 Hz, 1H), 6.91 (d, J = 8.0 Hz, 1H), 5.12 (s, 2H), 4.81 (s, 2H); 13C NMR (125 MHz, DMSO-d6): δ 145.2, 143.7, 121.4, 119.5, 109.5, 69.1; CHN Analysis: (Found: C, 36.85%; H, 3.25%; N, 6.25%; C7H7BBrNO2 requires: C, 36.90%; H, 3.10%; N, 6.15%).
6-amino-5,7-dibromobenzo[c][1,2]oxaborol-1(3H)-ol (5)
To a stirred solution of 6-aminobenzoboroxole 3 (1mmol) in acetic acid (5 mL) was added bromine (2.5 mmol) and stirred for 5h. Upon completion (TLC), the reaction mixture was quenched by the addition of saturated NaHCO3 and extracted with ethyl acetate (3×15mL). The combined organic layers were dried over anhydrous MgSO4 and concentrated under vacuum and purified via silica gel column chromatography (hexanes: ethyl acetate) to obtain pure compound 5 (53%). 1H NMR (500 MHz, DMSO-d6): δ 9.11 (s, 1H), 7.51 (s, 1H), 5.24 (s, 2H), 4.85 (s, 2H); 13C NMR (125 MHz, DMSO-d6): δ 142.3, 142.0, 125.3, 112.3, 109.9, 68.7; CHN Analysis: (Found: C, 27.12%; H, 2.05%; N, 4.34%; C7H6BBr2NO2 requires: C, 27.41%; H, 1.97%; N, 4.57%)
6-(benzylamino)benzo[c][1,2]oxaborol-1(3H)-ol (6)
1H NMR (500 MHz, DMSO-d6): δ 8.91 (s, 1H), 7.37-7.24 (m, 4H), 7.22 (t, J = 7.0 Hz, 1H), 7.07 (d, J = 8.0 Hz, 1H), 6.87 (s, 1H), 6.76 (d, J = 8.0 Hz, 1H), 6.23 (br s, 1H), 4.82 (s, 2H), 4.28 (s, 2H); 13C NMR (125 MHz, DMSO-d6): δ 148.3, 142.0, 140.8, 128.7, 127.6, 127.0, 121.9, 117.0, 112.7, 70.0, 47.1; CHN Analysis: (Found: C, 70.56%; H, 5.78%; N, 5.62%; C14H14BNO2 requires: C, 70.33%; H, 5.90%; N, 5.86%)
6-((4-fluorobenzyl)amino)benzo[c][1,2]oxaborol-1(3H)-ol (7)
1H NMR (500MHz, DMSO-d6): δ 8.91 (s, 1H), 7.41-7.38 (m, 2H), 7.16-7.07 (m, 3H), 6.86 (s, 1H), 6.76 (d, J = 8.5 Hz, 1H), 6.24 (t, J = 6.0 Hz, 1H), 4.82 (s, 2H), 4.27 (d, J = 6.0 Hz, 2H); 13C NMR (125 MHz, DMSO-d6): δ 161.6 (d, JF = 300.0 Hz), 148.1, 142.1, 136.9, 129.4, 122.0, 117.1, 115.4 (d, JF = 26.2 Hz), 112.8, 70.0, 46.4; CHN Analysis: (Found: C, 65.24%; H, 4.97%; N, 5.60%; C14H13BFNO2 requires: C, 65.41%; H, 5.10%; N, 5.45%).
6-((4-chlorobenzyl)amino)benzo[c][1,2]oxaborol-1(3H)-ol (8)
1H NMR (500MHz, DMSO-d6): δ 8.92 (s, 1H), 7.37-7.22 (m, 4H), 7.07 (d, J = 8.0 Hz, 1H), 6.84 (s, 1H), 6.74 (d, J = 8.5 Hz, 1H), 6.28 (t, J = 6.2 Hz, 1H), 4.82 (s, 2H), 4.28 (d, J = 6.0 Hz, 2H); 13C NMR (125 MHz, DMSO-d6): δ 148.0, 142.2, 140.0, 131.5, 129.4, 128.7, 122.0, 117.0, 112.8, 70.0, 46.4; CHN Analysis: (Found: C, 61.39%; H, 4.85%; N, 4.95%; C14H13BClNO2 requires: C, 61.48%; H, 4.79%; N, 5.12%).
6-((4-bromobenzyl)amino)benzo[c][1,2]oxaborol-1(3H)-ol (9)
1H NMR (500MHz, DMSO-d6): δ 8.92 (s, 1H), 7.51 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 7.08 (d, J = 8.5 Hz, 1H), 6.83 (s, 1H), 6.74 (d, J = 8.5 Hz, 1H), 6.28 (br s, 1H), 4.82 (s, 2H), 4.26 (s, 2H); 13C NMR (125 MHz, DMSO-d6): δ 148.0, 142.2, 140.4, 131.6, 129.8, 122.0, 119.9, 117.1, 112.8, 70.0, 46.5; CHN Analysis: (Found: C, 52.72%; H, 4.02%; N, 4.49%; C14H13BBrNO2 requires: C, 52.88%; H, 4.12%; N, 4.41%).
6-((furan-2-ylmethyl)amino)benzo[c][1,2]oxaborol-1(3H)-ol (10)
1H NMR (500MHz, DMSO-d6) δ 8.94 (s, 1H), 7.57 (d, J = 2.0 Hz, 1H), 7.10 (d, J = 8.5 Hz, 1H), 6.95 (s, 1H), 6.81-6.84 (m, 1H), 6.39-6.28 (m, 2H), 6.06 (t, J = 6.2 Hz, 1H), 4.84 (s, 2H), 4.25 (d, J = 6.0 Hz, 2H); 13C NMR (125 MHz, DMSO-d6) δ 153.9, 147.9, 142.3, 121.9, 117.1, 112.9, 110.8, 107.3, 70.0, 31.2; CHN Analysis: (Found: C, 62.79%; H, 5.37%; N, 6.20%; C12H12BNO3 requires: C, 62.93%; H, 5.28%; N, 6.12%).
6-(diallylamino)benzo[c][1,2]oxaborol-1(3H)-ol (11)
To a stirred solution of aminobenzoboroxole 3 (1mmol) in DMSO (5 mL) was added allyl bromide (6 mmol) and K2CO3 (3.0 mmol). The reaction mixture was stirred for 12h. Upon completion (TLC), reaction mixture was extracted with ethyl acetate and water. The combined organic layers were dried over anhydrous MgSO4, concentrated under vacuum and purified via silica gel column chromatography (hexanes: ethyl acetate) to obtain pure compound 10 (76%). 1H NMR (500MHz, DMSO-d6): δ 8.97 (s, 1H), 7.17 (d, J = 8.0 Hz, 1H), 7.03 (s, 1H), 6.84 (d, J = 8.5 Hz, 1H), 5.90-5.84 (m, 2H), 5.18-5.12 (m, 4H), 4.87 (s, 2H), 3.94-3.91 (m, 4H); 13C NMR (125 MHz, DMSO-d6): δ 147.9, 141.9, 134.9, 128.6, 122.0, 116.3, 113.5, 70.0, 53.1; CHN Analysis: (Found: C, 68.25%; H, 6.92%; N, 6.02%; C13H16BNO2 requires: C, 68.16%; H, 7.04%; N, 6.11%).
6-(di(prop-2-yn-1-yl)amino)benzo[c][1,2]oxaborol-1(3H)-ol (12)
1H NMR (500MHz, DMSO-d6): δ 9.08 (s, 1H), 7.30-7.27 (m, 2H), 7.11 (d, J = 8.5 Hz, 1H), 4.92 (s, 2H), 4.14 (s, 4H), 2.51 (s, 2H); 13C NMR (125 MHz, DMSO-d6): δ 147.1, 145.1, 122.1, 119.7, 116.9, 80.3, 75.6, 70.0, 40.8; CHN Analysis: (Found: C, 69.23%; H, 5.29%; N, 6.29%; C13H12BNO2 requires: C, 69.38%; H, 5.37%; N, 6.22%).
6-(pyrrolidin-1-yl)benzo[c][1,2]oxaborol-1(3H)-ol (13)
1H NMR (500MHz, DMSO-d6): δ 8.94 (s, 1H), 7.18 (d, J = 8.5 Hz, 1H), 6.86 (s, 1H), 6.69 (d, J = 8.5 Hz, 1H), 4.87 (s, 2H), 3.22 (t, J = 6.0 Hz, 4H), 1.97-1.95 (m, 4H); 13C NMR (125 MHz, DMSO-d6): δ 147.6, 141.1, 122.0, 115.7, 112.7, 70.0, 48.0, 25.4; CHN Analysis: (Found: C, 64.96%; H, 7.02%; N, 6.79%; C11H14BNO2 requires: C, 65.07%; H, 6.95%; N, 6.90%).
6-(dibenzylamino)benzo[c][1,2]oxaborol-1(3H)-ol (14)
1H NMR (500 MHz, DMSO-d6): δ 8.95 (s, 1H), 7.35-7.23 (m, 10H), 7.12 (d, J = 8.5 Hz, 1H), 7.07 (s, 1H), 6.84 (d, J = 8.0 Hz, 1H), 4.84 (s, 2H), 4.71 (s, 4H); 13C NMR (125 MHz, DMSO-d6): δ 148.0, 142.2, 139.4, 128.9, 127.2, 127.1, 122.1, 116.4, 113.6, 70.0, 54.8; CHN Analysis: (Found: C, 76.35%; H, 6.30%; N, 4.16%; C21H20BNO2 requires: C, 76.62%; H, 6.12%; N, 4.25%).
6-(bis(4-methylbenzyl)amino)benzo[c][1,2]oxaborol-1(3H)-ol (15)
1H NMR (500MHz, DMSO-d6): δ 8.97 (s, 1H), 7.21-7.07 (m, 10H), 6.83-6.85 (m, 1H), 4.85 (s, 2H), 4.62 (s, 4H), 2.28 (s, 6H); 13C NMR (125 MHz, DMSO-d6): δ 148.2, 142.2, 136.3, 129.5, 129.1, 127.1, 122.1, 116.6, 113.8, 70.0, 54.6, 21.2; CHN Analysis: (Found: C, 77.20%; H, 6.62%; N, 3.83%; C23H24BNO2 requires: C, 77.33%; H, 6.77%; N, 3.92%).
N-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)acetamide (16)
To a stirred solution of aminobenzoboroxole 3 (1mmol) in dioxane (5 mL) was added acetic anhydride (1.2 mmol) and stirred for 1h. Upon completion (TLC), reaction mixture was quenched by the addition of dilute HCl followed by extraction with ethyl acetate. The organic layers were dried under anhydrous MgSO4, concentrated under vacuum and purified via silica gel column chromatography (hexanes: ethyl acetate) to obtain amidobenzoboroxole 16 (67%).
N-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6yl)butyramide (17)
1H NMR (500MHz, DMSO-d6): δ 9.89 (s, 1H), 9.20 (s, 1H), 8.02 (s, 1H), 7.63 (d, J = 8.0 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 4.94 (s, 2H), 2.31 (t, J = 7.2 Hz, 2H), 1.66-1.61 (m, 2H), 0.94 (t, J = 7.2 Hz, 3H); 13C NMR (125 MHz, DMSO-d6): δ 171.5, 148.9, 138.6, 122.8, 121.9, 121.5, 70.2, 38.8, 19.1, 14.1; CHN Analysis: (Found: C, 60.13%; H, 6.38%; N, 6.45%; C11H14BNO3 requires: C, 60.32%; H, 6.44%; N, 6.39%).
2,2,2-trifluoro-N-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)acetamide (18)
1H NMR (500MHz, DMSO-d6): δ 11.30 (s, 1H), 9.32 (s, 1H), 8.07 (s, 1H), 7.68 (d, J = 8.5 Hz, 1H), 7.46 (d, J = 8.5 Hz, 1H), 4.99 (s, 2H); 13C NMR (125 MHz, DMSO-d6): δ 155.1 (q, JF = 36.0 Hz), 151.6, 135.5, 124.6, 123.6, 122.4, 118.5 (q, JF = 285.0 Hz), 70.2; CHN Analysis: (Found: C, 44.01%; H, 2.99%; N, 5.60%; C9H7BF3NO3 requires: C, 44.13%; H, 2.88%; N, 5.72%).
4-((1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)amino)-4-oxobutanoic acid (19)
1H NMR (500MHz, DMSO-d6): δ 12.13 (br s, 1H), 9.99 (s, 1H), 9.20 (s, 1H), 8.01 (s, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.33 (d, J = 8.5 Hz, 1H), 4.94 (s, 2H), 2.55 (m, 4H); 13C NMR (125 MHz, DMSO-d6): δ 174.4, 170.5, 148.9, 138.6, 122.6, 121.93, 121.3, 70.2, 31.5, 29.3; CHN Analysis: (Found: C, 53.18%; H, 4.74%; N, 5.49%; C11H12BNO5 requires: C, 53.05%; H, 4.86%; N, 5.62%).
2-((1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)carbamoyl)benzoic acid (20)
1H NMR (500MHz, DMSO-d6): δ 9.36 (s, 1H), 8.00-7.93 (m, 4H), 7.78 (s, 1H), 7.59-7.55 (m, 2H), 5.08 (s, 2H); 13C NMR (125 MHz, DMSO-d6): δ 167.7, 154.1, 135.2, 132.1, 131.2, 130.3, 129.8, 123.9, 122.5, 70.4; CHN Analysis: (Found: C, 64.38%; H, 3.44%; N, 5.18%; C15H10BNO4 requires: C, 64.56%; H, 3.61%; N, 5.02%).
N-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-4-nitrobenzene sulfonamide (21)
To a stirred solution of aminobenzoboroxole 3 (1mmol) in dioxane (5 mL) was added 4-nitrobenzenesulfonyl chloride (1.1 mmol) and stirred for 1h. Upon completion (TLC), reaction mixture was quenched by the addition of dilute HCl followed by extraction with ethyl acetate. The combined organic layers were dried over anhydrous MgSO4, concentrated under vacuum and purified by silica gel column chromatography (hexanes: ethyl acetate) to obtain pure compound 21 (40%). 1H NMR (500MHz, DMSO-d6): δ 9.34 (s, 1H), 7.99-7.90 (m, 4H), 7.76 (s, 1H), 7.57-7.52 (m, 2H), 5.06 (s, 2H); 13C NMR (125 MHz, DMSO-d6): δ 167.7, 154.1, 135.2, 132.1, 131.1, 130.3, 129.8, 123.9, 122.4, 70.4; CHN Analysis: (Found: C, 46.64%; H, 3.19%; N, 8.25%; C13H11BN2O6S requires: C, 46.73%; H, 3.32%; N, 8.38%).
Sulforhodamine-B assay for cytotoxicity
MCF-7 cells were cultured in Iscove’s Modified Eagle Medium containing 10% Hyclone-III and 1% antibiotic (500,000 units penicillin-streptomycin). The cells were seeded at a concentration of ~2×104 cells per well in 48 well plates and incubated for 18–24 hours at 37ºC and 5% CO2 atmosphere. The test compounds were initially diluted in DMSO and further diluted 1000 times in growth media so that the final DMSO concentration was <0.1%. Growth media was removed from 48 well plates and test compound in 400μL of growth media were added. Taxol was used as the positive control whereas DMSO and growth media were used as negative controls. All the compounds were tested in triplicates at 50 and 10μM. The plates were incubated for 72 hours and growth media was removed. The wells were washed with 1mL of 1% DPBS and the plates were dried. 100μL of 0.5% SRB (in 1% acetic acid) was added in each well and incubated at 37ºC for 45 minutes. The wells were washed 5 times with 1% acetic acid solution to remove residual SRB and the plates were dried. The cellular protein was dissolved in 400μL of 10mM tris base (pH 10.2) and absorbance was recorded at 540nm. %Survival was calculated using the formula (Absorbance of test compound/Absorbance of DMSO control) × 100%.
Bacterial strains, media and growth conditions
M. tuberculosis H37Rv was grown in Middlebrook 7H9 broth medium (Difco) supplemented with 0.2% (v/v) glycerol, 0.05% (v/v) tween 80 and albumin dextrose catalase (ADC): 5g/L bovine serum albumin (BSA); 2g/L dextrose; and 0.81g/L NaCl. M. tuberculosis was also cultured in GAST medium consisting of 0.3g/L bacto-casitone, 4g/L dibasic potassium phosphate, 2g/L citric acid, 1g/L L-alanine, 1.2g/L magnesium chloride hexahydrate, 0.6g/L potassium sulfate, 2g/L ammonium chloride, 1% glycerol (v/v) and 0.05% tween 80 (v/v) at pH 6.6. For generating non-replicating persistent cells M. tuberculosis was cultured in Middlebrook Dubos medium (Difco) supplemented with 5g/L BSA, 7.5g/L glucose and 0.81g/L NaCl. For determination of viable cell counts, M. tuberculosis cells were plated on Middlebrook 7H11 plates supplemented with 0.5% glycerol (v/v) and oleic albumin dextrose catalase (OADC): 0.06% oleic acid; 4mg/L NaOH; 50g/L BSA; 2g/L dextrose; and 0.81g/L NaCl. All cultures were incubated at 37ºC.
Minimum inhibitory concentration (MIC99)
MIC99 was determined in 7H9 as well as GAST medium by micro-dilution broth method. Briefly, M. tuberculosis was grown to an optical density at 650nm (OD650) of 0.2–0.3 in 7H9 or GAST medium. Cells were diluted to final OD650 of 0.0002 (1:1000 of parent culture) in desired medium. Ten-fold serial dilution of the test compounds was made in triplicate rows of a 96-well plate. The above dilution of cells was then added to all the wells of the 96-well plate. The assay was performed in duplicate and each compound dilution was set-up in triplicate rows per plate. The plates were incubated at 37ºC and observations were noted on day-7, and day-14. MIC99 was noted as the lowest concentration of test compound that inhibited visible growth after 14 days of incubation.
Minimum bactericidal concentration
M. tuberculosis was grown to OD650 0.2–0.3 in 7H9 medium and cells were then diluted to OD650 0.002 (1:100 of parent culture). For enumeration of viable numbers on day 0, serial dilutions of the diluted cultures were plated in duplicate on 7H11 plates (supplemented with OADC). 1mL aliquots of the diluted culture were then dispensed in duplicate wells of 24-well plates. Treatments with chosen test compounds were carried out at 4μM and 40μM for 7 days. Serial dilutions were then plated on 7H11 medium (supplemented with OADC) and colonies were counted after 3-week incubation at 37ºC and CFU/ml were determined.
Minimum anaerobicidal concentration
Non-replicating persistent (NRP) cells of M. tuberculosis were obtained by culturing the cells in Wayne model system.15 Briefly, M. tuberculosis was cultured in Dubos medium to an OD650 of 0.2–0.3. Cells were then diluted to OD650 0.005 and dispensed in glass bottles leaving a head-space ratio of 0.5. The bottles were sealed with wax and placed on a magnetic stir plate at 37ºC. The cells were allowed to stir for 21 days at which point the culture reached NRP2 state. The bottles were removed from the stir plate and opened in an anaerobic chamber. For determination of day 0 cell numbers, serial dilutions of the culture were plated on 7H11 plates (supplemented with OADC). 1mL cells were dispensed in duplicate wells of 24-well plates and treated with the desired concentrations of the candidate compounds for 7 days in the anaerobic chamber at 37ºC. After 7 days, the plates were removed from the chamber and serial dilutions were then made and plated on 7H11 plates (supplemented with OADC). Viable numbers were counted after 4 weeks of incubation at 37ºC and CFU/ml was determined.
Intracellular macrophage killing
Bone marrow derived macrophages were obtained from C57/BL6 mice. Briefly femurs obtained from the mice were flushed with RPMI supplemented with 10% heat-inactivated fetal bovine serum (RPMI+10% FBS). The collected material was then strained through a 70μm nylon strainer. The cells were counted in cellometer and plated at a density of 4×105 cells/ml in tissue culture petri-dishes in RPMI medium containing 20% FBS, 1% penicillin-streptomycin, 40% L-cell supernatant and allowed to adhere for 7 days at 37ºC with medium replenishment on day 3. After the monocytes had differentiated in to macrophages the adherent cells were removed by discarding the medium and rinsing the monolayer with ice-cold 1XPBS+3mM EDTA. The removed cells were collected by centrifugation at 400Xg for 10mins at 4ºC. The cells were resuspended in fresh RPMI+10%FBS and counted. The cells were then replated in wells of 24 well plates at a density of 5×105 cells/well and left overnight at 37ºC to adhere. Cells were then infected with a culture of Mtb grown in 7H9 to OD650=0.2–0.3. The bacterial culture was diluted to 5×105 CFU/ml (OD650=0.2 is approximately 2×108 CFU/ml) and added to the wells of the 24-well plates (m.o.i=1). Infection was allowed to establish for 24hrs at 37ºC and then the wells were rinsed with fresh medium to remove uninternalized bacteria. Treatments with the chosen compound 4 and its hydrochloride salt were initiated at the desired concentrations (4 and 40μM). For enumeration of viable bacterial numbers on day 0, macrophages in duplicate infected wells were lysed with 0.1% SDS and serial dilutions were plated on 7H11 plates (supplemented with OADC). After 6 days of treatment, all macrophages were lysed (by adding 0.1% SDS) and serial dilutions were plated on 7H11 plates (supplemented with OADC). Isoniazid (35μM) was used as a positive control and DMSO was used as a negative control.
Acknowledgments
The work was funded, in part by the Intramural Research Program at NIAID, NIH. We thank Dr. Clifton E. Barry and Dr. Helena Boshoff (NIAID, NIH) for their guidance with the study. We thank Dr. John Dahl, Department of Biology, University of Minnesota Duluth for kindly providing Mycobacterium smegmatis culture. We thank the Departments of Chemistry and Biochemistry at Rowan University and University of Minnesota Duluth as well as the Integrated Biosciences Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a)http://www.who.int/mediacentre/factsheets/fs104/en/Russell DG, Barry CE, Flynn JL. Science. 2010;328:852. doi: 10.1126/science.1184784.Russell DG. Nat Rev Microbiol. 2007;5:39. doi: 10.1038/nrmicro1538.Janin YL. Bioorg Med Chem. 2007;15:2479–2513. doi: 10.1016/j.bmc.2007.01.030.Kaufmann SHE, McMichael A. J Nat Med. 2005;11:578. doi: 10.1038/nm1221.Manabe YC, Bishai WR. Nat Med. 2000;6:1327. doi: 10.1038/82139.
- 2.(a) Dos Santos Fernandes GF, Jornada DH, de Souza PC, Chin CM, Pavan FR, Dos Santos JL. Curr Med Chem. 2015;22:3133–3161. doi: 10.2174/0929867322666150818103836. [DOI] [PubMed] [Google Scholar]; (b) Mathew B, Suresh J, Ahsan MJ, Mathew GE, Usman D, Subramanyan PN, Safna KF, Maddela S. Infect Disord Drug Targets. 2015;15:76–88. doi: 10.2174/1871526515666150724104411. [DOI] [PubMed] [Google Scholar]; (c) Primi MC, Segretti ND, Ferreira EI. Curr Clin Pharmacol. 2015;10:139–159. doi: 10.2174/1574884708666131229125103. [DOI] [PubMed] [Google Scholar]; (d) Ahsan MJ, Ansari MY, Yasmin S, Jadav SS, Kumar P, Garg SK, Aseri A, Khalilullah H. Infect Disord Drug Targets. 2015;15:32–41. doi: 10.2174/1871526514666140923153329. [DOI] [PubMed] [Google Scholar]; (e) Gengenbacher M, Dick T. Chem Biol. 2015;22:5–6. doi: 10.1016/j.chembiol.2014.12.005. [DOI] [PubMed] [Google Scholar]; (f) Chang KC, Yew WW, Sotgiu G. Int J Tuberc Lung Dis. 2015;19:1417–1427. doi: 10.5588/ijtld.15.0216. [DOI] [PubMed] [Google Scholar]; (g) Pandey S, Soni I, Topno N, Dasgupta A, Chopra S. Curr Respir Med Rev. 2014;10:88–96. [Google Scholar]; (h) Worley MV, Estrada SJ. Pharmacotherapy. 2014;34:1187–1197. doi: 10.1002/phar.1482. [DOI] [PubMed] [Google Scholar]; (i) Singh A, Budhraj A, Shrivastava A, Satyavana A, Gupta A, Gupta M, Wadhwa G, Sharma SK, Jain CK. Recent Pat Antiinfect Drug Discov. 2014;9:25–40. doi: 10.2174/1574891x09666140711111800. [DOI] [PubMed] [Google Scholar]; (j) Nidhi, Siddiqi MI. Curr Pharm Des. 2014;20:4418–4426. doi: 10.2174/1381612819666131118165059. [DOI] [PubMed] [Google Scholar]; (k) Mehanna MM, Mohyeldin SM, Elgindy NA. J Control Release. 2014;187:183–197. doi: 10.1016/j.jconrel.2014.05.038. [DOI] [PubMed] [Google Scholar]; (l) Sarangi MK. World J Pharm Sci. 2013;1:3–14. [Google Scholar]; (m) Branco FS, Pinto AC, Boechat N. Curr Top Med Chem. 2013;13:2808–2849. doi: 10.2174/15680266113136660201. [DOI] [PubMed] [Google Scholar]; (n) Asif M. Med Chem. 2012;2:151–167. [Google Scholar]
- 3.(a) Adamczyk-Woźniak A, Borys KM, Sporzyński A. Chem Rev. 2015;115:5224–5247. doi: 10.1021/cr500642d. [DOI] [PubMed] [Google Scholar]; (b) Liu CT, Tomsho JW, Benkovic SJ. Bioorg Med Chem. 2014;22:4462–4473. doi: 10.1016/j.bmc.2014.04.065. [DOI] [PubMed] [Google Scholar]
- 4.(a) Adamczyk-Woźniak A, Cyranski MK, Zubrowska A, Sporzynski A. J Organomet Chem. 2009;694:3533–3541. [Google Scholar]; (b) Dowlut M, Hall DG. J Am Chem Soc. 2006;128:4226–4227. doi: 10.1021/ja057798c. [DOI] [PubMed] [Google Scholar]; (c) Alexander C, Smith CR, Whitcombe MJ, Vulfson EN. J Am Chem Soc. 1999;121:6640–6651. [Google Scholar]; (d) Oshima K, Aoyama Y. J Am Chem Soc. 1999;121:2315–2316. [Google Scholar]; (e) Russell AP, Zepp CW. US5512246. US Patent. 1996; (f) Lowe CR, Sartain FK, Yang X. WO2006079843. PCT Int Appl. 2006; (g) Austin PW, Kneale CJ, Crowley PJ, Clough JM. WO9533754. PCT Int Appl. 1995
- 5.(a) Li X, Plattner JJ, Hernandez V, Ding CZ, Wu W, Yang Y, Xu M. Tetrahedron Lett. 2011;52:4924–4926. [Google Scholar]; (b) Xia Y, Cao K, Zhou Y, Alley MRK, Rock F, Mohan M, Meewan M, Baker SJ, Lux S, Ding CZ, Jia G, Kully M, Plattner JJ. Bioorg Med Chem Lett. 2011;21:2533–2536. doi: 10.1016/j.bmcl.2011.02.024. [DOI] [PubMed] [Google Scholar]; (c) Li X, Zhang S, Zhang YK, Liu Y, Ding CZ, Zhou Y, Plattner JJ, Baker SJ, Bu W, Liu L, Kazmierski WM, Duan M, Grimes RM, Wright LL, Smith GK, Jarvest RL, Ji JJ, Cooper JP, Tallant MD, Crosby RM, Creech K, Ni Z-J, Zou W, Wright J. Bioorg Med Chem Lett. 2011;21:2048–2054. doi: 10.1016/j.bmcl.2011.02.006. [DOI] [PubMed] [Google Scholar]; (d) Zhang Y-K, Plattner JJ, Freund YR, Easom EE, Zhou Y, Gut J, Rosenthal PJ, Waterson D, Gamo F-J, Angulo-Barturen I, Ge M, Li Z, Li L, Jian Y, Cui H, Wang H, Yang J. Bioorg Med Chem Lett. 2011;21:644–651. doi: 10.1016/j.bmcl.2010.12.034. [DOI] [PubMed] [Google Scholar]; (e) Jacobs RT, Plattner JJ, Nare B, Wring SA, Chen D, Freund Y, Gaukel EG, Orr MD, Perales JB, Jenks M, Noe RA, Sligar JM, Zhang YK, Bacchi CJ, Yarlett N, Don R. Future Med Chem. 2011;3:1259–1278. doi: 10.4155/fmc.11.80. [DOI] [PubMed] [Google Scholar]; (f) Ding D, Meng Q, Gao G, Zhao Y, Wang Q, Nare B, Jacobs R, Rock F, Alley MRK, Plattner JJ, Chen G, Li D, Zhou H. J Med Chem. 2011;54:1276–1287. doi: 10.1021/jm101225g. [DOI] [PubMed] [Google Scholar]; (g) Li X, Zhang YK, Liu Y, Zhang S, Ding CZ, Zhou Y, Plattner JJ, Baker SJ, Liu L, Bu W, Kazmierski WM, Wright LL, Smith GK, Jarvest RL, Duan M, Ji JJ, Cooper JP, Tallant MD, Crosby RM, Creech K, Ni ZJ, Zou W, Wright J. Bioorg Med Chem Lett. 2010;20:7493–7497. doi: 10.1016/j.bmcl.2010.10.007. [DOI] [PubMed] [Google Scholar]; (h) Ding CZ, Zhang YK, Li X, Liu Y, Zhang S, Zhou Y, Plattner JJ, Baker SJ, Liu L, Duan M, Jarvest RL, Ji J, Kazmierski WM, Tallant MD, Wright LL, Smith GK, Crosby RM, Wang AA, Ni ZJ, Zou W, Wright J. Bioorg Med Chem Lett. 2010;20:7317–7322. doi: 10.1016/j.bmcl.2010.10.071. [DOI] [PubMed] [Google Scholar]; (i) Ding D, Zhao Y, Meng Q, Xie D, Nare B, Chen D, Bacchi CJ, Yarlett N, Zhang YK, Hernandez V, Xia Y, Freund Y, Abdulla M, Ang KH, Ratnam J, McKerrow JH, Jacobs RT, Zhou H, Plattner JJ. Med Chem Lett. 2010;1:165–169. doi: 10.1021/ml100013s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Zhang YK, Plattner JJ, Akama T, Baker SJ, Hernandez VS, Sanders V, Freund Y, Kimura R, Bu W, Hold KM, Lu XS. Bioorg Med Chem Lett. 2010;20:2270–2274. doi: 10.1016/j.bmcl.2010.02.010. [DOI] [PubMed] [Google Scholar]; (k) Akama T, Baker SJ, Zhang YK, Hernandez V, Zhou H, Sanders V, Freund Y, Kimura R, Maples KR, Plattner JJ. Bioorg Med Chem Lett. 2009;19:2129–2132. doi: 10.1016/j.bmcl.2009.03.007. [DOI] [PubMed] [Google Scholar]; (l) Baker SJ, Zhang YK, Akama T, Lau A, Zhou H, Hernandez V, Mao W, Alley MRK, Sanders V, Plattner JJ. J Med Chem. 2006;49:4447–4450. doi: 10.1021/jm0603724. [DOI] [PubMed] [Google Scholar]
- 6.Alley MRK, Hernandez VS, Plattner JJ, Li X, Barros-Aguirre D, Giordano I. WO2015021396 A2. PCT Int Appl. 2015
- 7.(a) Suman P, Patel BP, Kasibotla AV, Solano LN, Jonnalagadda SC. J Organomet Chem. 2015;798:125–131. [Google Scholar]; (b) Tekkam S, Alam MA, Just MJ, Johnson JL, Jonnalagadda SC, Mereddy VR. Anti-Cancer Agents in Med Chem. 2013;13:1514–1530. doi: 10.2174/18715206113139990097. [DOI] [PubMed] [Google Scholar]; (c) Kumar JS, Alam MA, Gurrapu S, Nelson GL, Williams M, Corsello MA, Johnson JL, Jonnalagadda SC, Mereddy VR. J Het Chem. 2013;50:814–820. [Google Scholar]; (d) Kumar JS, Bashian CM, Corsello MA, Jonnalagadda SC, Mereddy VR. Tetrahedron Lett. 2010;51:4482–4485. doi: 10.1016/j.tetlet.2010.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kumar JS, Jonnalagadda SC, Mereddy VR. Tetrahedron Lett. 2010;51:779–782. doi: 10.1016/j.tetlet.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Jonnalagadda SC, Verga SR, Patel PD, Reddy AV, Tekkam S, Scott PM, Mereddy VR. Appl Organomet Chem. 2010;24:294–300. [Google Scholar]; (g) Jonnalagadda SC, Cruz J, Connell RJ, Scott P, Mereddy VR. Tetrahedron Lett. 2009;50:4314–4317. doi: 10.1016/j.tetlet.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Gunasekera DS, Gerold DA, Aalderks NA, Jonnalagadda SC, Kiprof P, Zhdankin VV, Reddy MVR. Tetrahedron. 2007;63:9401–9405. [Google Scholar]; (i) Reddy VJ, Chandra JS, Reddy MVR. Org Biomol Chem. 2007;5:889–891. doi: 10.1039/b618750a. [DOI] [PubMed] [Google Scholar]
- 8.(a) Lennarz WJ, Snyder HR. J Am Chem Soc. 1960;82:2172–2175. [Google Scholar]; (b) Lin Mingchang, Chen Guosong, Jiang Ming. Polymer Chemistry. 2014;5:234–240. [Google Scholar]; (c) Fu Zhengyan, He Jiangpeng, Tong Aiping, Xie Yongmei, Wei Yuquan. Synthesis. 2013;45:2843–2852. [Google Scholar]; (d) Sanders V, Maples K, Plattner JJ, Bellinger-Kawahara C. 20070286822. US Pat Appl. 2007
- 9.(a) Zhou Y, Zhang Y, Akama T, Jarnagin K. WO 2010028005 A1 20100311. PCT Int Appl. 2010; (b) Akama T, Zhang Y, Ding CZ, Plattner JJ, Maples KR, Freund Y, Sanders V, Xia Y, Baker SJ, Niemann JA, et al. WO 2009111676 A2 20090911. PCT Int Appl. 2009; (c) Sanders V, Maples KR, Plattner JJ, Bellinger-Kawahara C. US 20070286822 A1 20071213. US Pat Appl Publ. 2007; (d) Baker SJ, Sanders V, Akama T, Bellinger-Kawahara C, Freund Y, Maples KR, Plattner JJ, Zhang Y, Zhou H, Hernandez VS. WO 2007095638 A2 20070823. PCT Int Appl. 2007; (e) Baker SJ, Akama T, Alley MRK, Benkovic SJ, Dipierro M, Hernandez VS, Hold KM, Kennedy I, Likhotvorik I, Mao W, et al. WO 2007078340 A2 20070712. PCT Int Appl. 2007; (f) Baker SJ, Akama T, Alley MRK, Benkovic SJ, Dipierro M, Hernandez VS, Hold KM, Kennedy I, Likhotvorik I, Mao W, et al. US 20070155699 A1 20070705. US Pat Appl Publ. 2007
- 10.(a) Jacobs R, Orr M, Wring S, Chen D, Zhou H, Ding D, Feng Y, Ye L, Hernandez VS, Zhang Y, et al. WO 2010045503 A1 20100422. PCT Int Appl. 2010; (b) Akama T, Zhang Y, Ding CZ, Plattner JJ, Maples KR, Freund Y, Sanders V, Xia Y, Baker SJ, Niemann JA, et al. WO 2009111676 A2 20090911. PCT Int Appl. 2009; (c) Sanders V, Maples KR, Plattner JJ, Bellinger-Kawahara C. US 20070286822 A1 20071213. US Pat Appl Publ. 2007; (d) Baker SJ, Sanders V, Akama T, Bellinger-Kawahara C, Freund Y, Maples KR, Plattner JJ, Zhang Y, Zhou H, Hernandez VS. WO 2007095638 A2 20070823. PCT Int Appl. 2007; (e) Baker SJ, Akama T, Alley MRK, Benkovic SJ, Dipierro M, Hernandez VS, Hold KM, Kennedy I, Likhotvorik I, Mao W, et al. WO 2007078340 A2 20070712. PCT Int Appl. 2007; (f) Baker SJ, Akama T, Alley MRK, Benkovic SJ, Dipierro M, Hernandez VS, Hold KM, Kennedy I, Likhotvorik I, Mao W, et al. US 20070155699 A1 20070705. US Pat Appl Publ. 2007
- 11.(a) Hanson GJ. WO 2013142087 A1 20130926. PCT Int Appl. 2013; (b) Lennarz WJ, Snyder HR. J Am Chem Soc. 1960;82:2172–2175. [Google Scholar]
- 12.Xia Y, Alley MRK, Zhou Y, Hernandez VS, Plattner JJ, Ding CZ, Cao K, Zhang Y, Akama T, Sligar J, et al. WO 2009140309 A2 20091119. PCT Int Appl. 2009
- 13.(a) Altaf M, Miller CH, Bellows DS, O’Toole R. Tuberculosis. 2010;90:333–337. doi: 10.1016/j.tube.2010.09.002. [DOI] [PubMed] [Google Scholar]; (b) Chaturvedi V, Dwivedi N, Tripathi RP, Sinha S. J Gen Appl Microbiol. 2007;53:333–337. doi: 10.2323/jgam.53.333. [DOI] [PubMed] [Google Scholar]; Tyagi JS, Sharma D. Trends Microbiol. 2002;10:68–69. doi: 10.1016/s0966-842x(01)02296-x. [DOI] [PubMed] [Google Scholar]
- 14.Franzblau SG, DeGroote MA, Cho SH, Andries K, Nuermberger E, Orme IM, Mdluli K, Angulo-Barturen I, Dick T, Dartois V, Lenaerts AJ. Tuberculosis. 2012;92:453–488. doi: 10.1016/j.tube.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Wayne LG. In: Mycobacterium tuberculosis Protocols. Parish T, Stoker NG, editors. Humana Press Inc; Totowa, NJ: 2001. pp. 247–270. [Google Scholar]